Abstract
RNA interference holds the promise to knock down expression of every cancer gene. Both academic laboratories and pharmaceutical companies have committed heavily on manpower and financial resources to develop siRNA cancer therapeutics over the last decade. While significant advances have been made in the design of siRNA therapeutics and mechanism of action on cancer cell killing, there are still many hurdles to overcome including effective delivery of therapeutics in vivo. Nanotechnology has played an important role in the development of delivery vectors so far. This article summarizes current nanovectors for siRNA delivery, discusses technical challenges in overcoming biological barriers, and introduces the multistage vector system for tumor-specific delivery.
Keywords: nanovector, multistage, delivery, siRNA, cancer
INTRODUCTION
Ever since its discovery,(1) small interfering ribonucleic acid (siRNA) has been widely recognized to be capable of silencing many or perhaps all genes, thereby modulating or selectively blocking the biological processes that are the defining hallmarks of cancer.(2, 3) A tremendous amount of effort has been spent on developing siRNA cancer therapeutics over the last 10 years, and much progress has been achieved on research and development, both in academics and the pharmaceutical industry. However, no product has been launched into the market, and there are still many challenges to be overcome in this field. The current review will update recent achievements in siRNA nanotherapeutics, summarize challenges on effective tumor delivery, and discuss new directions in the design of delivery systems.
ADVANCES IN siRNA THERAPEUTICS
Nanovectors for siRNA delivery
Most siRNA oligonucleotides (oligos) for in vivo use are chemically synthesized duplexes of 21 to 23 oligos based on the original work of Tuschl and colleagues.(4, 5) Although chemical modifications have been developed to improve their stability in vivo, siRNA oligos are still vulnerable to degradation by plasma and tissue nucleases. The delivery of nucleic acids to target organs or cells is challenging because they are relatively large in size and do not readily diffuse across the cellular membranes. Consequently, siRNA oligos are commonly delivered to tumor tissues in nano-scale delivery vehicles (nanovectors). A good nanovector should meet the following minimal criteria: 1) to protect the siRNA from degradation, 2) to enrich siRNA in the target organ, and 3) to facilitate the cellular uptake of siRNA.
Nanotechnology has played an important role in siRNA cancer therapy, and many nanovector strategies have been developed for siRNA delivery. Current nanovectors can be divided into three groups: lipid-based, non-lipid organic-based, and inorganic (Table 1).
Table 1.
Nanovectors for siRNA delivery
| Class | Type of nanovectors | References |
|---|---|---|
| Lipid-based nanovectors | Liposomes | Fenske and Collis(6) Wu et al.(7) Ozpolat et al.(8) |
| Stable nucleic acid lipid particles | Judge et al.(11) | |
| Lipidoid nanoparticles | Akinc et al.(12) Love et al.(13) | |
| Non-lipid organic-based nanovectors | Chitosan | Kim et al.(14) |
| Cyclodextrin-containing polycations | Davis et al.(15) | |
| Dendrimers | Monteagudo et al.(16) | |
| Polyethylenimines | Cubillos-Ruiz et al.(17) | |
| Other polymer conjugates | Rozema et al.(18) | |
| Inorganic particles | Gold nanoparticle | Braun et al.(19) Lu et al.(20) |
Most research laboratories and biotech/pharmaceutical companies have used lipid-based nanovectors for siRNA delivery so far. Liposomes have been the default choice for the majority of the studies. Advances in liposomes as carriers for experimental therapy of cancer and other diseases have been summarized by recent review articles.(6-8) Many products are being tested in preclinical studies. Using liposomes to package siRNA targeting the PKN3 gene encoding protein kinase N3, scientists at Silence Therapeutics have successfully demonstrated knockdown of gene expression in tumor vascular endothelium, and subsequently have shown inhibition of lung metastasis in animal tumor models.(9, 10) The product has been approved for evaluation in a human clinical trial.
The first nonhuman primate study on siRNA delivery was carried out with stable nucleic acid lipid particles (SNALPs).(21) SNALPs are PEGylated (poly-ethylene glycol-conjugated) liposomes comprised of siRNA encapsulated inside a lipid bilayer of cationic lipids, neutral lipids, and PEG-lipid fusion regulators.(22) SNALP-formulated siRNA nanoparticles have a longer half-life in plasma and liver comparing to conventional liposomal siRNA. Both Tekmira Pharmaceuticals and Alnylam Pharmaceuticals have applied SNALPs to develop siRNA therapeutics. Interestingly, both APOB siRNA (21, 23) and KSP siRNA (11) from these companies target liver-related diseases, taking advantage of liver enrichment of this delivery vector.
