Abstract
Oral fluid (OF) is an increasingly accepted matrix for drug testing programs, but questions remain about its usefulness for monitoring cannabinoids. Expectorated OF specimens (n=360) were obtained from 10 adult daily cannabis smokers before, during, and after 37 20-mg oral Δ9-tetrahydrocannabinol (THC) doses over 9 days to characterize cannabinoid disposition in this matrix. Specimens were extracted and analyzed by gas chromatography– mass spectrometry with electron-impact ionization for THC, 11-hydroxy-THC, cannabidiol, and cannabinol, and negative chemical ionization for 11-nor-9-carboxy-THC (THCCOOH). Linear ranges for THC, 11-hydroxy-THC, and cannabidiol were 0.25–50 ng/mL; cannabinol 1–50 ng/mL; and THCCOOH 5–500 pg/mL. THCCOOH was the most prevalent analyte in 344 specimens (96.9%), with concentrations up to 1,390.3 pg/mL. 11-hydroxy-THC, cannabidiol, and cannabinol were detected in 1, 1, and 3 specimens, respectively. THC was detected in only 13.8% of specimens. The highest THC concentrations were obtained at admission (median 1.4 ng/mL, range 0.3–113.6) from previously self-administered smoked cannabis. A total of 2.5 and 3.7% of specimens were THC-positive at the recommended Substance Abuse and Mental Health Services Administration (2 ng/mL) and Driving Under the Influence of Drugs, Alcohol and Medicines (DRUID) (1 ng/mL) confirmation cutoffs, respectively. THC is currently the only analyte for monitoring cannabis exposure in OF; however, these data indicate chronic therapeutic oral THC administration and illicit oral THC use are unlikely to be identified with current guidelines. Measurement of THCCOOH may improve the detection and interpretation of OF cannabinoid tests and minimize the possibility of OF contamination from passive inhalation of cannabis smoke.
Keywords: Oral fluid, Expectoration, Cannabinoids, Δ9-tetrahydrocannabinol, Dronabinol
Introduction
Self-reported illicit drug use history may be inaccurate, leading to misclassification of drug exposure [1, 2]. Measurement of markers in any of a wide variety of biological matrixes provides objective data, with each matrix offering a different perspective on drug use and specific advantages and limitations.
Oral fluid (OF) is an increasingly well-accepted biological matrix for roadside, workplace, pain management, forensic, and clinical drug testing programs. Sampling OF is less invasive for the donor than blood collection, and is particularly advantageous when serial samples are required. OF may be obtained by direct expectoration (spitting) or absorption onto a permeable pad or sponge, with or without an agent that stimulates OF production.
Commercial OF collection devices employ buffers to extract drugs from the absorbent pad or sponge, diluting OF and reducing assay sensitivity. Expectoration provides undiluted OF, an approach that may be superior for directly determining drug concentrations in excreted saliva. Another advantage of direct expectoration is the considerably lower sampling cost, as collection devices are not required [3]. A disadvantage is that expectorated OF can be viscous and difficult to handle due to the presence of glycosaminoglycans and mucoproteins, and is often contaminated with food particles or other mouth debris [4]. Also, many drugs reduce salivary flow, making expectoration collection difficult and of low volume. Furthermore, salivary drug excretion can be greatly influenced by drug exposure itself, and by factors that stimulate salivary flow [5, 6].
Commercial OF collection devices resolve many of these problems by filtering saliva and physically collecting material during wiping of the oral mucosa. These devices also contain buffers that stabilize drug analytes in solution, reduce OF viscosity (thereby improving measurement accuracy), and reduce adsorption onto container surfaces during laboratory transport. Disadvantages of collection devices as compared to expectorated samples include variable amounts of collected OF and buffer, variable recovery from the device, and increased matrix effects from buffers that may interfere with liquid chromatography–tandem mass spectrometry analytical methods [7]. Thus, unless consistent amounts of OF are collected and buffer amounts are constant, it is not possible to accurately determine drug concentrations with OF collection devices.
Cannabinoid OF analysis is especially problematic due to the lipophilic nature of cannabinoids and their propensity to adsorb to collection devices. Manufacturers focused on improving cannabinoid adsorption onto the pad and in selecting better buffers and solvents to elute cannabinoids from the device. However, identification of cannabinoids in OF still varies greatly with the collection procedure [8, 9].
To our knowledge, cannabinoid distribution in expectorated OF specimens has not been investigated after controlled Δ9-tetrahydrocannabinol (THC) administration. This is of concern as regulatory agencies are requesting data to guide policy making regarding OF testing. Furthermore, drug treatment, workplace drug testing, pain management, and parole programs need data on windows of cannabinoid detection in OF. The present study aims to provide such data. An assay for simultaneous, sensitive identification and quantification of THC, 11-hydroxy-THC (11-OH-THC), 11-nor-9-carboxy-THC (THCCOOH), cannabidiol (CBD), and cannabinol (CBN) in expectorated OF was developed and validated. This assay was employed to quantify cannabinoids in expectorated OF after self-administered smoked cannabis, following single and multiple daily oral THC administrations and after cannabinoid abstinence initiation to determine cannabinoid OF disposition in chronic daily cannabis smokers.
