Abstract
The flavanone hesperetin is known to decrease basal glucose uptake, although the inhibitory mechanism is largely unknown. Here, we used MDA-MB-231 breast cancer cells to investigate the molecular pathways affected by hesperetin. The results indicate that the suppression of glucose uptake is caused by the down-regulation of glucose transporter 1 (GLUT1). Hesperetin was also found to inhibit insulin-induced glucose uptake through impaired cell membrane translocation of glucose transporter 4 (GLUT4). In addition, the phosphorylation of the insulin receptor-beta subunit (IR-beta) and Akt was suppressed. Hesperetin also decreased cellular proliferation, which is likely due to the inhibition of glucose uptake. Cancer cells are highly dependent on glucose and hesperetin may, therefore, have potential application as an anticancer agent.
Keywords: hesperetin, glucose uptake, GLUT1, GLUT4, breast cancer cells
INTRODUCTION
Cancer cells have high rates of proliferation and commonly overexpress glucose transporters to meet their increased need for biosynthetic building blocks.1–3 Glucose provides most of the carbon that is used for constructing essential molecules for daughter cells, such as, amino acids, fatty acids and nucleotides.3 In the basal state, glucose transporter 1 (GLUT1) appears to be the predominant glucose transporter in breast cancer cells,4–10 although the expression of other glucose transporters has also been reported. 8,11–14
The glucose transporter dynamics change when a cell is stimulated by a ligand, such as insulin.15,16 Insulin signaling plays a crucial role in muscle and adipose tissues17,18 as well as other tissues. Several cancer cells have also been shown to actively use the insulin pathway.19,20 Insulin binding to target receptors causes auto-phosphorylation of intracellular receptor regions. This process results in the phosphorylation of downstream substrates, such as PI3K and Akt.21,22 Akt activation triggers the translocation of glucose transporter 4 (GLUT4) to the cell surface, where it is responsible for glucose trafficking.23 The activation of GLUT4 enhances glucose uptake and increases the amount of intracellular glucose available for metabolic conversion, thereby promoting enhanced cell proliferation.24–26
Hesperidin is a flavanone glycoside that can be found in citrus fruit. Hesperidin is deglycosylated by enzymatic hydrolysis upon entering the digestive tract, forming hesperetin (4′-methoxy-3′,5,7-trihydroxyflavanone).27,28 Hesperetin has previously been reported to decrease basal glucose uptake in U937 monocytic cells.29 However, the inhibitory mechanism of this compound has remained elusive. Furthermore, it was unknown whether hesperetin also affects insulin-stimulated glucose uptake. In the present work, we investigated the role of hesperetin on basal and insulin-induced glucose uptake in MDA-MB-231 breast cancer cells. It has previously been shown that by drinking orange juice (8 ml kg–1), one can reach a plasma concentration of hesperetin in the range of 0.5–6 μM.30 In addition, hesperetin is metabolized to other compounds, such as aglycone glucuronides and sulfates, which can also exert pharmacological activity.31,32 We have therefore examined the cellular effects of hesperetin in the range of 5–100 μM.
MATERIALS AND METHODS
Materials
Insulin was purchased from Roche Diagnostics Corporation (Indianapolis, IN). Hesperetin was acquired from Sigma and GLUT1 and GLUT4 antibodies from Abcam. Alexa 488–conjugated antimouse IgG was purchased from Invitrogen, and phospho-insulin receptor and phospho-AKT antibodies were obtained from Cell Signaling Technology. A phosphatase inhibitor was acquired from Thermo Scientific and a HALT protease inhibitor cocktail from Thermo Scientific.
Cell lines and culture
Human breast cancer cells (MDA-MB-231) were maintained in DMEM (Gibco) containing 10% fetal bovine serum (FBS, Hyclone) at 37 °C and 5% CO2. Fifth to tenth passage cells at a confluency of 80% were used for the experiments.
Glucose uptake assay
For the basal glucose uptake assay, MDA-MB-231 cells were seeded in 96-well plates (1 × 104 cells per well) for 24 h, washed with phosphate-buffered saline (PBS) and incubated for 24 h with serum free media. Cells were thereafter treated with 0.1% dimethyl sulfoxide (DMSO) (vehicle) or hesperetin (25–100 μM) for 24 h and then washed with PBS. Glucose uptake was measured using a glucose uptake cell-based assay kit (Cayman) according to the manufacturer's instructions. For the insulin-stimulated glucose uptake assay, cells were treated as described previously, followed by a 30-min incubation with 100 nM of insulin. Three replicates were used for each group, and the experiments were repeated three times to confirm the results.