Lipidoids are lipid-like delivery molecules. The lipidoid nanoparticles contain lipidoids, cholesterol, and PEG-modified lipids specific for siRNA delivery.(12, 13, 24) Like SNALPs, lipidoid-siRNA nanoparticles tend to accumulate in the liver.(13, 24) One of the advantages of lipidoids over conventional liposomes for systemic delivery is that only a fraction of the siRNA oligos is needed to achieve the same extent of knockdown efficiency.(13) The safety and efficacy of lipidoids have been evaluated in mice, rats, and nonhuman primates.(12, 13)
Although some non-lipid-based nanovectors are still in early phases of development and existing forms of this technology are not as widely used as liposomes, non-lipid-based nanovectors have made a very significant impact on siRNA therapy. For example, cyclodextrin nanoparticles successfully delivered siRNA directed at the M2 subunit of ribonucleotide reductase (RRM2).(15, 25) The nanoparticle delivery system consists of a linear, cyclodextrin-based polymer as the building block with hydrophilic PEG molecules linked to them via adamantine. Human transferrin ligands were conjugated to some of the PEG molecules as the tumor-targeting moiety, as cancer cells overexpress transferrin receptor. The system has been tested in a previous study with mouse tumor models.(26) Although surface conjugation of the affinity moiety did not alter biodistribution comparing to the transferring-free particles, it did improve tumor cell uptake.
In recent years, integrated nanovectors have been developed to combine the unique features of different types of non-lipid-based vectors. The Dynamic PolyConjugates (DPC) of siRNA contain carboxylated dimethyl maleic acid and a polymeric amine, providing for a cell membrane lytic ability that can disrupt the endosome and release siRNA in response to the reducing environment of the cytoplasm.(18) The solid lipid-PEI (poly-ethylenimine) hybrid nanovector takes advantages of the high transfection performance of linear PEI and the controlled release properties of solid lipid components.(27) In glycol chitosan-PEI nanoparticles, a strong positively-charged surface complexes tightly with negatively charged siRNA.(28) The particles showed higher tumor-targeting ability in vivo. Bhatia and colleagues recently developed gold nanoparticles that incorporated both signaling and receiving modules for tissue-specific drug delivery.(29) Such new devices have the potential to integrate multi-functional agents together for enhanced drug delivery.
Advances in clinical studies
The success of siRNA therapeutics for human cancer treatment has been demonstrated by recent progress in clinical trials. The first-in-cancer evidence of siRNA modulation of gene expression was reported by Davis and colleagues.(15) The phase I trial was initiated in 2008 to determine the tolerability, safety profile, and maximum tolerated dose of the RRM2 siRNA drug CALAA-01 (http://www.clinicaltrials.gov/ct2/show/NCT00689065). The authors were able to demonstrate successful delivery of targeted nanoparticles to tumor tissues in all three cancer patients in this study.(15) Moreover, knockdown of RRM2 was confirmed in tumor biopsies from these patients by quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR), and by immunohistochemical staining in the patient treated with the highest dosage.(15) This study demonstrated the feasibility of systemic delivery of siRNA therapeutics for the treatment of human cancers.
More products are on their way to clinic. Alnylam Pharmaceutics initiated a phase I dose-escalation trial for SNALP/siRNA targeting VEGF and KSP1 (ALN-VSP02) in 2008 (http://www.clinicaltrials.gov/ct2/show/NCT01158079). This was the first dual targeted siRNA drug for a clinical trial. It was hypothesized that targeting two important genes for tumor growth would have an increased therapeutic efficacy on liver tumors. Interim safety, pharmacokinetic and pharmacodynamic report showed that ALN-VSP02 was generally well tolerated at the highest dose (1.25 mg/kg). A phase II efficacy evaluation with a 1 mg/kg dose has been planned. Silence Therapeutics initiated a phase I, dose-finding study with Atu027 (siRNA targeting the PKN3 gene encoding protein kinase N3) given as single treatment followed by repeated treatments in 2009 (http://www.clinicaltrials.gov/ct2/show/NCT00938574). Silenseed Ltd is conducting a phase I trial to assess the safety of the implantation of a single siG12D LODER (Local Drug EluteR targeting G12D K-Ras mutations) in patients diagnosed with operable adenocarcinoma of the pancreas or with high evidence of operable adenocarcinoma of the pancreas (http://www.clinicaltrials.gov/ct2/show/NCT01188785). K-Ras mutations are found in approximately one-third of all human malignancies,(30) and are responsible for therapy resistance in multiple tumor types.(31) Evidence of therapeutic efficacy on targeting mutant K-Ras will significantly impact the treatment of multiple cancer types. Both trials will be completed in the summer of 2012. They will be followed by a phase I dose escalation study sponsored by the National Cancer Institute on hepatic intra-arterial administration of TKM 080301 (lipid nanoparticles containing siRNA against the PLK1 gene encoding polo-like kinase 1 in multiple cancer types http://www.clinicaltrials.gov/ct2/show/NCT01437007). This study is expected to complete in 2014.