Materials and methods
Chemicals and reagents
THC, 11-OH-THC, THCCOOH, CBD, and CBN for calibrators and quality control samples and corresponding internal standards (d3-THC, d3-11-OH-THC, d3-THCCOOH, d3-CBD) were purchased from Cerilliant Corporation (Round Rock, TX). N,O-Bis(trimethylsilyl) trifluoroacetamide (BSTFA) with 1% trimethylchlorosilane was obtained from Thermo Fisher Scientific Inc. (Rockford, IL). Trifluoroacetic anhydride (TFAA) and hexafluoroisopropanol (HFIP) were acquired from Campbell Science (Rockton, IL), and CEREX® Polycrom™ THC (3 cc/ 35 mg) extraction columns were from SPEware Corporation (Baldwin Park, CA).
Participants
Participants' inclusion criteria were age 18–45 years, self-reported cannabis smoking at least 1 year, average daily cannabis use at least 3 months prior to study entry, and a cannabinoid-positive urine sample upon admission. Exclusion criteria included any clinically significant current medical or psychiatric disease, history of seizures or psychosis or cannabis-related adverse effect, consuming more than 6 alcoholic drinks/day at least 4 times/week, or allergy to sesame oil.
The National Institute on Drug Abuse, University of Maryland Baltimore, and Maryland Department of Health and Mental Hygiene Institutional Review Boards approved the study. All participants provided written informed consent.
Oral THC dosing and sample collection
Participants resided on a secure clinical research unit under continuous observation while participating in a protocol investigating cannabis tolerance and withdrawal. Participants were admitted the evening prior to the first oral THC dose and discharged 9 days later, 22.5 h after the last THC dose.
Over 8 days, 37 doses of 20 mg synthetic THC (dronabinol, Marinol®; Unimed Pharmaceuticals, Marietta, GA) were orally administered with increasing frequency (every 4–8 h) for total daily dosages of 40–120 mg/day. Five daily THC doses were administered on days 2–4 (total 100 mg), six on days 5–7 (total 120 mg), and two on day 8. Dose frequency was increased, rather than dose amount, to minimize adverse events previously reported with 30-mg THC doses [10]. This dosing regimen was designed to standardize cannabis tolerance in daily, heavy cannabis smokers. In clinical use the maximum recommended doses are 20 mg/day for appetite stimulation and 15 mg/m2 4–6 times daily (175 mg/day assuming a typical 1.95-m2 body surface area) for anti-emesis [11].
Three OF samples were obtained prior to the first THC dose: one at admission, one 7 h before, and one just before dosing. THC administration always preceded OF collection when both were scheduled concurrently. The first THC dose (20 mg) was administered at 15:00 on day 1 (16.9–19.3 h after admission), followed by 5-hourly samples to examine single-dose oral cannabinoid pharmacokinetics. During continuous dosing, OF specimens were collected daily about 10:00, 20:00, and 22:00. After the last dose (day 8), collections occurred every 1.5–3 h.
Participants were asked to expectorate into polypropylene tubes until a minimum of 3 mL OF was collected, or for 5 min, whichever occurred first. OF was centrifuged and stored at −20 °C in Nunc® cryotubes until analysis.
Oral fluid analysis
OF specimens were analyzed by a previously published validated two-dimensional gas chromatography–mass spectrometry (2D-GCMS) method for THC, 11-OH-THC, THCCOOH, CBD, and CBN with minor modifications [12]. THC, 11-OH-THC, CBD, and CBN were eluted and derivatized separately from THCCOOH and injected on two independent analytical systems with different ionization techniques. The method was fully validated for expectorated OF; the original method utilized OF collected with the Quantisal™ device, with a 1:4 dilution in the elution/stabilizing buffer.
The method for expectorated OF was validated for specificity, linearity, limits of detection (LOD) and quantification (LOQ), analytical recovery, intra- and interday imprecision, extraction efficiency, carryover, and stability. LOQ and dynamic ranges for THC and CBD were 0.25–50 ng/mL, 1–50 ng/mL for CBN, 0.25–25 ng/mL for 11-OH-THC, and 5–500 pg/mL for THCCOOH. Samples exceeding the linear range were diluted with blank OF and reanalyzed. Intra- and inter-assay imprecision were less than 6.5% and analytical recovery was within ±15.2% of target. Extraction efficiencies ranged between 54.4 and 97.4%. Supplementary Material Table S1 provides further details method validation parameters.
Briefly, 0.5 mL drug-free OF was fortified with calibrator or quality control solution. Deuterated internal standards [d3-THC, d3-11-OH-THC, d3-CBD (5 ng/mL), and d3-THCCOOH (0.05 ng/mL); CBN utilized d3-THC] were added to calibrators, controls, and authentic OF samples. To reduce viscosity and precipitate proteins, expectorated specimens were diluted with deionized water (1 mL) and ice-cold acetonitrile (0.75 mL) prior to extraction. Following vortexing and centrifugation, supernatants were decanted onto preconditioned CEREX® Polycrom™ THC extraction columns and washed with deionized water/acetonitrile/ammonium hydroxide (84:15:1, v/v/v; 3 mL). THC, 11-OH-THC, CBD, and CBN were eluted with hexane/acetone/ethyl acetate (60:30:20, v/v/v; 3 mL) and evaporated to dryness. THCCOOH was eluted into separate tubes with 3 mL hexane/ethyl acetate/glacial acetic acid (75:25:2.5, v/v/v). Eluates were evaporated to dryness under nitrogen at 35 °C for 15 min. THC, 11-OH-THC, CBD, and CBN were derivatized with BSTFA (20 μL) for 30 min (65 °C) prior to 2D-GCMS with electron ionization. THCCOOH was derivatized with HFIP (20 μL) and TFAA (40 μL) for 30 min at 65 °C. After evaporation and reconstitution in 20 μL toluene, extracts were subjected to 2D-GCMS with negative chemical ionization and pure ammonia as reagent gas. Electronic Supplementary Material Fig. S1 depicts cannabinoid analyte chromatograms at their LOQ concentrations.