Real-time RT-PCR
Cells were seeded in 12-well plates (1 × 105 cells per well) and serum starved for 24 h, followed by hesperetin treatment for 24 h. RNA extraction was performed using the ALLPrep RNA Kit (QIAGEN) according to manufacturer's instructions, and quantification was performed by absorption spectroscopy. Real-time RT-PCR was conducted as previously described.33 The GLUT1 primer sequences for quantitative PCR were as follows: sense 5′-GGG CAA GTC CTT TGA GAT GC-3′ and antisense 5′-AAG GCT GTG GGT GAC ACT TCA-3′. The GLUT1 mRNA expression was normalized by measuring the housekeeping gene coding for glyceraldehyde-3-phosphate (GAPDH).
Western blot assay
MDA-MB-231 cells were seeded in six-well plates (2 × 105 cells per well) and serum starved for 24 h and then treated with 0.1% DMSO (vehicle) or hesperetin (25–100 μM) for 24 h. For the measurement of Akt and insulin receptor phosphorylation, serum-starved cells were pretreated with 0.1% DMSO (vehicle) or 100 μM hesperetin for 30 min, followed by insulin stimulation (100 nM) for 30 min. The treated cells were washed and incubated with lysis buffer containing protease and phosphatase inhibitors. Protein lysates were mixed with SDS loading buffer and heated at 95 °C for 5 min. Samples were separated by electrophoresis with Any kD gels (Bio-Rad) and transferred to PVDF membranes. Membranes were blocked for 1 h in 5% nonfat milk in Tris-buffered saline with 0.1% Tween-20, incubated with desired primary antibody overnight, washed and incubated with HRP-conjugated secondary antibody for 1 h. Membranes were washed and protein bands were detected by enhanced chemiluminescence (Amersham Life Sciences, Amersham, UK).
Immunofluorescence imaging
MDA-MB-231 cells were grown in 35 mm dishes (2 × 105 cells per well) for 24 h, washed with PBS and incubated with fresh serum-free medium for 24 h. For detection of GLUT1, cells were treated with 0.1% DMSO (vehicle) or hesperetin (25–100 μM) for 24 h. For the detection of GLUT4, cells were treated with 0.1% DMSO (vehicle) or hesperetin (25–100 μM) for 30 min and then stimulated by insulin (100 nM) for 30 min. The treated cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. Nonspecific sites were blocked with 1% BSA in PBS containing 0.1% Tween-20. The fixed cells were incubated with GLUT1 and GLUT4 monoclonal mouse primary antibodies overnight (1:200 dilution), followed by washing and incubation with Alexa 488–conjugated antimouse IgG secondary antibody for 1 h at room temperature (1:500 dilution). All antibodies were diluted in PBS containing 0.1% Tween-20 solution containing 1% BSA. Fluorescence imaging was performed by confocal microscopy (Olympus IX81). Analysis of GLUT4 associated with the plasma membrane was performed as previously described.23 Each group was represented by five replicates and the experiment was repeated three times to confirm the results.
Cell proliferation assay
MDA-MB-231 cells were seeded in 96-well plates (1 × 104 cells per well) with complete medium for 24 h, washed and incubated with serum free medium for 24 h. In the basal cell proliferation assay, cells were treated with vehicle (0.1% DMSO) or 25–100 μM of hesperetin for 24 h. In the insulin-stimulated cell proliferation assay, cells were treated with vehicle (0.1% DMSO) or 25–100 μM of hesperetin in the absence or presence of insulin for 24 h. Cellular proliferation was quantified using the CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay (Promega Corporation) according to the manufacturer's instructions. Each group was represented by three replicates and the experiment was repeated three times to confirm the initial result.
Statistical analysis
Data were measured by analysis of variance, followed by Student's t-test (unpaired and two-tailed) (Microsoft Excel). Data were performed as mean ±S.E.