Cancer immunotherapy has also seen an influx of siRNA approaches. For example, Duke University has sponsored a phase I trial to test the hypothesis that targeting the immunoproteasome beta subunits LMP2, LMP7, and MECL1 with siRNA would alter proteasome-mediated antigen processing by dendritic cells, thereby inducing enhanced anti-melanoma immune responses from tumor antigens (http://www.clinicaltrials.gov/ct2/show/NCT00672542).
CHALLENGES IN siRNA THERAPY
Despite all the promising advances in preclinical and clinical studies, there are still many technical hurdles to overcome. Lack of efficacy has always been a concern with siRNA therapeutics. Cancer is a complicated disease with genetic and epigenetic alterations. Dependent on the cancer type, there could be as high as 100 mutations that result in amino acid changes in a certain tumor tissue.(32) A single therapeutic agent is unlikely to cure cancer. Moreover, overloading the system with siRNA oligos could easily trigger an innate immune response and produce toxic effects, although chemical modifications can offer some benefit to evade immune detection.(33) After a few setbacks, several big pharmaceutical companies decided to abandon or dramatically reduce their development programs in 2010-2011.
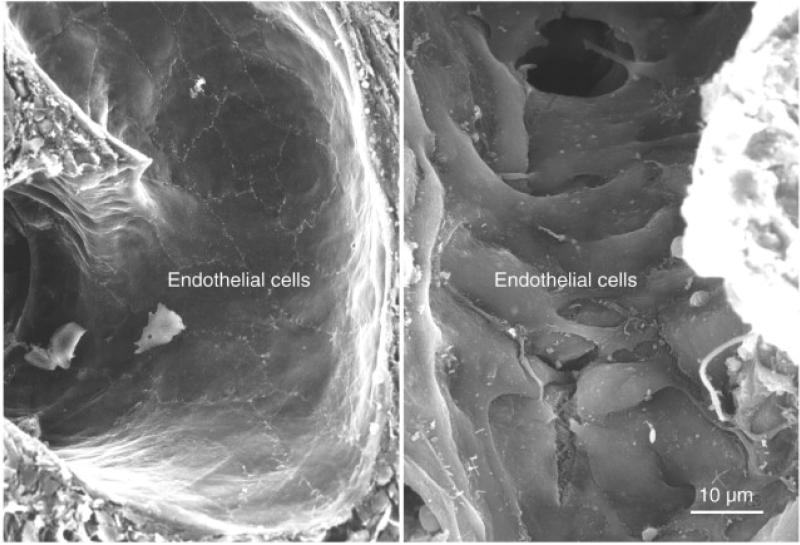
The central issue has been tissue-specific delivery of siRNA therapeutics. Multiple biological barriers inside the body prevent circulating nanoparticles from accumulating in the tumor. Most current nanovectors used in drug development and in clinical trials are based on the enhanced permeability and retention (EPR) effect of tumor tissues.(34, 35) The tumor vasculature is discontinuous and the endothelial cells are poorly aligned with large fenestrations – 100-500 nanometers in size (Fig. 1). Thus the nanoparticles, if sufficiently small, would passively cross the fenestrations and eventually accumulate in the tumor interstitium. In a series of seminal papers, Jain and collaborators showed that liposomes and latex beads smaller than 300–400 nm in diameter accumulated more efficiently in tumors than larger beads via passive extravasation at the tumor fenestrations.(36, 37)
Figure 1. Scanning electron micrograph of blood vessels from normal and tumor tissues.
Left: Smooth, tight endothelial cell monolayer covering the luminal surface of a normal blood vessel. Right: Disorganized endothelium of a tumor blood vessel. A gap is apparent at an open endothelial cell juncture. (Reproduced with permission from (61). Courtesy of Elsevier)
However, tumor vessel endothelium is only one of these barriers. En route to tumor tissues, the systemically administered nanoparticles need to overcome a serial of biological barriers.(38) A few of the major ones are discussed below.
Plasma and tissue nucleases
High activity of nucleases can be detected in blood circulation and in tumor tissues. The exterior surface of nanovectors serves as a shield to protect siRNA from degradation. To enhance the chance of fenestration, nanovectors are often PEGylated to increase their circulation time. Over half of the current siRNA cancer drugs in clinical trials fall into this category (CALAA-01 from Calando Pharmaceuticals, ALN-VSP02 from Alnylam Pharmaceuticals, Atu027 from Silence Therapeutics, TKM 080301 from Tekmira Pharmaceuticals), and more drug candidates will be added onto this list in the coming years. Extended circulation time is accompanied with an increased chance of siRNA degradation by plasma nucleases. Digestion of the building blocks of the nanovectors by lipases both in circulation and in tumor interstitium also exposes siRNA oligos to nucleases.
