Abstract
Recent evidence has been presented demonstrating that group III mechanoreceptors comprise an important part of the sensory arm of the exercise pressor reflex, which in turn functions to increase arterial blood flow to contracting skeletal muscles. Although Group III afferents are stimulated by mechanical distortion of their receptive fields, they are also stimulated by bradykinin, which is produced by skeletal muscle when it contracts. Moreover, blockade of B (bradykinin)2 receptors has been shown to decrease the magnitude of the exercise pressor reflex. Nevertheless, the effect of blockade of B2 receptors on responses of group III afferents to contraction is not known. We therefore determined the effect of B2 receptor blockade with HOE 140 (40 μg/kg) on the responses to both static and intermittent contraction of group III afferents with endings in the triceps surae muscle of decerebrated unanesthetized cats. We found that HOE 140 significantly attenuated (P= 0.04) the responses of 14 group III afferents to static contraction, but did not significantly attenuate (P= 0.16) the responses of 16 group III afferents to intermittent contraction. The attenuation induced by HOE 140 was present throughout the static contraction period, and led us to speculate that blockade of B2 receptors on the endings of group III afferents decreased their sensitivity to mechanical events occurring in the working muscles.
Keywords: HOE 140, exercise pressor reflex, mechanoreception, bradykinin, locomotion
1. Introduction
The exercise pressor reflex, which is evoked by contracting skeletal muscles, increases arterial pressure, heart rate, sympathetic nerve discharge in animals and humans [1] [2] [3] [4] [5] [6] [7] [8] [9]. The afferent arm of the reflex arc is comprised of thinly myelinated group III afferents, which respond primarily to mechanical stimuli, and unmyelinated group IV afferents, which respond primarily to metabolic stimuli [2] [10] [11] [12]. The afferents evoking the reflex were believed to signal that the oxygen/blood supply to the exercising muscles was not adequate to meet its metabolic demand [5] [13]. The exercise pressor reflex, in turn, was believed to increase perfusion of the contracting muscle by increasing cardiac output and vascular resistance to non-exercising muscles and viscera [14] [15]. These findings resulted in investigations focusing on the responses of group IV afferents to administration of putative metabolites believed to be generated by a mismatch between oxygen/blood supply and demand during exercise [16] [17] [18] [19] [20].
Recent evidence has shown that group III mechanoreceptors also play a significant role in the generation of the exercise pressor reflex [4]. For example, gadolinium-induced blockade of mechanogated channels on group III afferents attenuated the reflex pressor and sympathetic nerve responses to static contraction [21] [22] [23]. Although gadolinium attenuated the responses of group III mechanoreceptors to contraction and stretch, it had no effect on their responses to bradykinin [23]. In addition, gadolinium had no effect on the responses of group IV metaboreceptors to contraction, a finding which further supported the selective effect of this agent on mechanogated channels [23]. Nevertheless, the mechanical sensitivity of group III afferents can be influenced by the chemical milieu surrounding their endings. For example, the responses of group III mechanoreceptors to static contraction were attenuated by indomethacin, an agent which decreased the production of prostaglandins and thromboxane by the exercising muscles [24] [25] [26]. Likewise, the responses of group III afferents to mechanical distortion of their receptive fields can be increased by prior administration of bradykinin [27], an autocoid whose concentration in skeletal muscle is increased by its contraction [28].
Several lines of evidence indicate that bradykinin plays a role in the generation of the exercise pressor reflex. For example, blockade of the enzyme that synthesized bradykinin in skeletal muscle attenuated the exercise pressor reflex [29]. Moreover, blockade of the enzyme that degraded bradykinin exaggerated the reflex [29]. In addition, blockade of bradykinin 2 receptors (B2) in contracting skeletal muscles attenuated the exercise pressor reflex [30]. Last, injection of bradykinin into the arterial circulation of hindlimb muscles of cats stimulated group III afferents, which were also mechanosensitive, [10] [17] as well as evoked reflex pressor responses [31]. Despite these findings, the role played by bradykinin in stimulating group III afferents during contraction is not known. We were prompted, therefore, to determine the effect of blockade of B2 receptors on the responses of group III afferents to static and intermittent contraction of the triceps surae muscles.