Data analysis
Statistical calculations utilized SPSS® 14.0 for Windows (SPSS, Chicago, IL). THC decrease (%) from admission to predosing baseline was determined as [(admission–baseline)/admission]×100%. Median concentration reflects positive (≥LOQ) samples only. For comparative statistical analyses, OF specimens below the LOQ were assigned values of 0.5×LOQ (0.125 ng/mL for THC and 2.5 pg/mL THCCOOH). Concentration variations before the first dose, after the first and last dose, and on each of days 2–7 were examined by nonparametric Friedman's ANOVA (χ2) after verifying absence of normal distribution (Kolmogorov–Smirnov normality test) and homogeneity (Levene's test) of variances. A two-tailed P value less than 0.05 defined significance for all comparisons.
Results
Demographic and drug use characteristics for the 10 participants are shown in Table 1. All participants self-reported cannabis smoking in the 24 h prior to admission, and had a positive cannabinoid urine test upon admission. A total of 360 OF specimens were collected over 9 days. Five specimens were not processed due to low volume and high viscosity, yielding 355 cannabinoid OF results. Thirty of 355 specimens contained only 0.5 mL OF, the minimum volume for our analysis.
Table 1. Demographic and self-reported drug use characteristics of 10 chronic daily cannabis smokers.
| Participant | Mean±SD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| A | B | C | D | E* | F | G* | H | I* | J* | ||
| Age (years) | 20 | 32 | 26 | 18 | 23 | 21 | 25 | 27 | 25 | 22 | 23.9±4.0 |
| BMI (kg/m2) | 17.8 | 29.8 | 25.1 | 23.7 | 21.1 | 22.9 | 30.6 | 28.7 | 30.5 | 29.7 | 26.0±4.5 |
| Cannabis joints/day | 6 | 3 | 24 | 3 | 6 | 1 | 3 | 9 | 21 | 3 | 7.9±8.0 |
| Cannabis use in past 14 days | 13 | 13 | 14 | 12 | 14 | 14 | 14 | 14 | 13 | 14 | 13.5±0.7 |
| Last cannabis use (days) | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0.7±0.5 |
| Lifetime cannabis use (years) | 4 | 18 | 13 | 4 | 10 | 4 | 11 | 16 | 13 | 10 | 10.3±5.0 |
| Alcoholic drinks/week | 0.5 | 0.08 | 1 | 0.25 | 2 | 3 | 0.25 | 2 | 0.5 | 0.25 | 1.0±1.0 |
| Tobacco cigarettes/week | 70 | 3 | 76 | 10.5 | 0 | 21 | 105 | 42 | 140 | 14 | 48.2±47.9 |
| Other drug usea | NA | NA | A | O | O | O,C,T,H | A,O,H, | A,O,C | A,O | NA | |
BMI body mass index
Participants with THC-positive oral fluid specimens between 2nd and last THC dose
NA none admitted, A amphetamines, O opiates, C cocaine, T tranquilizers, H hallucinogens
THC in oral fluid
THC was present in only 49 (13.8%) of 355 specimens; concentrations generally decreased from those at admission. Eight participants were THC-positive at admission, likely from previously self-administered smoked cannabis. One participant was THC-negative (<LOQ 0.25 ng/mL) on admission and one participant's admission specimen could not be processed due to high sample viscosity. THC was present in 20 of 29 specimens collected between admission and the first oral THC dose, with concentrations ranging from 0.25 to 113.6 ng/mL (Table 2). Mean THC concentrations decreased 92.4±11.8% from admission to the first THC dose (χ2(2)=10.8, P=0.005); three participants' OF specimens were continuously THC-positive prior to the first dose, one was continuously THC-negative, five had a least one THC-negative specimen during this time interval, and one had a single THC-positive specimen on admission.
Table 2. Median (range) concentrations of positive Δ9-tetrahydrocan-nabinol (THC) expectorated oral fluid specimens per subject (n=10), between admission and the first 20-mg oral THC dose (predose), first and second THC doses, during 7 days of round-the-clock THC (36 doses), and after the last THC dose.