Reticulo-endothelial system (RES)
The RES organs, such as liver and spleen, are characterized by an intricate vascular architecture with vessel fenestrations and are densely populated by specialized cells of the immune system. In the liver, Kupffer cells line the blood vessels resting firmly over the endothelium as sentinels; and specialized macrophages accumulate in the splenic white pulp. These features allow such organs to function as natural filters sequestering foreign objects from the circulation, such as pathogens and macromolecules. While PEGylation of particles creates a stealth effect that reduces RES uptake, this modification does not fully protect the nanoparticles. Not surprisingly, a large portion of systemically injected nanoparticles accumulates in these major RES organs where siRNA oligos will be degraded.
The immune system
It is well established that certain motifs in siRNA oligos can trigger an innate immune response through recruitment of toll-like receptors (TLR) 3, 7 and 8.(39, 40) It is important to design siRNA oligos in combination with delivery strategies to evade cells of the immune system. The dicer-substrate siRNAs have been proposed to have enhanced potency as they need to be processed by the RNAi-induced silencing complex (RISC).(41) However, a recent study indicated that they were more immunostimulatory than the canonical siRNAs.(42) It is interesting to note that under certain circumstances the innate immune response could be beneficial. The success of Bevasiranib (VEGFA siRNA) in treating choroidal neovascularization by intraocular injection has at least partially been attributed to non-sequence-specific TLR3 activation.(43)
Heterogeneity of tumor vasculature
Tumor blood vessels differ dramatically from those in normal tissues, characterized by their abnormal structure, abnormal blood flow, high interstitial pressure, and pathophysiological microenvironment. It is not uncommon to identify regions from a single tumor tissue with different vascularity, different angiogenesis status (and thus different pH value), and different metabolic rate.(44, 45) Consequently, not every region shares the same accessibility by nanoparticles, and hence same extent of knockdown of key genes by siRNA. As a result, complete eradication of the tumor tissue is difficult.
Endosomes/Lysosomes
Phagocytosis is a mechanism for the cell to remove pathogens and cell debris. The endocytic vesicles will travel through the endosomal compartments, and eventually fuse with the lysosomes where the content will be hydrolyzed. Vesicle transport is also the main route for most nanoparticles once they are internalized by tumor cells. A large portion of the siRNA oligos will be digested during this process after a long journey to reach cancer cells.
Improvements have been made by the scientific community to develop the ideal nanovector for drug delivery. However, much of the efforts have been focused on solving one problem at a time, while creating another problem. Surface conjugation of affinity ligand proteins, for example, increases the chance to bind the tumor surface receptor; in the mean time, the process also increases the overall size of the nanoparticle. However, the size of the nanoparticle is a key determinant of tissue accessibility in many cancer types, such as pancreatic cancer.(46) Out of the drug-loaded micelles in the range of 30-100 nm in diameter, only the 30 nm particles could penetrate the poorly permeable tumor tissues to achieve an anti-tumor effect. Besides, the particles with increased sizes will more likely be captured by the filtering organs and be less capable of transport through the tumor interstitium.(45, 47, 48) Surface PEGylation increases particle circulation time at the expense of reduced targeting specificity, since PEG molecules sterically disrupt selective conjugation.(38) Thus, new strategies should be introduced in the design of next generation drug delivery systems.
MULTISTAGE VECTOR-MEDIATED siRNA DELIVERY
The multistage vector (MSV) delivery system is comprised of two delivery carriers: 1) the first stage micrometer-size porous silicon particle, and 2) the second stage nanoparticles (e. g., liposomes) incorporated with therapeutic agents such as siRNA or miRNA (the third stage component).(49) The second stage particles are loaded into the nanopores of the first stage porous silicon for drug delivery. This is a particle-in-particle system, with each stage designed to provide transport across a set of sequential biological barriers, and provide associated levels of targeting specificity. The drug-loaded first stage carrier travels in the circulation, and settles at tumor vasculature where the second stage nanoparticles get released and enter the tumor interstitium (Fig. 2). This system has been successfully applied for siRNA delivery,(50), enhanced tumor thermotherapy,(51) and improved tumor imaging.(52)
Figure 2. Schematic illustration of siRNA delivery with multistage vector.
(a) The 1st stage porous silicon loaded with siRNA nanoparticles travels in the circulation, and attaches to the tumor vascular endothelium. (b) The 1st stage particle releases 2nd stage carrier nanoparticles through the vascular endothelium into tumor interstitium where they are taken up by tumor cells. (c) Possible routes of siRNA entry into tumor cells.
Tumor targeting
Mathematical models have been applied in the design of MSVs incorporating parameters of vascular structure and flow dynamics in tumor tissues.(53, 54) Optimal tumor enrichment of MSVs can be achieved in particles with the right combination of size, shape, and surface chemical modifications.(55) The discoidal particles accumulate more effectively in tumor vasculature than the spherical or cylindrical particles, as this type of particles has enhanced interaction with the vessel wall and is more resistant to internalization by macrophages. Interestingly, conjugation with tumor-targeting affinity moieties, such as the RGD peptide, onto the surface of discoidal particles did not improve tumor accumulation of the micrometer-size particles as significantly as the sub-micrometer particles.(56)
Since the porous silicon microparticles can be fabricated with any size, shape, and porosity,(57) the MSV can be designed to optimize siRNA delivery based on tumor vasculature and disease stage for each individual patient. Thus, in contrast to conventional siRNA therapy which solely relies on the EPR effect of tumor vasculature for drug delivery, targeted therapy with this new approach is achieved through two layers of accuracy: personalized delivery of drugs by MSVs and targeted knockdown of cancer genes by siRNA.