RESULTS
Hesperetin inhibits basal and insulin-stimulated glucose uptake
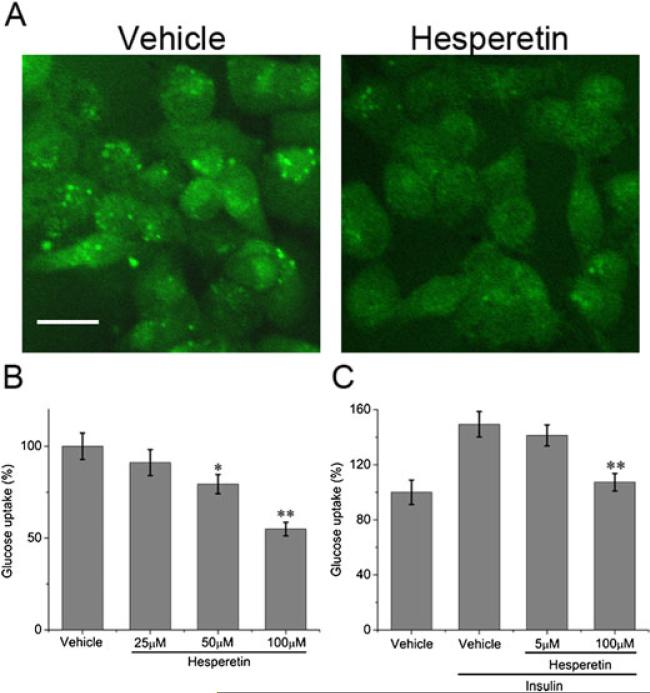
To investigate the effect of hesperetin on basal glucose uptake in MDA-MB-231 breast cancer cells, glucose transport was monitored using a fluorescent 2-deoxyglucose analogue (2-NBDG). As shown in Figures 1A and 1B, hesperetin caused a decrease in glucose uptake. At the highest concentration (100 μM), glucose uptake was reduced by approximately 45%.
Figure 1.
Effect of hesperetin on basal and insulin-stimulated glucose uptake in MDA-MB-231 cells. (A) Confocal fluorescence microscope images of cells treated with vehicle (0.1% DMSO) or 100 μM hesperetin. Fluorescently labelled deoxyglucose (2-NDBG) is visible in green. Scale bar: 20 μm. (B and C) Fluorescence intensity of deoxyglucose compared with vehicle. (B) Cells were treated with vehicle or 100 μM hesperetin for 24 h. (C) Cells were treated with 5 μM or 100 μM hesperetin in the presence of insulin (100 nM). Results represent the means ±S.E. of three replicates. * P<0.05, ** P<0.01.
To further determine the effect of hesperetin on insulin-stimulated glucose uptake, cells were pretreated with a high dose (100 μM) or a low dose (5 μM) of hesperetin and then stimulated with insulin. Insulin-stimulated glucose uptake was reduced by approximately 40 % in the high dose group and 8% in the low dose group (Figure 1C).
Hesperetin inhibits GLUT1 protein and mRNA expression
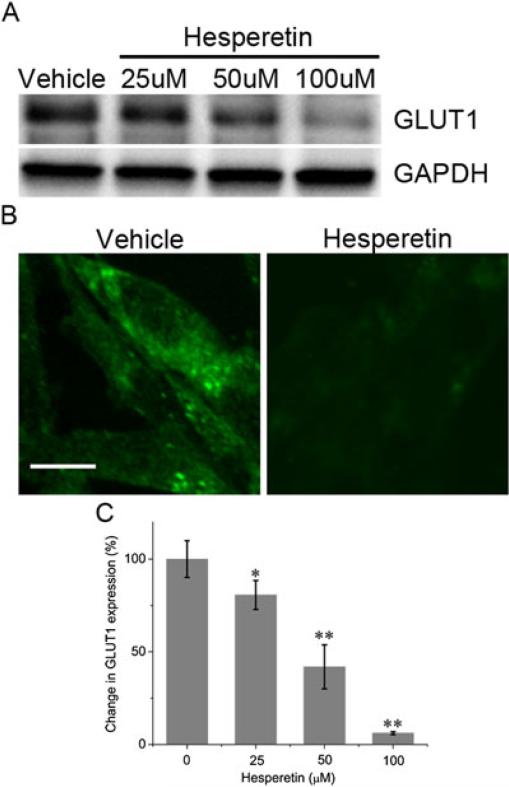
We performed Western blot, fluorescence imaging and RT-PCR to evaluate whether hesperetin induces changes in GLUT1 expression. Western blot analysis revealed that hesperetin caused down-regulation of GLUT1 protein expression in a concentration-dependent fashion (Figure 2A). These results were supported by fluorescence images showing that hesperetin (100 μM) significantly inhibited GLUT1 expression (Figure 2B). RT-PCR analysis indicated a concentration-dependent reduction of GLUT1 mRNA levels by hesperetin. The mean value of GLUT1 expression relative to controls was decreased to 80.6% ± 7.8% at 25 μM, 41.8% ± 11.9% at 50 μM and 6.1% ± 0.8% at 100 μM (Figure 2C).
Figure 2.
GLUT1 expression in MDA-MB-231 cells treated with vehicle (0.1% DMSO) or hesperetin. (A) Western blot analysis of cells. (B) Confocal fluorescence microscope image of GLUT1 detected with fluorescent antibody (green). Scale bar: 20 μm. (C) GLUT1 mRNA expression. Results are expressed as the mean ±SE of the percent change in GLUT1.* P<0.05, ** P<0.01.