| Subject | THC, ng/mL | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Predose (n=3) | After first dose (n=5) | During 36 doses (n=20) | After last dose (n=8) | |||||
|
|
|
|
|
|||||
| Positive (%) | Median (range) | Positive (%) | Median (range) | Positive (%) | Median (range) | Positive (%) | Median (range) | |
| A | 3 (100) | 1.6 (0.38–11.1) | 1 (20) | 0.3 | 0 | ND | 0 | ND |
| B | 3 (100) | 0.8 (0.34–113.6) | 3 (60) | 0.3 (0.25–0.34) | 0 | ND | 0 | ND |
| C | 2 (67) | 0.9 (0.90–0.92) | 3 (60) | 0.8 (0.29–2.9) | 0 | ND | 0 | ND |
| D | 1 (33) | 0.3 | 0 | ND | 0 | ND | 0 | ND |
| E* | 3 (100) | 0.8 (0.52–1.8) | 2 (40) | 0.5 (0.32–0.59) | 5 (25) | 0.7 (0.35–10.3) | 0 | ND |
| F | 0 | ND | 0 | ND | 0 | ND | 0 | ND |
| G* | 2 (67) | 6.0 (4.0–8.1) | 3 (60) | 0.5 (0.33–0.54) | 1 (5) | 0.9 | 0 | ND |
| H | 2 (67) | 0.3 (0.25–0.34) | 0 | ND | 0 | ND | 0 | ND |
| I* | 2 (67) | 1.9 (0.51–3.3) | 5 (100) | 0.3 (0.25–1.1) | 3 (15) | 0.4 (0.31–0.72) | 2 (25) | 0.5 (0.32–0.61) |
| J* | 2 (67) | 0.7 (0.34–1.1) | 0 | ND | 1 (5) | 9.5 | 0 | ND |
| Total | 20 (76.7) | 17 (34) | 10 (5.0) | 2 (2.5) | ||||
| Mediana | 0.8 | 0.3 | 0.8 | 0.5 | ||||
ND none detected, n number of specimens
Participants with THC-positive oral fluid specimens between 2nd and last THC dose
Data are median of individual medians for each study period
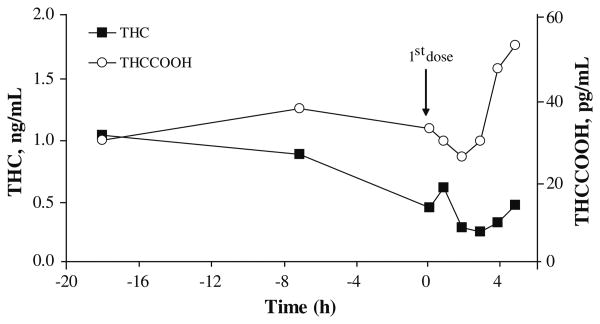
Only 17 of 50 (34.0%) OF specimens obtained within the first 5 h following the first oral THC dose were THC-positive (range 0.25–2.9 ng/mL). THC concentrations did not decrease significantly (χ2(4)=4.2, P=0.380) over this time interval (Fig. 1). All participants had at least one THC-negative specimen during the first day of oral administration (mean time from admission to first negative specimen 18.6±3.4 h).
Fig. 1. Median Δ9-tetrahydrocannabinol (THC) (■) and 11-nor-9-carboxy-THC (THCCOOH) (○) expectorated oral fluid concentrations collected on admission, prior to and up to 5 h after the first 20-mg THC dose.
Of 196 OF specimens, only 10 (5.1%) were THC-positive over the 7 days between the second and final oral THC doses (Fig. 2). All THC-positive specimens were from 4 participants. Negative OF specimens were dispersed among the 10 positive specimens, yielding a median THC concentration of 0.7 ng/mL (range 0.31–10.3). THC was present in only 2 of 80 (2.5%) OF specimens collected up to 22.5 h following the last THC dose, both from the same participant (Table 2).
Fig. 2. Δ9-Tetrahydrocannabinol (THC) expectorated oral fluid concentrations in 4 participants who produced occasional THC-positive specimens after the second 20-mg oral THC administration.
THCCOOH in oral fluid
THCCOOH was the primary analyte identified in 344 of 355 specimens (96.9%), with concentrations up to 1,390.3 pg/mL. THCCOOH concentrations tended to increase throughout the study (Fig. 3). Eight participants' specimens were positive for THCCOOH at admission, one was negative, and one was not suitable for measurement. THCCOOH was quantifiable in 28 of 29 predose collections at an LOQ of 5 pg/mL (Table 3), but concentration variations differed substantially across subjects. Six participants' THCCOOH OF concentrations consistently decreased (mean 41.4±24.2%) from admission, two were variable, and two consistently increased (80.8±14.1%), suggesting differences in time of last cannabis smoking. There were no significant differences in mean THCCOOH concentrations prior to THC dosing (χ2(2)=5.0, P=0.082).
Fig. 3. Median 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THCCOOH) concentrations in expectorated oral fluid collected from 10 chronic daily cannabis smokers over 9 days before, during, and after 37 20-mg oral THC doses. Arrows indicate the number of 20-mg THC doses ingested between collections.
Table 3. Median (range) concentrations of positive 11-nor-9-carboxy-Δ9-tetrahydro-cannabinol (THCCOOH) expectorated oral fluid specimens per subject (n=10), between admission and the first 20-mg oral THC dose (predose), first and second THC doses, during 100 and 120 mg/day, and after the last THC dose.