Tumor uptake
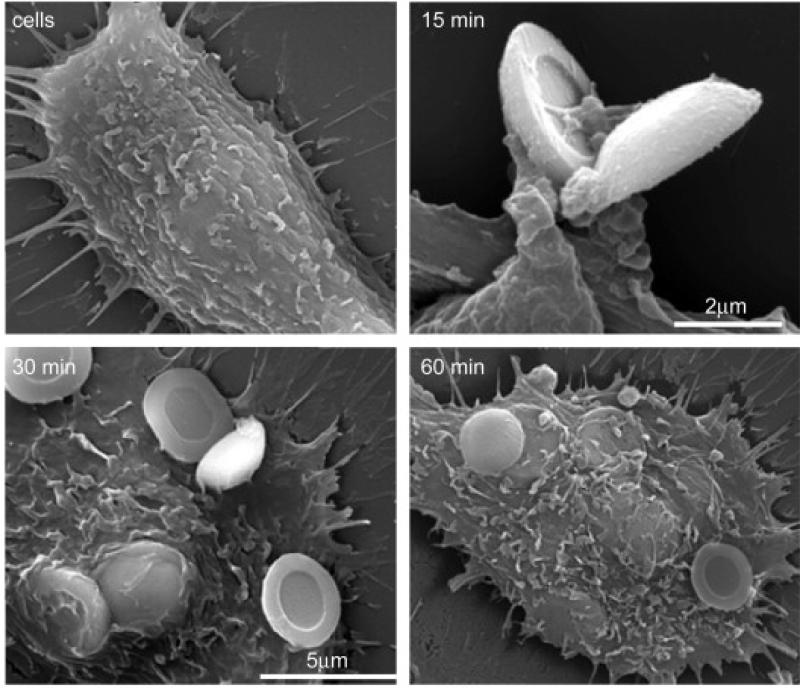
Once in tumor vasculature, MSVs are actively internalized by vessel endothelial cells. In an in vitro study, Serda et al. examined particle uptake by human umbilical vein endothelial cells (HUVECs).(58) Scanning electron microscopy (SEM) images showed that particle uptake started as early as 15 minutes in incubation, and most particles had been internalized within an hour (Fig. 3). They further analyzed mechanisms of particle internalization, and found that both phagocytosis and macropinocytosis were involved in the process.(58) They also tested the impact of surface chemical modification on particle internalization by endothelial cells. While both oxidized and 3-aminopropyl-triethoxysilane (APTES)-modified particles were efficiently internalized by HUVECs, surface conjugation with PEG-5000 effectively suppressed particle uptake by the same cells. In another study, they showed that chemical modification of the second stage nanoparticles determined their fate on subcellular localization and trafficking.(59) The amine-functionalized nanoparticles were released from MSV and entered the vesicular transport. Some vesicular bodies were secreted from one cell and passed to another cell. However, the chitosan-coated nanoparticles escaped the endosome pathway and entered cytosol directly once they were released from the first stage particle.
Figure 3. Uptake of microparticles by endothelial cells.
HUVECs were incubated with 3.2 μm oxidized silicon microparticles at a ratio of 1:10 (cell/particle) for 15-60 min in a serum-free medium. Uptake of particles by HUVEC cells was imaged by SEM. (Reproduced with permission from (58). Courtesy of Elsevier)
Tumor therapy
Therapeutic efficacy of MSV-delivered siRNA has been validated in mouse models of human ovarian cancers.(50) The EphA2 oncoprotein is overexpressed in the vasculature of most ovarian tumors.(60) EphA2 siRNA was incorporated into 30-40 nm neutral liposomes that were loaded into the 60-80 nm pores in the first stage porous silicon. Modification with APTES provided stability of the MSVs inside the body for up to 3 weeks, dependent on the microenvironment of individual tissues/organs. Knockdown of EphA2 expression was maintained for up to three weeks after one treatment of MSV/EphA2 siRNA (Fig. 4). Consequently, tumor growth was effectively inhibited. The extent of inhibition on tumor growth from a single dose of MSV/EphA2 siRNA was comparable to, if not more effective than, treatment of the tumor mice with free liposomal EphA2 siRNA every three days.(50) This study demonstrated the power of MSVs to maintain sustained siRNA delivery in tumor tissues.
Figure 4. Systemic delivery of MSV/EphA2 siRNA results in long-lasting in vivo gene silencing.