2. Methods
All procedures were approved by the Institutional Care and Use Committee of the Pennsylvania State College of Medicine.
Surgical preparation
Anesthesia was induced in 39 female cats (2.9 ± 0.1 kg) with 5% isofluorane in oxygen. The trachea was cannulated, and the lungs were ventilated mechanically with 3% isofluorane in oxygen. Catheters were placed in the right jugular vein and common carotid artery, with the latter being used to measure arterial pressure.
The cat was placed in a Kopf stereotaxic and spinal unit and then given dexamethasone (4 mg IV). A midcollicular decerebration was performed, and all neural tissue rostral to the section was removed. The cranial vault was filled with agar (37°C). A laminectomy was performed, the left triceps surae muscles were isolated, and the calcaneal bone was cut. The free end of the calcaneal tendon was attached to a force transducer to measure tension development. All visible branches of the left sciatic nerve innervating the thigh and hip as well as the left femoral nerve were cut. Anesthesia was terminated after the surgery was completed, but in some instances where the cat displayed excessive activity when the ventral roots were stimulated, we ventilated the lungs with 0.5% isofluorane in oxygen.
2.1 Recording of impulse activity from group III and IV afferents
The impulse activity from group III afferents with endings in the left triceps surae muscles was recorded from filaments dissected from the L7 or S1 dorsal roots. The afferent signals were passed through a high-impedance probe, amplified and filtered (100 3,000 Hz). Action potentials were displayed on a computer monitor (Spike 2).
The conduction velocity of an afferent was calculated by dividing the conduction distance between the recording electrode and the stimulating electrode on the tibial nerve by the conduction time. Afferents conducting impulses between 2.5 and 30 m/s were classified as group III afferents [10]. The receptive fields of the afferents were located in the triceps surae muscles by either gently stroking the muscle or squeezing it in a non-noxious manner. Afferents conducting impulses greater than 30 m/s were tested for their responses to muscle twitch which was evoked by single pulse stimulation of the ventral roots; the ones that were stimulated by twitch were classified as group Ib afferents (i.e., Golgi tendon organs) and the ones that were inhibited were classified as group Ia or II afferents (i.e., spindles) and were discarded.
The triceps surae muscles were contracted by electrical stimulation (15 20 Hz; 0.1 ms; 2–3 time motor threshold) of the cut peripheral ends of the L7 and S1 ventral roots. Two types of contraction were used. The first was static contraction, which was evoked by a maintained stimulation of the ventral roots for 45 seconds. The second was intermittent contraction, which was evoked by stimulation of the ventral roots for 0.5 seconds followed by no stimulation for 0.5 seconds until 45 seconds had elapsed.
2.2. Experimental protocol
We recorded group III and Ib afferent activity during both static and intermittent contraction. The order of the two types of contraction was varied randomly and the interval between the two contractions was at least 10 minutes. If an afferent appeared to be stimulated by either contraction, we injected the B2 receptor antagonist, HOE 140 (40 μg/ kg) into the right carotid artery. HOE 140 is more than 1000 fold more selective for the B2 receptor than for the B1 receptor [32]. The dose of HOE 140 used was greater than that used previously (i.e., 30 μg/ kg) [30]. Twenty minutes after injecting HOE 140, we initiated the two contraction protocols. In previous experiments in cats, we demonstrated that the responses of group III afferents to contraction of the triceps surae muscles were repeatable if no antagonist was given [33].
2.3. Data analysis
Baseline activity of each afferent was counted for the 30 second period immediately preceding either static or intermittent contraction. Likewise, activity of the afferent was counted for the 45 second period of contraction. Our criterion for stimulation of a group III afferent by either type of contraction was an increase in activity of >12 impulses in 45 s. The tension time index (TTI) [34] was calculated by integrating the area between the tension trace and its baseline level. All values are expressed as means ± SE. Two-way repeated-measures ANOVA followed by Scheffe post hoc tests were used to determine statistical significance, the criterion for which was P < 0.05.
