Abstract
Methamphetamine use is a growing problem among pregnant women in the United States. Many negative consequences of methamphetamine use have been documented for the users, but little research has examined the long-term association between prenatal methamphetamine exposure (PME) and childhood outcomes. The current study examined the extent to which PME was predictive of childhood neurobehavioral disinhibition (ND), as well as the extent to which early adversity mediated this relationship. A sample of 320 mother–infant dyads (162 PME) was followed from birth through 6.5 years of age. ND was conceptualized as a two factor model consisting of deficits in (a) behavioral and emotional control, and (b) executive function. PME was associated with behavioral and emotional control at 5 years, which was associated with executive function deficits at 6.5 years. Early adversity (birth through year 3) significantly mediated the relationship between PME and ND. Associations with previous research and implications for prevention are discussed.
Keywords: methamphetamine, prenatal, adversity, disinhibition
Methamphetamine use represents a continuing and serious public health concern in the United States, with the 2010 estimates from the National Surveys on Drug Use and Health indicating 353,000 individuals in the United States used methamphetamine in the past month (Substance Abuse and Mental Health Services Administration, 2011). Methamphetamine is a neurotoxic para-sympathetic stimulant (Salisbury, Ponder, Padbury, & Lester, 2009) whose use is associated with a host of negative consequences for the users (e.g., Berman, O’Neill, Fears, Bartzokis, & London, 2008; Block, Erwin, & Ghoneim, 2002; Chang, Alicata, Ernst, & Volkow, 2007; Sommers, Baskin, & Baskin-Sommers, 2006). Although the negative consequences of methamphetamine are experienced by the majority of users, pregnant women represent a particularly important subpopulation, as research has shown wide-ranging effects of prenatal methamphetamine exposure (PME) on the developmental outcomes of the child. A recent study in the United States estimated that, between 2002 and 2004, an average of approximately 19,000 women annually used methamphetamine while pregnant (Colliver, Kroutil, Dai, & Gfroerer, 2006). Moreover, 24% of pregnant women admitted to federally funded substance abuse treatment centers were there for methamphetamine abuse (Terplan, Smith, Kozloski, & Pollack, 2009).
A surprisingly small but growing body of literature has shown cognitive and behavioral effects of PME on the developing child (Lester & Lagasse, 2010; Wouldes, LaGasse, Sheridan, & Lester, 2004), as well as effects on brain structure and neurochemistry. Sowell et al. (2010) found—in their sample of children ages 5 to 15 years who were prenatally exposed to (a) methamphetamine use only, (b) heavy alcohol use only, (c) methamphetamine and some alcohol use, and (d) neither substance—that PME children displayed lower full scale IQ scores than control individuals. Using magnetic resonance imaging, these researchers also found that each of the exposed groups displayed deficits in the striatal and thalamic regions, right prefrontal cortex, and left occipitoparietal cortex, but the “methamphetamine and some alcohol exposure” group displayed significantly smaller volumes than even the heavy-alcohol exposure group (Sowell et al., 2010), which implies a potentially unique risk of PME beyond other known teratogens.
PME effects, whether direct or indirect, on overall IQ have not been observed in most studies, but others have shown PME negative effects on aspects of memory (e.g., Chang et al., 2004; Lu et al., 2009; Piper et al., 2011), attention (Chang et al., 2004), inhibitory control (Derauf et al., 2012), and motor control (Chang et al., 2009; Smith et al., 2011). Chang et al. (2009) also found, using proton magnetic spectroscopy, that PME infants have abnormal brain metabolite levels at age 4 years (i.e., creatine, N-acetyl compounds, and glutamate + glutamine, myoinositol), and levels of thalamic myoinositol were linked with poorer visual motor integration. In addition, the memory and attention deficits among PME children from 3 to 16 years of age observed in Chang et al. (2004) were linked with smaller volume subcortical structures (i.e., putamen, globus pallidus, hippocampus, and caudate).