Mice bearing human SKOV3ip1 orthotopic ovarian tumors were dosed once with MSV/ePhA2 siRNA. (A) Knockdown of EphA2 gene expression was confirmed by Western blot analysis. (B) Densitometric analysis to normalize EphA2 expression by β-actin. (C) Immunohistochemical analysis of EphA2 expression in tumor tissues. (Reproduced with permission from (50). Courtesy of American Association for Cancer Research)
CONCLUSION
Tumor-specific delivery of siRNA has great potential for successful targeted cancer therapy. The shift of drug biodistribution towards tumor tissues not only improves therapeutic efficacy, but also reduces unwanted side effects when properly formulated. While improvements are being pursued for individual nanovectors, it is critical to integrate multiple features so that the new delivery system will be empowered to negotiate through multiple biological barriers.
ACKNOWLEDGEMENT
The authors acknowledge financial support from the following sources: Department of Defense grant DODW81XWH-09-1-0212, National Institute of Health grants NIH RO1CA128797, NIH U54CA143837, and NIH U54CA151668.
REFERENCES
- 1.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998 Feb 19;391(6669):806–11. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000 Jan 7;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011 Mar 4;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001 May 24;411(6836):494–8. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 5.Elbashir SM, Lendeckel W, Tuschl T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 2001 Jan 15;15(2):188–200. doi: 10.1101/gad.862301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fenske DB, Cullis PR. Liposomal nanomedicines. Expert Opin Drug Deliv. 2008 Jan;5(1):25–44. doi: 10.1517/17425247.5.1.25. [DOI] [PubMed] [Google Scholar]
- 7.Wu SY, McMillan NA. Lipidic systems for in vivo siRNA delivery. AAPS J. 2009 Dec;11(4):639–52. doi: 10.1208/s12248-009-9140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozpolat B, Sood AK, Lopez-Berestein G. Nanomedicine based approaches for the delivery of siRNA in cancer. J Intern Med. 2010 Jan;267(1):44–53. doi: 10.1111/j.1365-2796.2009.02191.x. [DOI] [PubMed] [Google Scholar]
- 9.Santel A, Aleku M, Keil O, Endruschat J, Esche V, Fisch G, et al. A novel siRNA-lipoplex technology for RNA interference in the mouse vascular endothelium. Gene Ther. 2006 Aug;13(16):1222–34. doi: 10.1038/sj.gt.3302777. [DOI] [PubMed] [Google Scholar]
- 10.Santel A, Aleku M, Keil O, Endruschat J, Esche V, Durieux B, et al. RNA interference in the mouse vascular endothelium by systemic administration of siRNA-lipoplexes for cancer therapy. Gene Ther. 2006 Sep;13(18):1360–70. doi: 10.1038/sj.gt.3302778. [DOI] [PubMed] [Google Scholar]
- 11.Judge AD, Robbins M, Tavakoli I, Levi J, Hu L, Fronda A, et al. Confirming the RNAi-mediated mechanism of action of siRNA-based cancer therapeutics in mice. J Clin Invest. 2009 Mar;119(3):661–73. doi: 10.1172/JCI37515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akinc A, Zumbuehl A, Goldberg M, Leshchiner ES, Busini V, Hossain N, et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol. 2008 May;26(5):561–9. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Love KT, Mahon KP, Levins CG, Whitehead KA, Querbes W, Dorkin JR, et al. Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci U S A. 2010 Feb 2;107(5):1864–9. doi: 10.1073/pnas.0910603106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim HS, Han HD, Armaiz-Pena GN, Stone RL, Nam EJ, Lee JW, et al. Functional roles of Src and Fgr in ovarian carcinoma. Clin Cancer Res. 2011 Apr 1;17(7):1713–21. doi: 10.1158/1078-0432.CCR-10-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010 Apr 15;464(7291):1067–70. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monteagudo S, Perez-Martinez FC, Perez-Carrion MD, Guerra J, Merino S, Sanchez-Verdu MP, et al. Inhibition of p42 MAPK using a nonviral vector-delivered siRNA potentiates the antitumor effect of metformin in prostate cancer cells. Nanomedicine (Lond) 2011 Oct 13; doi: 10.2217/nnm.11.61. [DOI] [PubMed] [Google Scholar]
- 17.Cubillos-Ruiz JR, Engle X, Scarlett UK, Martinez D, Barber A, Elgueta R, et al. Polyethylenimine-based siRNA nanocomplexes reprogram tumor-associated dendritic cells via TLR5 to elicit therapeutic antitumor immunity. J Clin Invest. 2009 Aug;119(8):2231–44. doi: 10.1172/JCI37716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rozema DB, Lewis DL, Wakefield DH, Wong SC, Klein JJ, Roesch PL, et al. Dynamic PolyConjugates for targeted in vivo delivery of siRNA to hepatocytes. Proc Natl Acad Sci U S A. 2007 Aug 7;104(32):12982–7. doi: 10.1073/pnas.0703778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braun GB, Pallaoro A, Wu G, Missirlis D, Zasadzinski JA, Tirrell M, et al. Laser-Activated Gene Silencing via Gold Nanoshell-siRNA Conjugates. ACS Nano. 2009 Jul 28;3(7):2007–15. doi: 10.1021/nn900469q. [DOI] [PubMed] [Google Scholar]