3. Results
We recorded the impulse activity of 22 group III afferents with endings in the triceps surae muscles (conduction velocity: 13.8 ± 1.5 m/s; range: 2.7–26.2 m/s). The responses to static contraction were tested in each of the 22 group III afferents; likewise, the responses to intermittent contraction were tested in 19 of the 22 afferents.
3.1 Effects of HOE 140 on the responses of group III and group Ib afferents to static contraction
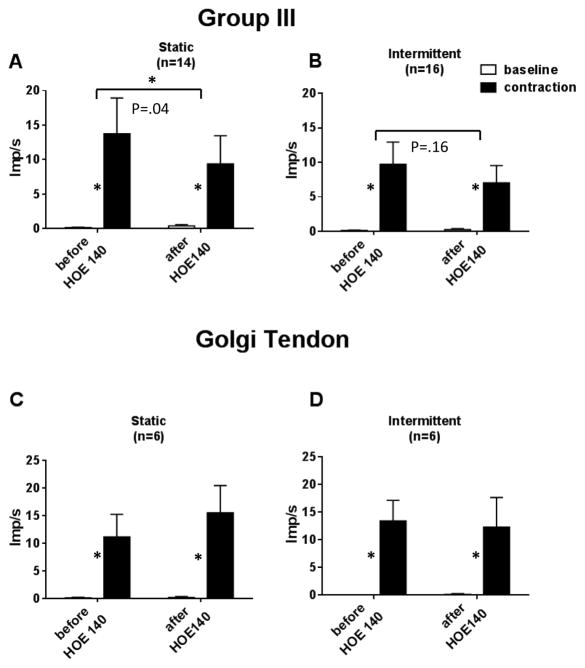
Static contraction of the triceps surae muscles stimulated 14 of the 22 group III afferents tested. On average, B2 receptor blockade significantly decreased the responses of the 14 group III afferents responding to static contraction (P= 0.04); nevertheless, contraction still increased the discharge of the 14 afferents over their baseline levels after injection of HOE 140 (P= 0.02; Figures 1, 2 and 3). On an individual basis, B2 receptor blockade attenuated the responses to static contraction of 12 of the 14 afferents tested. The tension time indices before and after HOE 140 averaged 165 ± 20 kg seconds before and 164 ± 17 kg seconds afterwards (P= 0.93; n=14). The conduction velocity of the 14 group III afferents responding to contraction averaged 15.1 ± 2.0 m/s, whereas the conduction velocity of the eight group III afferents not responding to contraction averaged 11.5 ± 2.0 m/s (P= 0.25). The attenuating effect of HOE 140 was present throughout the entire contraction period (Figure 3).
Figure 1.
Summary data showing the responses of group III and Ib afferents to static and intermittent contraction before and after HOE 140 (40μg/kg). Open bars represent baseline activity. Filled bars represent the discharge of the afferents during the contraction period. Asterisks represent a significant (P< 0.05) contraction-induced increase in activity over baseline levels. Horizontal brackets connect differences between responses to contraction and baseline before and after HOE 140. Note that HOE 140 significantly reduced (P< 0.04) the group III responses to static contraction (bracket with asterisk), but had no statistical effect on the group III responses to intermittent contraction (bracket with no asterisk).