Hesperetin inhibits insulin-induced redistribution of GLUT4 and impairs phosphorylation of the insulin receptor and Akt
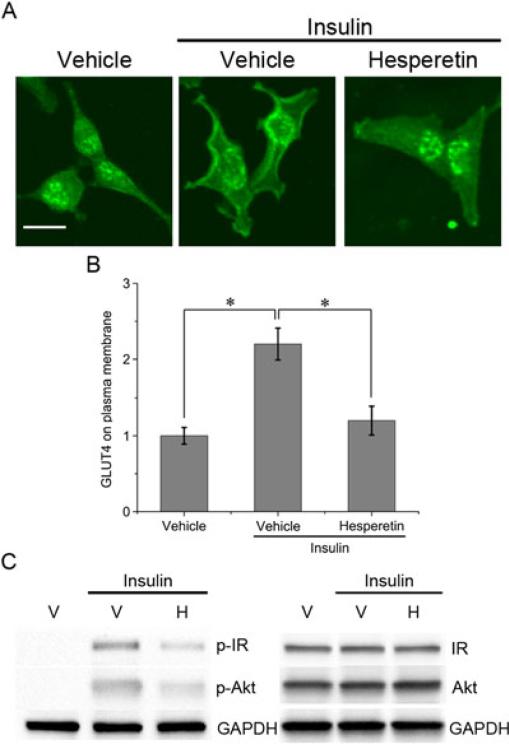
We investigated the mechanism of hesperetin inhibition on insulin-stimulated glucose uptake. Under basal conditions, GLUT4 displayed a perinuclear distribution, whereas insulin stimulation caused a redistribution of GLUT4 to the cell periphery (Figure 3A). By analyzing the fluorescence intensity of the plasma membrane, it was shown that insulin caused a 2.2-fold increase in the amount of GLUT4 in comparison with basal conditions (p < 0.05) (Figure 3C). Pretreatment with hesperetin significantly decreased the amount of GLUT4 that accumulated at the cell surface in response to insulin (p < 0.05) (Figures 3A and 3B).
Figure 3.
Effect of hesperetin on insulin-stimulated signaling in MDA-MB-231 cells. (A) Confocal images of immunofluorescent detection of GLUT4. Cells were treated with vehicle (0.1% DMSO) or 100 μM hesperetin and then incubated in the absence or presence of insulin. (B) Fluorescent intensity analysis of GLUT4 associated with the plasma membrane. In each group five cells were analyzed. Data are expressed as the mean fold change in fluorescent intensity (compared with the vehicle group) ± SE. Scale bar, 20 μm. * P<0.05. (C) Western blot analysis of phosphorylated insulin receptor (p-IR), phosphorylated Akt (p-Akt), insulin receptor (IR) and Akt. Cells were pretreated with 0.1% DMSO (vehicle, V) or 100 μM hesperetin (H) and stimulated with insulin for 30 min.
Hesperetin was also shown to suppress the phosphorylation of the insulin receptor β subunit, which is involved in the initial steps of the insulin pathway (Fig 3C). Moreover, we confirmed that hesperetin significantly reduced the phosphorylation of Akt, a downstream effector in the insulin pathway (Fig 3C). In contrast to GLUT1, hesperetin did not induce any changes in GLUT4 protein levels (data not shown).
Hesperetin inhibits cell proliferation
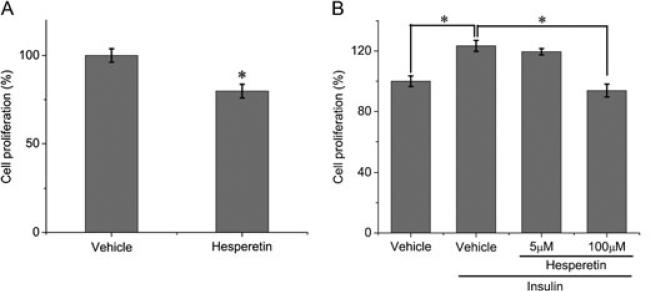
To examine whether hesperetin treatment causes a decrease in cell proliferation, cells were incubated in serum-free DMEM in the presence of vehicle or hesperetin (100 μM) for 24 h. The results indicate that hesperetin inhibited cell proliferation under basal conditions (Fig 4A). We also determined the effect of hesperetin on insulin-stimulated proliferation and found that a high dose (100 μM) reduced proliferation by approximately 30%, whereas a low dose (5 μM) reduced cell proliferation by 4% (Fig 4B).
Figure 4.
Cell proliferation assay (MTS). (A) MDA-MB-231 cells were incubated with serum-free medium and treated with 0.1% DMSO (vehicle) or 100 μM hesperetin. (B) MDA-MB-231 cells were incubated with 0.1% DMSO (vehicle) or hesperetin in the presence of insulin. Results represent the means ± SE of three replicates. *P<0.05.