- 20.Lu W, Zhang G, Zhang R, Flores LG, 2nd, Huang Q, Gelovani JG, et al. Tumorsite-specific silencing of NF-kappaB p65 by targeted hollow gold nanospheremediated photothermal transfection. Cancer Res. 2010 Apr 15;70(8):3177–88. doi: 10.1158/0008-5472.CAN-09-3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004 Nov 11;432(7014):173–8. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 22.Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W, et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol. 2005 Aug;23(8):1002–7. doi: 10.1038/nbt1122. [DOI] [PubMed] [Google Scholar]
- 23.Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006 May 4;441(7089):111–4. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
- 24.Akinc A, Goldberg M, Qin J, Dorkin JR, Gamba-Vitalo C, Maier M, et al. Development of lipidoid-siRNA formulations for systemic delivery to the liver. Mol Ther. 2009 May;17(5):872–9. doi: 10.1038/mt.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heidel JD, Yu Z, Liu JY, Rele SM, Liang Y, Zeidan RK, et al. Administration in non-human primates of escalating intravenous doses of targeted nanoparticles containing ribonucleotide reductase subunit M2 siRNA. Proc Natl Acad Sci U S A. 2007 Apr 3;104(14):5715–21. doi: 10.1073/pnas.0701458104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc Natl Acad Sci U S A. 2007 Sep 25;104(39):15549–54. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xue HY, Wong HL. Solid lipid-PEI hybrid nanocarrier: an integrated approach to provide extended, targeted, and safer siRNA therapy of prostate cancer in an all-in-one manner. ACS Nano. 2011 Sep 27;5(9):7034–47. doi: 10.1021/nn201659z. [DOI] [PubMed] [Google Scholar]
- 28.Huh MS, Lee SY, Park S, Lee S, Chung H, Choi Y, et al. Tumor-homing glycol chitosan/polyethylenimine nanoparticles for the systemic delivery of siRNA in tumor-bearing mice. J Control Release. 2010 Jun 1;144(2):134–43. doi: 10.1016/j.jconrel.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 29.von Maltzahn G, Park JH, Lin KY, Singh N, Schwoppe C, Mesters R, et al. Nanoparticles that communicate in vivo to amplify tumour targeting. Nat Mater. 2011 Jul;10(7):545–52. doi: 10.1038/nmat3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989 Sep 1;49(17):4682–9. [PubMed] [Google Scholar]
- 31.Pao W, Wang TY, Riely GJ, Miller VA, Pan Q, Ladanyi M, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005 Jan;2(1):e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wood LD, Parsons DW, Jones S, Lin J, Sjoblom T, Leary RJ, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007 Nov 16;318(5853):1108–13. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 33.Kariko K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005 Aug;23(2):165–75. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986 Dec;46(12 Pt 1):6387–92. [PubMed] [Google Scholar]
- 35.Maeda H. Tumor-selective delivery of macromolecular drugs via the EPR effect: background and future prospects. Bioconjug Chem. 2010 May 19;21(5):797–802. doi: 10.1021/bc100070g. [DOI] [PubMed] [Google Scholar]
- 36.Yuan F, Leunig M, Huang SK, Berk DA, Papahadjopoulos D, Jain RK. Microvascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res. 1994 Jul 1;54(13):3352–6. [PubMed] [Google Scholar]
- 37.Yuan F, Dellian M, Fukumura D, Leunig M, Berk DA, Torchilin VP, et al. Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 1995 Sep 1;55(17):3752–6. [PubMed] [Google Scholar]
- 38.Ferrari M. Frontiers in cancer nanomedicine: directing mass transport through biological barriers. Trends Biotechnol. 2010 Apr;28(4):181–8. doi: 10.1016/j.tibtech.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hornung V, Guenthner-Biller M, Bourquin C, Ablasser A, Schlee M, Uematsu S, et al. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med. 2005 Mar;11(3):263–70. doi: 10.1038/nm1191. [DOI] [PubMed] [Google Scholar]
- 40.Judge AD, Sood V, Shaw JR, Fang D, McClintock K, MacLachlan I. Sequencedependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol. 2005 Apr;23(4):457–62. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- 41.Amarzguioui M, Lundberg P, Cantin E, Hagstrom J, Behlke MA, Rossi JJ. Rational design and in vitro and in vivo delivery of Dicer substrate siRNA. Nat Protoc. 2006;1(2):508–17. doi: 10.1038/nprot.2006.72. [DOI] [PubMed] [Google Scholar]