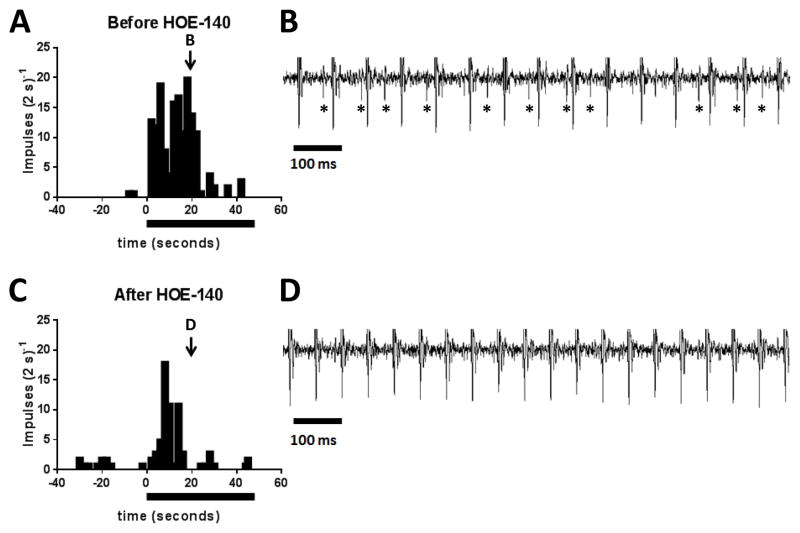
Figure 2.
The effect of HOE 140 (40μg/kg) on the responses to static contraction by a group III afferent (conduction velocity: 4.2 m/s). A and C plot the discharge of the group III afferent before, during, and after static contraction, which is signified by the filled horizontal bar. B and D show the recording of the afferent’s discharge at the same time points of the contraction period and is depicted by the arrow and corresponding letter in A and C. Note that the large vertical, equally spaced lines in B and D are stimulus artifact induced by electrical stimulation of the ventral roots. In B the ventral roots were stimulated at 15 Hz, whereas in D the ventral roots were stimulated at 20 Hz. The increase in frequency was necessitated to match tension development. Asterisks (*) signify impulses generated by the group III afferent.
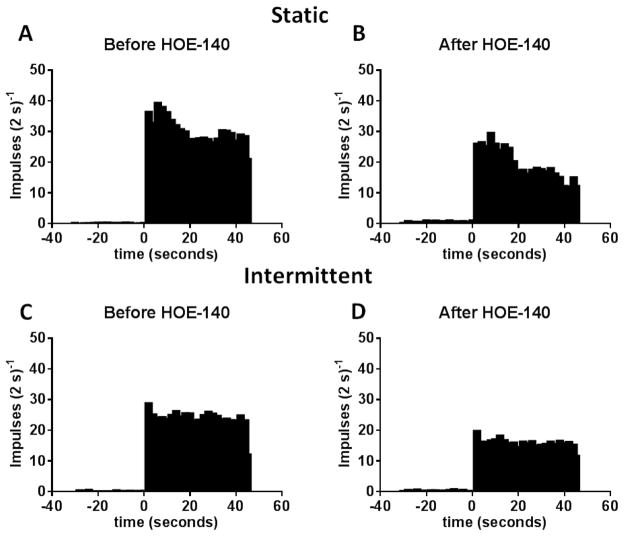
Figure 3.
A and B represent cumulative plots of the effects of HOE 140 (40 μg/kg) on the discharge of the 12 of the 14 group III afferents whose responses to static contraction were attenuated by B2 receptor blockade. Likewise C and D represent cumulative plots of the effects of HOE 140 (40 μg/kg) on the discharge of the 12 of the 16 group III afferents whose responses to intermittent contraction were attenuated by B2 receptor blockade. Note that the contraction period started at time zero and continued for 45 seconds.
HOE 140 had no effect on the responses of six group Ib afferents to static contraction (conduction velocity: 46 ± 3 m/s). The TTIs before and after HOE 140 averaged 237 ± 53 kg s and 218 ± 57 kg s, respectively (P= 0.42).
3.2 Effects of HOE 140 on the responses of group III and group Ib afferents to intermittent contraction
Intermittent contraction of the triceps surae muscles stimulated 16 of the 19 group III afferents tested. On average, B2 receptor blockade had no significant effect on the responses of the 16 group III afferents responding to intermittent contraction (P=0.16). On an individual basis, B2 receptor blockade appeared to decrease the responses of 12 of the 16 group III afferents, but the effect was small and overall was not statistically significant (Figures 1 and 3). The conduction velocity of the 16 group III afferents responding to intermittent contraction averaged 12.1 ± 1.6 m/s, whereas the conduction velocity of the three group III afferents not responding to contraction averaged 13.2 ± 3.1 m/s (P= 0.79). The tension time indices before and after HOE 140 averaged 89 ± 18 kg seconds before and 99 ± 19 kg seconds afterwards (P= 0.12; n=16).