DISCUSSION
Cancer cells have high rates of glucose uptake and metabolism, which are essential for tumour growth. This is partly due to the increased activity of enzymes, such as hexokinases,34–36 and partly the result of overexpressed glucose transporters.1,2 Therefore, compounds that target these transporters have the potential to be used as inhibitors for tumour progression. In addition, it has been shown that the inhibition of glucose37 or glucose metabolism38,39 can sensitize cells to chemotherapeutics, thereby overcoming required resistance to these agents.
More than 20 glucose transporters have been characterized, of which GLUT1 and GLUT4 are the predominant ones found in carcinoma.9,10,37,40 In the present study, we found that hesperetin down-regulates expression of GLUT1 at both the transcriptional and translational level as well as reducing insulin-induced relocation of GLUT4. By reducing glucose uptake through multiple mechanisms, hesperetin may have an advantage compared with agents that inhibit a single pathway of glucose uptake.41,42 We have also shown that hesperetin reduces cancer cell proliferation, which is likely due to the impairment of glucose uptake, as the suppression of cell division by low levels of glucose is a common phenomenon reported in the literature.2,26,43 According to a study conducted by Erlund et al.30, the physiological dose of hesperetin, attainable from drinking orange juice, is in the range of 0.5–6 μM. We have shown that insulin-stimulated glucose uptake was inhibited by approximately 8% and proliferation by approximately 4% at 5 μM of hesperetin, although these results were not statistically significant. However, at a higher concentration (100 μM), the suppression is much stronger, reaching 45% inhibition of glucose uptake and 30% inhibition of proliferation. Therefore, we conclude that there could be a possible therapeutic benefit of regular consumption of citrus juice, although higher concentrations of hesperetin are necessary to bring about more dramatic effects. Higher plasma levels of hesperetin could potentially be obtained by using alternative routes of administration instead of oral uptake. Metabolized versions of hesperetin that arise in vivo, which were not accounted for in our experiments, could also influence therapeutic efficacy. It should also be noted that the natural form of hesperetin mainly exists as an S-stereoisomer, whereas the commercially available version, used in this work, is a mixture of both S- and R-stereoisomers.44,45
Previous reports also demonstrate that hesperetin induces apoptosis46–48 and inhibits angiogenesis.46,49 Moreover, it has been shown that hesperetin glucuronides can act as inhibitors or substrates for xenobiotic transporters, such as BCRP and MRP3.50 The presence of such substrates inhibits the efflux of other compounds through these transporters,50 suggesting that hesperetin could potentially suppress multi-drug resistance. These effects, combined with the inhibition of glucose uptake, suggest a potential role for hesperetin in cancer therapy.
ACKNOWLEDGEMENTS
This research was supported by funds from the Methodist Hospital Research Institute. Partial funds are from the following grants: Ernest Cockrell Jr. Distinguished Endowed Chair (M.F.), US Department of Defense (grant no. W81XWH-09-1-0212) (M.F.), National Institutes of Health (grants no. U54CA143837 and U54CA151668) (M.F.) and Nylands nation Finland (J.W.). The authors thank the reviewers of this manuscript for insightful comments.
Footnotes
CONFLICT OF INTEREST
The authors have declared that there is no conflict of interest.