| Subject | THCCOOH, pg/mL | ||||
|---|---|---|---|---|---|
|
| |||||
| Predose (n=3) | After first dose (n=5) | 100 mg/day 2–4 days (n=9) | 120 mg/day 5–7 days (n=9) | Last dose (n=8) | |
| Aa | 27.8 (26.7–29.0) | 16.9 (16.0–17.8) | 21.8 (5.5–96.7) | 22.6 (6.9–105.8) | 26.3 (5.4–181.1) |
| B | 37.1 (31.0–75.0) | 47.0 (32.3–54.0) | 93.2 (6.9–136.9) | 137.5 (5.3–254.0) | 249.5 (75.4–273.6) |
| C | 14.8 (7.5–53.1) | 25.5 (10.0–59.4) | 70.8 (5.9–111.3) | 51.0 (20.0–80.9) | 115.9 (19.1–213.9) |
| Db | 30.9 (20.9–35.1) | 17.4 (13.5–32.8) | 38.4 (5.2–137.5) | 84.0 (15.6–152.1) | 65.1 (25.7–271.5) |
| E* | 40.3 (11.8–40.7) | 19.5 (13.1–36.8) | 76.3 (10.9–137.7) | 91.4 (52.6–237.2) | 70.2 (26.6–131.2) |
| Fc | 21.9 (15.8–27.1) | 34.4 (16.1–78.6) | 126.0 (5.5–357.7) | 42.7 (11.4–195.3) | 359.4 (196.1–630.1) |
| G* | 85.2 (76.3–172.1) | 60.1 (28.0–80.3) | 158.2 (61.6–333.9) | 202.4 (109.9–482.6) | 287.0 (156.3–727.3) |
| H | 55.2 (34.1–58.1) | 40.1 (26.0–82.2) | 88.9 (69.9–143.5) | 87.6 (33.4–155.7) | 76.6 (38.9–127.4) |
| I*d | 635.0 (189.3–1,080.7) | 234.4 (142.2–248.3) | 415.7 (84.6–670.6) | 435.9 (63.3–780.4) | 648.9 (325.6–1,386.7) |
| J* | 24.3 (10.3–33.7) | 8.4 (5.1–11.8) | 42.9 (7.1–85.3) | 44.3 (11.1–201.5) | 54.2 (13.2–122.4) |
| Mediane | 34.0 | 30.0 | 82.6 | 85.8 | 96.3 |
n number of specimens
Participants with THC-positive oral fluid specimens between 2nd and last THC dose
Subject A had 1 sub-LOQ predose specimen, 3 after first dose, and 3 over 2–4 days
Subject D had 1 sub-LOQ specimen over the 2–4 days
Subject F had 1 sub-LOQ specimen over the 5–7 days
Subject I had 1 sub-LOQ specimen over the 5–7 days
Data are median of individual medians for each study period
Forty-seven of 50 OF specimens (94.0%) were THCCOOH-positive within 5 h following the first THC dose (Table 3), with no significant change in mean THCCOOH concentrations over that time interval (χ2(4)= 3.4, P=0.502) (Fig. 1).
THCCOOH was positive in 94.5% of specimens collected between the second (day 1) and last (day 8) THC doses (Table 3), with no significant change in THCCOOH concentrations, despite continuous dosing (χ2(5)=7.6, P=0.181) (Fig. 3). During days 2–4 (100 mg THC/day), median THCCOOH concentration was 82.6 pg/mL (range 5.2–670.6), and during days 5 through 7 (120 mg/day), the median (range) THCCOOH concentration was 85.8 pg/mL (5.3–780.4). Median Tmax for THCCOOH was 161 h (range 115–175 h) after the first THC dose or after 36 oral THC doses (range 25–37 doses).
THCCOOH was present in all 80 specimens collected after the last oral THC dose (Table 3). The median THCCOOH concentration at the time of the last dose was 85.0 pg/mL (range 6.2–978.0), increasing to 98.0 pg/mL (range 5.4–1,386.7) 22.5 h later, a nonsignificant change (χ2(7)=6.3, P=0.505) during abstinence initiation.
11-OH-THC, CBD, and CBN in oral fluid
11-OH-THC (LOQ=0.25 ng/mL) was present (0.5 ng/mL) in only one specimen, 161 h after the first THC dose, the time of the highest THCCOOH concentrations. CBD was present (0.4 ng/mL) in only one specimen, at admission. CBN was present in 3 specimens collected prior to the first THC dose (1.2–14.8 ng/mL) from 3 participants.
Discussion
Participants had a difficult time providing adequate OF due to dry mouth and decreased salivation during THC administration, requiring additional collection time. This difficulty would be especially relevant at the roadside, when time required to collect a specimen is critical. Cannabinoid quantification in expectorated OF also was analytically challenging. In the current study, 5 specimens could not be processed due to low specimen volume and high viscosity, one of which occurred at admission from a participant who self-reported cannabis smoking just prior to collection. Although participants were requested to provide 3-mL expectorations, actual volumes collected were much lower, potentially due to reduced salivary flow resulting from repeated oral THC administration. More froth than liquid OF was sometimes provided, yielding low-volume, viscous specimens. Other factors such as diet, licit and illicit drugs, disease, and smoking or alcohol consumption may alter salivary flow rate [13, 14]. Thus, acquiring an adequate and consistent amount of OF, and a specimen of appropriate viscosity may be problematic for expectorated specimens collected after oral or smoked cannabis.
Mucous in the OF specimen can plug frits in the solid-phase extraction (SPE) column, preventing continuous flow and effective analyte binding to the sorbent bed. Centrifuging specimens and freezing and thawing specimens prior to analysis minimize these problems, but may produce changes in concentrations that have not been well characterized [15-17]. We employed cold acetonitrile and centrifugation to effectively precipitate mucous and mouth debris in expectorated OF, substantially improving SPE performance, also reported previously [18].