HOE 140 (40μg/kg) had no effect on the responses of six group Ib afferents to intermittent contraction (conduction velocity: 46 ± 3 m/s). The TTIs before and after HOE 140 averaged 75 ± 9 kg s and 97 ± 26 kg s, respectively (P= 0.52).
4. Discussion
Bradykinin stimulates two types of receptors on primary afferents. The first, termed B1, is inducible; under normal conditions its level is low, although during inflammation or injury its level increases. The second receptor, termed B2, is constitutive and is therefore found on primary afferents in normal conditions [35] [36]. Only the B2 receptor has been shown to play a role in the generation of autonomic reflexes arising from skeletal muscle [30] [37]. Group III afferents comprise part of the afferent arm of the exercise pressor reflex [2] [12]. The role played by bradykinin in stimulating group III afferents during contraction is unknown. However, injection of bradykinin into the arterial supply of muscle stimulated the same group III afferents that are stimulated by contraction [10] [38] [27] [17]. These findings prompted us to investigate the effect of blockade of the B2 receptor on the responses of group III afferents to contraction. We found that blockade of B2 receptors with HOE 140 significantly decreased the responses of group III afferents to static contraction, but had no significant effect on their responses to intermittent contraction.
The doses of bradykinin used previously to stimulate group III afferents seemed large, raising the possibility that they created concentrations in the muscle that exceeded those created during contraction [10] [38] [17]. Our findings using a B2 receptor antagonist demonstrated that bradykinin significantly contributed towards stimulating these thinly myelinated afferents during static contraction. Our findings, nevertheless, suggest that this contribution is modest because much of the response to static contraction by these afferents remained after administration of HOE 140. One interpretation of this finding is that blockade of B2 receptors prevented bradykinin from sensitizing group III afferents to static contraction. Bradykinin-induced sensitization of group III afferents has been shown for other stimuli such as stretch and mechanical distortion of receptive fields [27]
We can only speculate as to why blockade of B2 receptors in our experiments reduced the responses of group III afferents to static contraction, but had no statistical effect on their responses to intermittent contraction. One explanation is that static contraction produced more bradykinin in the working muscles than did intermittent contraction. Support for our speculation comes from the fact that static contraction resulted in a higher TTI than did intermittent contraction. As a consequence, metabolism was likely to be higher and arterial blood flow was likely to be lower in muscles contracting statically than in muscles contracting intermittently. Both factors probably contributed to the generation of bradykinin by contracting skeletal muscle [28]. A second explanation is that the higher blood flow to the triceps surae muscles during intermittent contraction was higher than during static contraction and resulted in more washout of bradykinin.
Group Ib afferents are known to be stimulated by contraction, but their stimulation has no reflex autonomic effects. Group Ib afferents, therefore, do not play a role in evoking the exercise pressor reflex [2] [12]. In addition, group Ib afferents are not stimulated by doses of bradykinin that vigorously stimulate group III afferents [38]. These findings lead to the prediction that HOE 140 would have no effect on the responses of group Ib afferents to either static or intermittent contraction. We confirmed this prediction in our experiments.
The contraction-induced release of bradykinin by skeletal muscle can stimulate group III afferents by two mechanisms, namely by a direct action on B2 receptors and an indirect action by producing cyclooxygenase metabolites of arachidonic acid. Substantial evidence exists that cyclooxygenase products, such as prostaglandin E2 and thromboxane A2, either directly stimulate group III muscle afferents or sensitize them to static contraction [16] [33] [39]. Nevertheless, bradykinin-induced prostaglandin and thromboxane production requires activation of B2 receptors [40], which were blocked by HOE 140 in our experiments. Consequently, any bradykinin-induced prostaglandin and thromboxane production by the contracting muscles was probably prevented by HOE 140. Nevertheless, prostaglandin and thromboxane production by mechanisms other than bradykinin release by muscle still were available to stimulate group III afferents during contraction.