REFERENCES
- 1.Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol. 2005;202:654–662. doi: 10.1002/jcp.20166. [DOI] [PubMed] [Google Scholar]
- 2.Medina RA, Owen GI. Glucose transporters: expression, regulation and cancer. Biol Res. 2002;35:9–26. doi: 10.4067/s0716-97602002000100004. [DOI] [PubMed] [Google Scholar]
- 3.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alo PL, Visca P, Botti C, et al. Immunohistochemical expression of human erythrocyte glucose transporter and fatty acid synthase in infiltrating breast carcinomas and adjacent typical/atypical hyperplastic or normal breast tissue. Am J Clin Pathol. 2001;116:129–134. doi: 10.1309/5Y2L-CDCK-YB55-KDK6. [DOI] [PubMed] [Google Scholar]
- 5.Zimmerman RL, Goonewardene S, Fogt F. Glucose transporter Glut-1 is of limited value for detecting breast carcinoma in serous effusions. Mod Pathol. 2001;14:748–751. doi: 10.1038/modpathol.3880384. [DOI] [PubMed] [Google Scholar]
- 6.Hao LS, Ni Q, Jia GQ, et al. Expression of glucose transporter 1 in human breast carcinoma and its clinical significance. Sichuan Da Xue Xue Bao Yi Xue Ban. 2009;40:44–47. [PubMed] [Google Scholar]
- 7.Kang SS, Chun YK, Hur MH, et al. Clinical significance of glucose transporter 1 (GLUT1) expression in human breast carcinoma. Jpn J Cancer Res. 2002;93:1123–1128. doi: 10.1111/j.1349-7006.2002.tb01214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Younes M, Brown RW, Mody DR, Fernandez L, Laucirica R. GLUT1 expression in human breast carcinoma: correlation with known prognostic markers. Anticancer Res. 1995;15:2895–2898. [PubMed] [Google Scholar]
- 9.Binder C, Binder L, Marx D, Schauer A, Hiddemann W. Deregulated simultaneous expression of multiple glucose transporter isoforms in malignant cells and tissues. Anticancer Res. 1997;17:4299–4304. [PubMed] [Google Scholar]
- 10.Grover-McKay M, Walsh SA, Seftor EA, Thomas PA, Hendrix MJ. Role for glucose transporter 1 protein in human breast cancer. Pathol Oncol Res. 1998;4:115–120. doi: 10.1007/BF02904704. [DOI] [PubMed] [Google Scholar]
- 11.Godoy A, Ulloa V, Rodriguez F, et al. Differential subcellular distribution of glucose transporters GLUT1-6 and GLUT9 in human cancer: ultrastructural localization of GLUT1 and GLUT5 in breast tumour tissues. J Cell Physiol. 2006;207:614–627. doi: 10.1002/jcp.20606. [DOI] [PubMed] [Google Scholar]
- 12.Rogers S, Docherty SE, Slavin JL, Henderson MA, Best JD. Differential expression of GLUT12 in breast cancer and normal breast tissue. Cancer Lett. 2003;193:225–233. doi: 10.1016/s0304-3835(03)00010-7. [DOI] [PubMed] [Google Scholar]
- 13.Rivenzon-Segal D, Rushkin E, Polak-Charcon S, Degani H. Glucose transporters and transport kinetics in retinoic acid-differentiated T47D human breast cancer cells. Am J Physiol Endocrinol Metab. 2000;279:E508–519. doi: 10.1152/ajpendo.2000.279.3.E508. [DOI] [PubMed] [Google Scholar]
- 14.Zamora-Leon SP, Golde DW, Concha II, et al. Expression of the fructose transporter GLUT5 in human breast cancer. Proc Natl Acad Sci U S A. 1996;93:1847–1852. doi: 10.1073/pnas.93.5.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sweeney G, Somwar R, Foster L, et al. Intracellular delivery of phosphatidylinositol(3,4,5)trisphosphate and phosphatidylinositol(3,4) bisphosphate cause translocation and incorporation of GLUT4 to the plasma membrane without increasing glucose transport in muscle and fat cells. Diabetes. 2001;50:A19–A19. doi: 10.1074/jbc.M402897200. [DOI] [PubMed] [Google Scholar]
- 16.Pessin JE, Thurmond DC, Elmendorf JS, Coker KJ, Okada S. Molecular basis of insulin-stimulated GLUT4 vesicle trafficking. J Biol Chem. 1999;274:2593–2596. doi: 10.1074/jbc.274.5.2593. [DOI] [PubMed] [Google Scholar]
- 17.Resh MD. Development of insulin responsiveness of the glucose transporter and the (Na+,K+)-adenosine triphosphatase during in vitro adipocyte differentiation. J Biol Chem. 1982;257:6978–6986. [PubMed] [Google Scholar]
- 18.Muretta JM, Mastick CC. How insulin regulates glucose transport in adipocytes. Vitam Horm. 2009;80:245–286. doi: 10.1016/S0083-6729(08)00610-9. [DOI] [PubMed] [Google Scholar]