THC in oral fluid
OF THC at admission reflected self-administered smoked cannabis, yielding highly variable cannabinoid concentrations due to different times since last administration and different doses. Participants' self-reported drug use histories indicated that their last smoking event occurred from just prior to admission to 1 day earlier, explaining the wide range in predose concentrations. THC expectorated OF concentrations before the first oral THC dose were lower than those collected with the Quantisal™ OF collection device (range 0.5–481.9 ng/mL) at the same times from the same participants [19]. With OF collection devices, the OF is diluted in an extraction/stabilization buffer, reducing the problem of specimen viscosity. Others also reported higher THC concentrations after controlled cannabis smoking in OF specimens collected with devices [20, 21]. We suggest several reasons for the lower expectorated concentrations. Lower specimen volume following THC may lead to viscous OF composition. Cannabinoids may bind to mouth debris or precipitated material during centrifugation and be lost when the supernatant is transferred for SPE. In addition, cannabinoids may be bound to or entrapped in the mucous components found to a greater extent in expectorated specimens, thereby reducing adsorption and retention onto the SPE sorbent surface, further reducing concentrations.
THC OF concentrations decreased variably, possibly due to differences in tissue body burden from frequency, amount, and duration of self-administered smoked cannabis. However, there were no significant differences in age, height, weight, or amount or duration of self-reported smoked cannabis between those positive for THC only on day 1 and those occasionally positive later in the study. Six participants' OF specimens were THC-negative (<LOQ 0.25 ng/mL) within the first day, despite administration of 20 mg oral THC. Expectorated specimens from the other four participants were occasionally THC-positive (Fig. 2) after the second oral THC dose. Participant I, who self-reported smoking 21 joints/day for 13 years, had THC-positive OF on day 9; however, concentrations in these occasional positive specimens were below (range 0.3–0.7 ng/mL) those proposed by the Substance Abuse and Mental Health Services Administration (SAMHSA) 2 ng/mL cutoff for OF THC confirmation [22], and the 1 ng/mL THC cutoff utilized in the Driving Under the Influence of Drugs, Alcohol and Medicines (DRUID) European study [23]. A total of 2.5% and 3.7% of all specimens were THC-positive at the recommended SAMHSA and DRUID confirmation cutoffs, respectively. Two specimens with unexpectedly high THC concentrations were obtained from two participants (E and J) in occasional THC-positive expectorated specimens. This possibly could have been due to accidental biting before swallowing of the soft, gelatin capsule, abruptly releasing THC and providing rapid absorption across the oral mucosa. There is low probability of this occurring as the capsules were enclosed within a second capsule to maintain the double blind design.
THCCOOH in oral fluid
In contrast to THC, THCCOOH was detected in almost all specimens (96.9%), albeit with a much lower LOQ of 5 pg/mL. If THC had a similar LOQ, a much longer detection window might have been achieved for this analyte. However, a similar THC LOQ could yield further interpretive difficulties. For instance, it is unknown if detection at a lower concentration could differentiate active cannabis smoking from passive OF contamination resulting from THC in environmental cannabis smoke. Additionally, residual OF THC detection windows are currently unknown for chronic, daily cannabis smokers, as prolonged detection of THC for 7 days after initiation of cannabis abstinence was recently reported in whole blood [24] and plasma [25].
Relatively low THCCOOH concentrations were noted on admission, with decreasing concentrations in 6 participants before the first THC dose. Two participants displayed the opposite pattern, with THCCOOH concentrations increasing after admission. This increase might indicate recent cannabis smoking just prior to admission. Participant I, a heavy chronic cannabis user, reported his most recent smoking within 1 day before admission. Although on admission his OF was one of five nonmeasurable specimens, 7 h before the first THC dose his OF THCCOOH concentration was 20 times higher than the mean concentration from the other nine participants. In general, his THCCOOH concentrations were on average 5 times higher than mean concentrations of the other participants throughout the study, indicative of rapid THC metabolism and long elimination patterns.
Mean THCCOOH concentrations increased significantly (p=0.005) from the first to the last day of the study (Fig. 3), potentially because of THCCOOH accumulation and/or increases in THC dosing throughout the study. Some variability in THCCOOH concentrations in sequential specimens can be explained by OF collections at different times after dosing (Fig. 3). THCCOOH concentrations in expectorated specimens were similar to those in specimens collected concurrently with the Quantisal OF collection device [19].
11-OH-THC, CBD, and CBN in oral fluid
This is the first evidence of 11-OH-THC presence in OF. 11-OH-THC is a pharmacologically active intermediate in THC first-pass metabolism that is oxidized further to inactive THCCOOH. A single 11-OH-THC-positive specimen contained the highest THCCOOH (Cmax=1,390.3 pg/mL) concentration and was one of the occasional THC-positive (0.72 ng/mL) specimens in participant I's OF. In plasma, 11-OH-THC concentrations range from 50 to 100% of THC concentrations following oral cannabinoid administration, compared to only 10% after smoked administration [26]. Therefore, analysis of 11-OH-THC in OF with a lower LOQ similar to that employed for THCCOOH would most likely yield many more 11-OH-THC-positive specimens. Further research should address the value of inclusion of 11-OH-THC in the interpretation of OF cannabinoid results.
In the present study, CBD and CBN were detected in only 4 specimens that had the highest THC concentrations and were measurable on average for 11 h after admission, indicating they likely derived from recent cannabis smoking. Others found CBN in OF for only 4 h (range 0.9–4.1 ng/mL) after a single smoking session; our findings confirm the short detection window for this analyte [27].