Our experiments have two important limitations. The first is that we did not challenge the efficacy of blockade by HOE 140. The dose of HOE 140 used, however, was one third larger than that used previously to abolish the pressor response to bradykinin injection into the arterial supply of hind limb muscle in cats [30]. The second limitation is that we did not examine the effect of B2 receptor blockade on the responses of group III afferents to ischemic contraction. We have previously shown, however, that ischemia does not increase the discharge of group III afferents to static contraction [11]. In fact, ischemia sometimes decreased their responses to static contraction because a lack of arterial blood flow decreased the ability of the muscles to develop tension, which in turn decreased the amount of mechanical distortion to the afferents’ receptive fields. We speculate that the decrease in mechanical stimulus to the group III afferents countered the increase in bradykinin production induced by ischemia [28].
In summary, we have shown that blockade of B2 receptors on the presumptive endings of group III afferents attenuated their responses to static contraction, but had minimal effect on their responses to intermittent contraction. Our findings confirm and extend previous evidence that bradykinin plays a role in evoking the exercise pressor reflex [28] [30]. This attenuating effect appears to present throughout the period of static contraction, and is consistent with the speculation that bradykinin production functions to sensitize group III afferents to mechanical stimulation.
Highlights.
The exercise pressor reflex is driven, in part, by group III mechanoreceptors.
Bradykinin2 receptor blockade attenuated group III responses to static contraction.
B2 blockade had no effect on group III responses to intermittent contraction.
Our data suggests that bradykinin helps evoke the exercise pressor reflex.
Acknowledgments
This work was supported by NIH grant HL 30710. We thank Ms. Joyce Kim for her technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol. 1971;215:789–804. doi: 10.1113/jphysiol.1971.sp009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell JH, Reeves DR, Rogers HB, Secher NH. Epidural anesthesia and cardiovascular responses to static exercise in man. J Physiol. 1989;417:13–24. doi: 10.1113/jphysiol.1989.sp017787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Victor RG, Rotto DM, Pryor SL, Kaufman MP. Stimulation of renal sympathetic activity by static contraction: evidence for mechanoreceptor-induced reflexes from skeletal muscle. Circ Res. 1989;64:592–599. doi: 10.1161/01.res.64.3.592. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: Its cardiovascular effects, afferent mechanisms, and central pathways. Ann Rev Physiol. 1983;45:229–242. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- 6.Sheriff DD, Wyss CR, Rowell LB, Scher AM. Does inadequate oxygen delivery trigger pressor response to muscle hypoperfusion during exercise? Am J Physiol. 1987;253:H1199–H1207. doi: 10.1152/ajpheart.1987.253.5.H1199. [DOI] [PubMed] [Google Scholar]
- 7.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol. 2010;109:966–976. doi: 10.1152/japplphysiol.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Leary DS, Sala-Mercado JA, Hammond RL, Ansorge EJ, Kim JK, Rodriguez J, Fano D, Ichinose M. Muscle metaboreflex-induced increases in cardiac sympathetic activity vasoconstrict the coronary vasculature. J Appl Physiol. 2007;103:190–194. doi: 10.1152/japplphysiol.00139.2007. [DOI] [PubMed] [Google Scholar]