- 19.Wang YF, Litzenburger B, Kuiatse I, et al. Expression and Activity of Both IGF and Insulin Signaling Pathways in a Large Panel of Breast Cancer Cell Lines. Cancer Res. 2009;69:762s–762s. [Google Scholar]
- 20.Noguchi Y, Sato S, Marat D, et al. Glucose uptake in the human gastric cancer cell line, MKN28, is increased by insulin stimulation. Cancer Lett. 1999;140:69–74. doi: 10.1016/s0304-3835(99)00054-3. [DOI] [PubMed] [Google Scholar]
- 21.Cheatham B, Vlahos CJ, Cheatham L, Wang L, Blenis J, Kahn CR. Phosphatidylinositol 3-Kinase Activation Is Required for Insulin Stimulation of Pp70 S6 Kinase, DNA-Synthesis, and Glucose-Transporter Translocation. Mol Cell Biol. 1994;14:4902–4911. doi: 10.1128/mcb.14.7.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanti JF, Gremeaux T, Grillo S, et al. Overexpression of a constitutively active form of phosphatidylinositol 3-kinase is sufficient to promote glut 4 translocation in adipocytes. J Biol Chem. 1996;271:25227–25232. doi: 10.1074/jbc.271.41.25227. [DOI] [PubMed] [Google Scholar]
- 23.Sweeney G, Garg RR, Ceddia RB, et al. Intracellular delivery of phosphatidylinositol (3,4,5)-trisphosphate causes incorporation of glucose transporter 4 into the plasma membrane of muscle and fat cells without increasing glucose uptake. J Biol Chem. 2004;279:32233–32242. doi: 10.1074/jbc.M402897200. [DOI] [PubMed] [Google Scholar]
- 24.Singh A, Purohit A, Hejaz HA, Potter BV, Reed MJ. Inhibition of deoxyglucose uptake in MCF-7 breast cancer cells by 2-methoxyestrone and 2-methoxyestrone-3-O-sulfamate. Mol Cell Endocrinol. 2000;160:61–66. doi: 10.1016/s0303-7207(99)00256-7. [DOI] [PubMed] [Google Scholar]
- 25.Okumura M, Yamamoto M, Sakuma H, et al. Leptin and high glucose stimulate cell proliferation in MCF-7 human breast cancer cells: reciprocal involvement of PKC-alpha and PPAR expression. Biochim Biophys Acta. 2002;1592:107–116. doi: 10.1016/s0167-4889(02)00276-8. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto M, Patel NA, Taggart J, Sridhar R, Cooper DR. A shift from normal to high glucose levels stimulates cell proliferation in drug sensitive MCF-7 human breast cancer cells but not in multidrug resistant MCF-7/ADR cells which overproduce PKC-beta II. Int J Cancer. 1999;83:98–106. doi: 10.1002/(sici)1097-0215(19990924)83:1<98::aid-ijc18>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 27.Tomas-Barberan FA, Clifford MN. Flavanones, chalcones and dihydrochalcones – nature, occurrence and dietary burden. J Sci Food Agric. 2000;80:1073–1080. [Google Scholar]
- 28.Walle T. Absorption and metabolism of flavonoids. Free Radic Biol Med. 2004;36:829–837. doi: 10.1016/j.freeradbiomed.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Park JB. Flavonoids are potential inhibitors of glucose uptake in U937 cells. Biochem Biophys Res Comm. 1999;260:568–574. doi: 10.1006/bbrc.1999.0890. [DOI] [PubMed] [Google Scholar]
- 30.Erlund I, Meririnne E, Alfthan G, Aro A. Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J Nutr. 2001;131:235–241. doi: 10.1093/jn/131.2.235. [DOI] [PubMed] [Google Scholar]
- 31.Brett GM, Hollands W, Needs PW, et al. Absorption, metabolism and excretion of flavanone from single portions of orange fruit and juice and effects of anthropometric variables and contraceptive pill use on flavanone excretion. Br J Nutr. 2009;101:664–675. doi: 10.1017/S000711450803081X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mullen W, Archeveque MA, Edwards CA, Matsumoto H, Crozier A. Bioavailability and metabolism of orange juice flavanones in humans: impact of a full-fat yogurt. J Agric Food Chem. 2008;56:11157–11164. doi: 10.1021/jf801974v. [DOI] [PubMed] [Google Scholar]
- 33.Chung FY, Huang MY, Yeh CS, et al. GLUT1 gene is a potential hypoxic marker in colorectal cancer patients. BMC Cancer. 2009;9:241. doi: 10.1186/1471-2407-9-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown RS, Goodman TM, Zasadny KR, Greenson JK, Wahl RL. Expression of hexokinase II and Glut-1 in untreated human breast cancer. Nucl Med Biol. 2002;29:443–453. doi: 10.1016/s0969-8051(02)00288-3. [DOI] [PubMed] [Google Scholar]