A majority of these studies were cross-sectional and did not address the potential influence of exposure on behavioral concerns. Notable exceptions are several studies on a small sample of Swedish amphetamine-exposed children (n = 66) followed prospectively from birth through age 14 years. This work has shown higher levels of aggression and behavior problems, in addition to poorer psychosocial well-being and academic achievement, in PME children (e.g., Billing, Eriksson, Larsson, & Zetterström, 1980; Billing, Eriksson, Steneroth, & Zetterström, 1985, 1988; Billing, Eriksson, Jonsson, Steneroth, & Zetterström, 1994; Eriksson & Zetterström, 1994). Limitations of this work include the lack of a control/comparison group, a very high rate of polydrug exposure (i.e., approximately 80% were prenatally exposed to alcohol and tobacco; 30% were prenatally exposed to heroin), and the primary route of administration was via injection (Lester & Lagasse, 2010). In the United States, methamphetamine is primarily consumed via snorting or smoking (Arria et al., 2006). The current study sought to prospectively examine both cognitive and behavioral consequences of PME using a more general theoretical representation of developmental outcomes.
Taken as a whole, the deficits associated with PME observed in the previous research discussed fall under the general description of neurobehavioral disinhibition (ND) described by Tarter and colleagues (2003). ND was described as a developmentally salient combination of interrelated deficits in executive cognitive functioning, emotion regulation, and behavior control (Tarter et al., 2003). Childhood ND has been shown to significantly increase the risk of substance use and abuse later in life (Chapman, Tarter, Kirisci, & Cornelius, 2007; Lester et al., 2012; McNamee et al., 2008; Tarter et al., 2003). Research into antecedents of ND has shown significant deficits associated with prenatal exposure to alcohol (Chapman et al., 2007) and multiple substances (i.e., cocaine, opiates, marijuana, alcohol, and/or tobacco; Fisher et al., 2011). Prenatal exposure to cocaine has also been found to be associated with increased ND across late childhood and early adolescence (Lester et al., 2012).
In the Fisher et al. (2011) study, postnatal environmental adversity was modeled as a mediator of the effects of drug exposure on later ND. The inclusion of early adversity (as indexed by postnatal drug exposure, low socioeconomic status, unstable home and caregiver environment, caregiver experiences of abuse, and psychopathology) as an additional predictor and mediator is intuitive given previously observed relationships both (a) between maternal substance use while pregnant and child exposure to adverse environments (e.g., Eiden, Foote, & Schuetze, 2007; Smith, Johnson, Pears, Fisher, & DeGarmo, 2007; Tronick & Beeghly, 1999), and (b) between adverse child environments and later negative outcomes (e.g., Bendersky, Bennett, & Lewis, 2006; Connell & Goodman, 2002; Eiden, 1999; Westbrook & Harden, 2010). Early adversity, as operationalized in the current study, closely corresponds to the contextual dimension of environmental chaos described in the developmental literature (e.g., Evans, Maxwell, & Hart, 1999; Wachs, Gurkas, & Kontos, 2004), with the primary exception being that early adversity is a more general concept that includes previously defined antecedents of environmental chaos (e.g., parental drug use). In regard to methamphetamine, studies imply that parental use is particularly predictive of an adverse environment (Terplan et al., 2009), with qualitative work indicating parents tend to report feeling they had created an unsafe and poorly nurturing environment for their children as the result of their use (Brown & Hohman, 2006).
Purpose of the Current Study
The current study seeks to expand upon the existing literature on PME in several important ways. First, the majority of work in this area has made use of small and geographically restricted samples of PME children. The current study utilized a much larger sample of children, including a comparison group, collected from multiple sites in the United States. Second, although research on exposure to other substances has made use of Tarter et al.’s (2003) ND framework when examining childhood outcomes, the current study represents the first to explicitly model ND in PME children. Third, the current study seeks to demonstrate PME findings similar to the prenatal cocaine exposure findings in Fisher et al. (2011) with regard to the potential mediating role of early adversity on the relationship between prenatal exposure and childhood ND. Although cocaine and methamphetamine produce similar euphoric feelings in users, methamphetamine has a half-life approximately 8 times the length of cocaine (Newton, De La Garza, Kalechstein, & Nestor, 2005), which greatly increases the potential for neurotoxic effects on the mother and the developing fetus. Furthermore, both cocaine and methamphetamine inhibit the reuptake of synaptic dopamine, but methamphetamine also increases the release of dopamine into the synapse, which vastly increases its potential for addiction and impact on the developing brain of the fetus.
Research Questions
In the current study, we explored two primary research questions: (a) Is PME associated with childhood ND at ages 5 and 6.5 years? (2) To what extent does early adversity mediate the relationship between PME and ND?