These data increase our knowledge of cannabinoid pharmacokinetics in expectorated OF after self-reported cannabis smoking, single oral THC dosing, and around-the-clock multiple THC doses. Low expectorated OF volume due to cannabinoid-induced dry mouth may hinder quantitative analysis. OF collection devices utilizing an absorption pad, stabilizing buffers, and volume adequacy indicator might help overcome these limitations.
We document that THC, CBD, and CBN in OF likely derive primarily from self-reported smoked cannabis, rather than oral THC. 11-OH-THC can be identified in OF, but a substantially lower LOQ would be required to quantify this cannabinoid consistently. The detection of THCCOOH at sub-nanogram concentrations in OF serves as a reliable marker of cannabinoid intake and reduces the possibility of passive cannabis smoke exposure. However, the duration of detection of residual THCCOOH concentrations in OF from chronic daily cannabis smokers is unknown and could complicate the interpretation of cannabinoid OF results in this population. Overall, these data from controlled oral THC administration indicate that chronic therapeutic oral THC administration or illicit oral cannabis use are not likely to be identified with OF monitoring with the current proposed SAMHSA or DRUID guidelines for OF testing.
Supplementary Material
Acknowledgments
Supported by the Intramural Research Program, NIH, National Institute on Drug and NIDA Residential Research Support Services Contract HHSN271200599091 CADB Contract No N01Da-5-9909. We thank clinical research teams from the NIDA clinical program, the Johns Hopkins Behavioral Pharmacology Research Unit, and the Maryland Psychiatric Research Center Treatment Research Program.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00216-011-5066-4) contains supplementary material, which is available to authorized users.
Contributor Information
Garry Milman, Chemistry and Drug Metabolism, Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Biomedical Research Center, 251 Bayview Boulevard, Room 05A-721, Baltimore, MD 21224, USA.
Allan J. Barnes, Chemistry and Drug Metabolism, Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Biomedical Research Center, 251 Bayview Boulevard, Room 05A-721, Baltimore, MD 21224, USA
David M. Schwope, Chemistry and Drug Metabolism, Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Biomedical Research Center, 251 Bayview Boulevard, Room 05A-721, Baltimore, MD 21224, USA
Eugene W. Schwilke, Chemistry and Drug Metabolism, Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Biomedical Research Center, 251 Bayview Boulevard, Room 05A-721, Baltimore, MD 21224, USA
Robert S. Goodwin, Chemistry and Drug Metabolism, Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Biomedical Research Center, 251 Bayview Boulevard, Room 05A-721, Baltimore, MD 21224, USA
Deana L. Kelly, Maryland Psychiatric Research Center, University of Maryland School of Medicine, Catonsville, MD 21228, USA
David A. Gorelick, Chemistry and Drug Metabolism, Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Biomedical Research Center, 251 Bayview Boulevard, Room 05A-721, Baltimore, MD 21224, USA
Marilyn A. Huestis, Email: mhuestis@intra.nida.nih.gov, Chemistry and Drug Metabolism, Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, Biomedical Research Center, 251 Bayview Boulevard, Room 05A-721, Baltimore, MD 21224, USA.
References
- 1.Ledgerwood DM, Goldberger BA, Risk NK, Lewis CE, Price RK. Comparison between self-report and hair analysis of illicit drug use in a community sample of middle-aged men. Addict Behav. 2008;33(9):1131–1139. doi: 10.1016/j.addbeh.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Musshoff F, Driever F, Lachenmeier K, Lachenmeier DW, Banger M, Madea B. Results of hair analyses for drugs of abuse and comparison with self-reports and urine tests. Forensic Sci Int. 2006;156(2–3):118–123. doi: 10.1016/j.forsciint.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Kim I, Barnes AJ, Oyler JM, Schepers R, Joseph RE, Jr, Cone EJ, Lafko D, Moolchan ET, Huestis MA. Plasma and oral fluid pharmacokinetics and pharmacodynamics after oral codeine administration. Clin Chem. 2002;48:1486–1496. [PubMed] [Google Scholar]
- 4.Crouch DJ. Oral fluid collection: the neglected variable in oral fluid testing. Forensic Sci Int. 2005;150(2–3):165–173. doi: 10.1016/j.forsciint.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 5.Samyn N, van Haeren C. On-site testing of saliva and sweat with Drugwipe and determination of concentrations of drugs of abuse in saliva, plasma and urine of suspected users. Int J Leg Med. 2000;113:150–154. doi: 10.1007/s004140050287. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman E, Lamster IB. The diagnostic applications of saliva—a review. Crit Rev Oral Biol Med. 2002;13(2):197–212. doi: 10.1177/154411130201300209. [DOI] [PubMed] [Google Scholar]