- 9.Sala-Mercado JA, Hammond RL, Kim JK, Rossi NF, Stephenson LW, O’Leary DS. Muscle metaboreflex control of ventricular contractility during dynamic exercise. Am J Physiol Heart Circ Physiol. 2006;290:H751–H757. doi: 10.1152/ajpheart.00869.2005. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol. 1983;55:105–112. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman MP, Rybicki KJ, Waldrop TG, Ordway GA. Effect of ischemia on responses of group III and IV afferents to contraction. J Appl Physiol. 1984;57:644–650. doi: 10.1152/jappl.1984.57.3.644. [DOI] [PubMed] [Google Scholar]
- 12.Coote JH, Pérez-González JF. The response of some sympathetic neurones to volleys in various afferent nerves. J Physiol. 1970;208:261–278. doi: 10.1113/jphysiol.1970.sp009118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, Section 12: Exercise: Regulation and Integration of Multiple Systems II. Control of Respiratory and Cardiovascular Systems. Oxford University Press; New York, NY: 1996. pp. 381–447. [Google Scholar]
- 14.O’Leary DS, Augustyniak RA, Ansorge EJ, Collins HL. Muscle metaboreflex improves O2 delivery to ischemic active skeletal muscle. Am J Physiol. 1999;276:H1399–H1403. doi: 10.1152/ajpheart.1999.276.4.H1399. [DOI] [PubMed] [Google Scholar]
- 15.Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR, Richardson RS. On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol. 2011;589:3855–3866. doi: 10.1113/jphysiol.2011.209353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rotto DM, Kaufman MP. Effects of metabolic products of muscular contraction on the discharge of group III and IV afferents. J Appl Physiol. 1988;64:2306–2313. doi: 10.1152/jappl.1988.64.6.2306. [DOI] [PubMed] [Google Scholar]
- 17.Mense S. Nervous outflow from skeletal muscle following chemical noxious stimulation. J Physiol. 1977;267:75–88. doi: 10.1113/jphysiol.1977.sp011802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinoway LI, Hill JM, Pickar JG, Kaufman MP. Effects of contraction and lactic acid on the discharge of group III muscle afferents in cats. J Neurophysiol. 1993;69:1053–1059. doi: 10.1152/jn.1993.69.4.1053. [DOI] [PubMed] [Google Scholar]
- 19.Hanna RL, Kaufman MP. Role played by purinergic receptors on muscle afferents in evoking the exercise pressor reflex. J Appl Physiol. 2003;94:1437–1445. doi: 10.1152/japplphysiol.01011.2002. [DOI] [PubMed] [Google Scholar]
- 20.Hayes SG, Kindig AE, Kaufman MP. Blockade of Acid Sensing Ion Channels attenuates the exercise pressor reflex in cats. J Physiol. 2007;581:1271–2323. doi: 10.1113/jphysiol.2007.129197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith SA, Mitchell JH, Naseem RH, Garry MG. Mechanoreflex mediates the exaggerated exercise pressor reflex in heart failure. Circulation. 2005;112:2293–2300. doi: 10.1161/CIRCULATIONAHA.105.566745. [DOI] [PubMed] [Google Scholar]
- 22.Kim JK, Hayes SG, Kindig AE, Kaufman MP. Thin-fiber mechanoreceptors reflexly increase renal sympathetic nerve activity during static contraction. Am J Physiol Heart Circ Physiol. 2007;292:H866–H873. doi: 10.1152/ajpheart.00771.2006. [DOI] [PubMed] [Google Scholar]
- 23.Hayes SG, McCord JL, Koba S, Kaufman MP. Gadolinium inhibits group III but not group IV muscle afferent responses to dynamic exercise. J Physiol. 2009;587:873–882. doi: 10.1113/jphysiol.2008.164640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rotto DM, Massey KD, Burton KP, Kaufman MP. Static contraction increases arachidonic acid levels in gastrocnemius muscles of cats. J Appl Physiol. 1989;66:2721–2724. doi: 10.1152/jappl.1989.66.6.2721. [DOI] [PubMed] [Google Scholar]