- 42.Foster DJ, Barros S, Duncan R, Shaikh S, Cantley W, Dell A, et al. Comprehensive evaluation of canonical versus Dicer-substrate siRNA in vitro and in vivo. RNA. 2012 Mar;18(3):557–68. doi: 10.1261/rna.031120.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, et al. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008 Apr 3;452(7187):591–7. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stohrer M, Boucher Y, Stangassinger M, Jain RK. Oncotic pressure in solid tumors is elevated. Cancer Res. 2000 Aug 1;60(15):4251–5. [PubMed] [Google Scholar]
- 45.Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000 May 1;60(9):2497–503. [PubMed] [Google Scholar]
- 46.Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M, et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat Nanotechnol. 2011;6(12):815–23. doi: 10.1038/nnano.2011.166. [DOI] [PubMed] [Google Scholar]
- 47.Jain RK. Transport of molecules, particles, and cells in solid tumors. Annu Rev Biomed Eng. 1999;1:241–63. doi: 10.1146/annurev.bioeng.1.1.241. [DOI] [PubMed] [Google Scholar]
- 48.Pluen A, Boucher Y, Ramanujan S, McKee TD, Gohongi T, di Tomaso E, et al. Role of tumor-host interactions in interstitial diffusion of macromolecules: cranial vs. subcutaneous tumors. Proc Natl Acad Sci U S A. 2001 Apr 10;98(8):4628–33. doi: 10.1073/pnas.081626898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tasciotti E, Liu X, Bhavane R, Plant K, Leonard AD, Price BK, et al. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nat Nanotechnol. 2008 Mar;3(3):151–7. doi: 10.1038/nnano.2008.34. [DOI] [PubMed] [Google Scholar]
- 50.Tanaka T, Mangala LS, Vivas-Mejia PE, Nieves-Alicea R, Mann AP, Mora E, et al. Sustained small interfering RNA delivery by mesoporous silicon particles. Cancer Res. 2010 May 1;70(9):3687–96. doi: 10.1158/0008-5472.CAN-09-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen H, You J, Zhang G, Ziemys A, Li Q, Bai L, et al. Cooperative, nanoparticle-enabled thermal therapy of breast cancer. Adv Healthcare Mater. 2011;1(1):84–9. doi: 10.1002/adhm.201100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ananta JS, Godin B, Sethi R, Moriggi L, Liu X, Serda RE, et al. Geometrical confinement of gadolinium-based contrast agents in nanoporous particles enhances T1 contrast. Nat Nanotechnol. 2010 Nov;5(11):815–21. doi: 10.1038/nnano.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Decuzzi P, Ferrari M. Design maps for nanoparticles targeting the diseased microvasculature. Biomaterials. 2008 Jan;29(3):377–84. doi: 10.1016/j.biomaterials.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 54.Lee SY, Ferrari M, Decuzzi P. Shaping nano-/micro-particles for enhanced vascular interaction in laminar flows. Nanotechnology. 2009 Dec 9;20(49):495101. doi: 10.1088/0957-4484/20/49/495101. [DOI] [PubMed] [Google Scholar]
- 55.Decuzzi P, Godin B, Tanaka T, Lee SY, Chiappini C, Liu X, et al. Size and shape effects in the biodistribution of intravascularly injected particles. J Control Release. 2010 Feb 15;141(3):320–7. doi: 10.1016/j.jconrel.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 56.van de Ven AL, Kim P, Haley O, Fakhoury JR, Adriani G, Schmulen J, et al. Rapid tumoritropic accumulation of systemically injected plateloid particles and their biodistribution. J Control Release. 2011 Oct 26; doi: 10.1016/j.jconrel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Godin B, Tasciotti E, Liu X, Serda RE, Ferrari M. Multistage nanovectors: from concept to novel imaging contrast agents and therapeutics. Acc Chem Res. 2011 Oct 18;44(10):979–89. doi: 10.1021/ar200077p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Serda RE, Gu J, Bhavane RC, Liu X, Chiappini C, Decuzzi P, et al. The association of silicon microparticles with endothelial cells in drug delivery to the vasculature. Biomaterials. 2009 May;30(13):2440–8. doi: 10.1016/j.biomaterials.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 59.Serda RE, Mack A, van de Ven AL, Ferrati S, Dunner K, Jr., Godin B, et al. Logic-embedded vectors for intracellular partitioning, endosomal escape, and exocytosis of nanoparticles. Small. 2010 Dec 6;6(23):2691–700. doi: 10.1002/smll.201000727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Landen CN, Jr., Chavez-Reyes A, Bucana C, Schmandt R, Deavers MT, Lopez-Berestein G, et al. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005 Aug 1;65(15):6910–8. doi: 10.1158/0008-5472.CAN-05-0530. [DOI] [PubMed] [Google Scholar]
- 61.Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev. 2005 Feb;15(1):102–11. doi: 10.1016/j.gde.2004.12.005. [DOI] [PubMed] [Google Scholar]