- 7.Bosker WM, Huestis MA. Oral fluid testing for drugs of abuse. Clin Chem. 2009;55(11):1910–1931. doi: 10.1373/clinchem.2008.108670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore C, Ross W, Coulter C, Adams L, Rana S, Vincent M, Soares J. Detection of the marijuana metabolite 11-nor-delta9-tetrahydrocannabinol-9-carboxylic acid in oral fluid specimens and its contribution to positive results in screening assays. J Anal Toxicol. 2006;30(7):413–418. doi: 10.1093/jat/30.7.413. [DOI] [PubMed] [Google Scholar]
- 9.Kauert GF, Iwersen-Bergmann S, Toennes SW. Assay of delta9-tetrahydrocannabinol (THC) in oral fluid-evaluation of the OraSure oral specimen collection device. J Anal Toxicol. 2006;30(4):274–277. doi: 10.1093/jat/30.4.274. [DOI] [PubMed] [Google Scholar]
- 10.Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following oral THC administration to humans. Psychopharmacol. 1999;141:385–394. doi: 10.1007/s002130050848. [DOI] [PubMed] [Google Scholar]
- 11.Food and Drug Administration (2004) MARINOL® (Dronabinol) Capsules. 500012 Rev Sep 2004 ed
- 12.Milman G, Barnes AJ, Lowe RH, Huestis MA. Simultaneous quantification of cannabinoids and metabolites in oral fluid by two-dimensional gas chromatography mass spectrometry. J Chromatogr A. 2010;1217:1513–1521. doi: 10.1016/j.chroma.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enberg N, Alho H, Loimaranta V, Lenander-Lumikari M. Saliva flow rate, amylase activity, and protein and electrolyte concentrations in saliva after acute alcohol consumption. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92(3):292–298. doi: 10.1067/moe.2001.116814. [DOI] [PubMed] [Google Scholar]
- 14.de Almeida PDV, Grégio AMT, Machado MÂN, de Lima AAS, Azevedo LR. Saliva composition and functions: a comprehensive review. J Contemp Dent Pract. 2008;9(3):72–80. [PubMed] [Google Scholar]
- 15.Kintz P, Samyn N. Use of alternative specimens: drugs of abuse in saliva and doping agents in hair. Ther Drug Monit. 2002;24:239–246. doi: 10.1097/00007691-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Hold KM, De Boer D, Zuidema J, Maes RAA. Saliva as an analytical tool in toxicology. Int J Drug Test. 1996;1:1–31. [Google Scholar]
- 17.Lillsunde P. Analytical techniques for drug detection in oral fluid. Ther Drug Monit. 2008;30(2):181–187. doi: 10.1097/FTD.0b013e3181685088. [DOI] [PubMed] [Google Scholar]
- 18.Teixeira H, Proenca P, Verstraete A, Corte-Real F, Vieira DN. Analysis of delta-9-tetrahydrocannabinol in oral fluid samples using solid-phase extraction and high-performance liquid chromatography-electrospray ionization mass spectrometry. Forensic Sci Int. 2005;150(2-3):205–211. doi: 10.1016/j.forsciint.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 19.Milman G, Barnes AJ, Schwope DM, Schwilke EW, Darwin WD, Goodwin RS, Kelly DL, Gorelick DA, Huestis MA. Disposition of cannabinoids in oral fluid after controlled around-the-clock oral THC administration. Clin Chem. 2010;56(8):1261–1269. doi: 10.1373/clinchem.2009.141853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kauert GF, Ramaekers JG, Schneider E, Moeller MR, Toennes SW. Pharmacokinetic properties of delta9-tetrahydrocannabinol in serum and oral fluid. J Anal Toxicol. 2007;31(5):288–293. doi: 10.1093/jat/31.5.288. [DOI] [PubMed] [Google Scholar]
- 21.Niedbala RS, Kardos KW, Fritch DF, Kardos S, Fries T, Waga J, Robb J, Cone EJ. Detection of marijuana use by oral fluid and urine analysis following single-dose administration of smoked and oral marijuana. J Anal Toxicol. 2001;25(5):289–303. doi: 10.1093/jat/25.5.289. [DOI] [PubMed] [Google Scholar]
- 22.DHHS. Proposed revisions to mandatory guidelines for federal workplace drug testing programs. Fed Regist. 2004;69(71):19673–19732. [Google Scholar]
- 23.Pil K, Raes E, Verstraete AG. The toxicological challenges in the European research project DRUID. Forensic Sci Int Suppl Ser. 2009;1(1):29–32. [Google Scholar]
- 24.Karschner E, Schwilke E, Lowe R, Darwin W, Pope H, Jr, Herning R, Cadet J, Huestis M. Do delta 9-tetrahydrocannabinol concentrations indicate recent use in chronic cannabis users? Addiction. 2009;104:2041–2048. doi: 10.1111/j.1360-0443.2009.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karschner E, Schwilke E, Lowe R, Darwin WD, Herning R, Cadet J, Huestis M. Implications of plasma delta9-tetrahydrocannabinol, 11-hydroxy-THC, and 11-nor-9-carboxy-THC concentrations in chronic cannabis smokers. J Anal Toxicol. 2009;33(8):469–477. doi: 10.1093/jat/33.8.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wall ME, Sadler BM, Brine D, Taylor H, Perez-Reyes M. Metabolism, disposition, and kinetics of delta-9-tetrahydrocannabinol in men and women. Clin Pharmacol Ther. 1983;34:352–363. doi: 10.1038/clpt.1983.179. [DOI] [PubMed] [Google Scholar]
- 27.Moore C, Rana S, Coulter C. Simultaneous identification of 2-carboxy-tetrahydrocannabinol, tetrahydrocannabinol, cannabinol and cannabidiol in oral fluid. J Chromatogr B Anal Technol Biomed Life Sci. 2007;852(1–2):459–464. doi: 10.1016/j.jchromb.2007.02.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.