- 25.Rotto DM, Hill JM, Schultz HD, Kaufman MP. Cyclooxygenase blockade attenuates the responses of group IV muscle afferents to static contraction. Am J Physiol. 1990;259:H745–H750. doi: 10.1152/ajpheart.1990.259.3.H745. [DOI] [PubMed] [Google Scholar]
- 26.Symons JD, Theodossy SJ, Longhurst JC, Stebbins CL. Intramuscular accumulation of prostaglandins during static contraction of the cat triceps surae. J Appl Physiol. 1991;71:1837–1842. doi: 10.1152/jappl.1991.71.5.1837. [DOI] [PubMed] [Google Scholar]
- 27.Mense S, Meyer H. Bradykinin-induced modulation of the response behavour of different types of feline group III and IV muscle receptors. J Physiol. 1988;398:49–63. doi: 10.1113/jphysiol.1988.sp017028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stebbins CL, Carretero OA, Mindroiu T, Longhurst JC. Bradykinin release from contracting skeletal muscle of the cat. J Appl Physiol. 1990;69:1225–1230. doi: 10.1152/jappl.1990.69.4.1225. [DOI] [PubMed] [Google Scholar]
- 29.Stebbins CL, Longhurst JC. Bradykinin in reflex cardiovascular response to static muscular contraction. J Appl Physiol. 1986;61:271–279. doi: 10.1152/jappl.1986.61.1.271. [DOI] [PubMed] [Google Scholar]
- 30.Pan H-L, Stebbins CL, Longhurst JC. Bradykinin contributes to the exercise pressor reflex: mechanism of action. J Appl Physiol. 1993;75:2061–2068. doi: 10.1152/jappl.1993.75.5.2061. [DOI] [PubMed] [Google Scholar]
- 31.Stebbins CL, Longhurst JC. Bradykinin-induced chemoreflexes from skeletal muscle: implications for the exercise reflex. J Appl Physiol. 1985;59:56–63. doi: 10.1152/jappl.1985.59.1.56. [DOI] [PubMed] [Google Scholar]
- 32.Dear JW, Wirth K, Scadding GK, Foreman JC. Characterization of the bradykinin receptor in the human nasal airway using the binding of [125I]-Hoe 140. Br J Pharmacol. 1996;119:1054–1062. doi: 10.1111/j.1476-5381.1996.tb15777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rotto DM, Schultz HD, Longhurst JC, Kaufman MP. Sensitization of group III muscle afferents to static contraction by products of arachidonic acid metabolism. J Appl Physiol. 1990;68:861–867. doi: 10.1152/jappl.1990.68.3.861. [DOI] [PubMed] [Google Scholar]
- 34.Perez-Gonzalez JF. Factors determining the blood pressure responses to isometric exercise. Circ Res. 1981;48:I-76–I-86. [PubMed] [Google Scholar]
- 35.Marceau F, Hess JF, Bachvarov DR. The B1 receptors for kinins. Pharmacol Rev. 1998;50:357–386. [PubMed] [Google Scholar]
- 36.Petho G, Reeh PW. Sensory and signaling mechanisms of bradykinin, eicosanoids, platelet-activating factor, and nitric oxide in peripheral nociceptors. Physiol Rev. 2012;92:1699–1775. doi: 10.1152/physrev.00048.2010. [DOI] [PubMed] [Google Scholar]
- 37.Lu J, Xing J, Li J. Bradykinin B2 receptor contributes to the exaggerated muscle mechanoreflex in rats with femoral artery occlusion. Am J Physiol Heart Circ Physiol. 2013;304:H1166–H1174. doi: 10.1152/ajpheart.00926.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaufman MP, Iwamoto GA, Longhurst JC, Mitchell JH. Effects of capsaicin and bradykinin on afferent fibers with endings in skeletal muscle. Circ Res. 1982;50:133–139. doi: 10.1161/01.res.50.1.133. [DOI] [PubMed] [Google Scholar]
- 39.Kenagy J, VanCleave J, Pazdernik L, Orr JA. Stimulation of group III and IV afferent nerves from the hindlimb by thromboxane A2. Brain Res. 1997;744:175–178. doi: 10.1016/s0006-8993(96)01211-5. [DOI] [PubMed] [Google Scholar]
- 40.Jenkins DW, Sellers LA, Feniuk W, Humphrey PP. Characterization of bradykinin-induced prostaglandin E2 release from cultured rat trigeminal ganglion neurones. Eur J Pharmacol. 2003;469:29–36. doi: 10.1016/s0014-2999(03)01732-1. [DOI] [PubMed] [Google Scholar]