- 35.Verhagen JN, Vanderheijden MCM, Rijksen G, Derkinderen PJ, Vanunnik JAM, Staal GEJ. Determination and Characterization of Hexokinase in Thyroid-Cancer and Benign Neoplasms. Cancer. 1985;55:1519–1524. doi: 10.1002/1097-0142(19850401)55:7<1519::aid-cncr2820550718>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 36.Yasuda S, Arii S, Mori A, et al. Hexokinase II and VEGF expression in liver tumors: correlation with hypoxia-inducible factor-1 alpha and its significance. J Hepatol. 2004;40:117–123. doi: 10.1016/s0168-8278(03)00503-8. [DOI] [PubMed] [Google Scholar]
- 37.Cao XH, Fang LY, Gibbs S, et al. Glucose uptake inhibitor sensitizes cancer cells to daunorubicin and overcomes drug resistance in hypoxia. Cancer Chemoth Pharm. 2007;59:495–505. doi: 10.1007/s00280-006-0291-9. [DOI] [PubMed] [Google Scholar]
- 38.Maschek G, Savaraj N, Priebe W, et al. 2-deoxy-D-glucose increases the efficacy of adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivo. Cancer Res. 2004;64:31–34. doi: 10.1158/0008-5472.can-03-3294. [DOI] [PubMed] [Google Scholar]
- 39.Sandulache VC, Skinner HD, Wang Y, et al. Glycolytic inhibition alters anaplastic thyroid carcinoma tumor metabolism and improves response to conventional chemotherapy and radiation. Mol Cancer Ther. 2012;11:1373–1380. doi: 10.1158/1535-7163.MCT-12-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown RS, Wahl RL. Overexpression of Glut-1 Glucose-Transporter in Human Breast-Cancer - an Immunohistochemical Study. Cancer. 1993;72:2979–2985. doi: 10.1002/1097-0142(19931115)72:10<2979::aid-cncr2820721020>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 41.Wojtaszewski JFP, Hansen BF, Urso B, Richter EA. Wortmannin inhibits both insulin- and contraction-stimulated glucose uptake and transport in rat skeletal muscle. J Appl Physiol. 1996;81:1501–1509. doi: 10.1152/jappl.1996.81.4.1501. [DOI] [PubMed] [Google Scholar]
- 42.Purcell SH, Chi MM, Moley KH. Insulin-Stimulated Glucose Uptake Occurs in Specialized Cells within the Cumulus Oocyte Complex. Endocrinology. 2012;153:2444–2454. doi: 10.1210/en.2011-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou SH, Fan J, Chen XM, Cheng KJ, Wang SQ. Inhibition of Cell Proliferation and Glucose Uptake in Human Laryngeal Carcinoma Cells by Antisense Oligonucleotides against Glucose Transporter-1. Head Neck-J Sci Spec. 2009;31:1624–1633. doi: 10.1002/hed.21137. [DOI] [PubMed] [Google Scholar]
- 44.Yanez JA, Remsberg CM, Miranda ND, Vega-Villa KR, Andrew PK, Davies NM. Pharmacokinetics of selected chiral flavonoids: hesperetin, narigenin and eriodictyol in rats and their content in fruit juice. Biopharm Drug Dispos. 2008;29:63–82. doi: 10.1002/bdd.588. [DOI] [PubMed] [Google Scholar]
- 45.Brand W, Shao J, Hoek-van den Hil EF, et al. Stereoselective conjugation, transport and bioactivity of s- and R-hesperetin enantiomers in vitro. J Agric Food Chem. 2010;58:6119–6125. doi: 10.1021/jf1008617. [DOI] [PubMed] [Google Scholar]
- 46.Nalini N, Aranganathan S, Kabalimurthy J. Chemopreventive efficacy of hesperetin (citrus flavonone) against 1,2-dimethylhydrazine-induced rat colon carcinogenesis. Toxicol Mech Methods. 2012;22(5):397–408. doi: 10.3109/15376516.2012.673092. [DOI] [PubMed] [Google Scholar]
- 47.Patil JR, Chidambara Murthy KN, Jayaprakasha GK, Chetti MB, Patil BS. Bioactive compounds from Mexican lime (Citrus aurantifolia) juice induce apoptosis in human pancreatic cells. J Agric Food Chem. 2009;57:10933–10942. doi: 10.1021/jf901718u. [DOI] [PubMed] [Google Scholar]
- 48.Sivagami G, Vinothkumar R, Preethy CP, et al. Role of hesperetin (a natural flavonoid) and its analogue on apoptosis in HT-29 human colon adenocarcinoma cell line--a comparative study. Food Chem Toxicol. 2012;50:660–671. doi: 10.1016/j.fct.2011.11.038. [DOI] [PubMed] [Google Scholar]
- 49.Choi EJ, Kim GD, Chee KM, Kim GH. Effects of hesperetin on vessel structure formation in mouse embryonic stem (mES) cells. Nutrition. 2006;22:947–951. doi: 10.1016/j.nut.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 50.Brand W, Oosterhuis B, Krajcsi P, et al. Interaction of hesperetin glucuronide conjugates with human BCRP, MRP2 and MRP3 as detected in membrane vesicles of overexpressing baculovirus-infected Sf9 cells. Biopharm Drug Dispos. 2011;32:530–535. doi: 10.1002/bdd.780. [DOI] [PubMed] [Google Scholar]