Method
Participants
Data come from the Infant Development, Environment, and Lifestyle (IDEAL) study (for more information on IDEAL, see Smith et al., 2007, 2008). In the first phase of the IDEAL study, mothers who recently delivered (i.e., within 48 hr) were approached and screened for eligibility in four U.S. sites in representative geographic areas known to have methamphetamine problems (Los Angeles, California; Des Moines, Iowa; Tulsa, Oklahoma; Honolulu, Hawaii). The institutional review boards at each site reviewed and approved the study protocol and consent forms. Exclusion criteria included the following: non-English speaking, maternal age <18 years, multiple births, maternal cognitive or psychological impairment, maternal use of opiates or LSD/hallucinogens/PCP, maternal cocaine use only, infant congenital anomalies/chromosomal abnormalities, and infants unlikely to survive. A National Institute on Drug Abuse certificate of confidentiality was obtained for the project that assured confidentiality of information regarding the mothers’ drug use, superseding mandatory reporting of illegal substance use. The study identified a total of 34,833 mother–infant dyads at the time of the infant’s birth. Of these, 26,999 mothers were available for contact and were screened for eligibility in the study, with a total of 9,038 dyads declared ineligible. A total of 3,705 eligible participants agreed to participate for a consent rate of 21%, which is consistent with other prospective studies that involve repeated and extensive assessments of mothers and infants.
Participant mothers were interviewed on demographics and substance use during pregnancy, and infant meconium was assayed for drug metabolites. A total of 204 infants were classified as PME (see Measures section), and 208 mother–infant dyads were matched within site on maternal race, infant birth weight, private versus public insurance, and maternal educational status. Matched nonexposed comparison participants were enrolled only with both maternal denial of methamphetamine use during pregnancy and a negative meconium screen for methamphetamine. As such, 412 mother-infant dyads were recruited into the longitudinal portion of the larger study on PME. Parents were provided with a $50 incentive for participation at each time point from birth.
The current study utilizes data from those participants retained with outcome data through the 5-year follow-up (n = 320; 162 exposed), and 290 participants had outcome data at the 6.5-year follow-up. The majority of participants who were not retained were not able to be contacted at the 5- and 6.5-year follow-up. There were no significant differences between retained participants and lost-to-follow-up participants on PME status, data collection site, infant sex, insurance status, maternal educational level, and socioeconomic status, or in terms of prenatal exposure to alcohol, tobacco, marijuana, and cocaine (ps > 0.10). Retention was very similar across the exposed (79%) and nonexposed (76%) participants. Table 1 presents the demographic characteristics of the sample by exposure status and overall.
Table 1.
Demographic Characteristics of the Sample by Exposure Status
| PME (n = 162) | Nonexposed (n = 158) | Overall (n = 320) | |
|---|---|---|---|
| Frequencies (%) | |||
| Data collection site | |||
| Los Angeles, CA | 59 (36%) | 56 (35%) | 115 (36%) |
| Des Moines, IA | 12 (7%) | 18 (11%) | 30 (9%) |
| Tulsa, OK | 20 (12%) | 24 (15%) | 44 (14%) |
| Honolulu, HI | 71 (44%) | 60 (38%) | 131 (41%) |
| Infant female sex | 76 (47%) | 77 (49%) | 153 (48%) |
| Public insurance (vs. private) | 154 (96%) | 156 (99%) | 310 (97%) |
| Maternal non-Hispanic White race | 61 (38%) | 64 (41%) | 125 (39%) |
| Maternal education ≤ High school degree | 88 (54%) | 100 (64%) | 188 (59%) |
| Means (SDs) | |||
| Maternal substance use while pregnant | |||
| Tobacco (cigarettes/day)b | 7.02 (8.37) | 1.56 (4.49) | 4.32 (7.26) |
| Alcohol (ounces of alcohol/day)a | 0.11 (0.52) | 0.00 (0.02) | 0.06 (0.38) |
| Marijuana (joints/day)a | 0.07 (0.22) | 0.01 (0.10) | 0.04 (0.18) |
| Maternal age in years | 25.41 (5.59) | 24.54 (5.63) | 24.98 (5.62) |
| Gestational age at birth (weeks)a | 38.24 (2.35) | 39.01 (1.80) | 38.62 (2.13) |
| Birth head circumference (cm) | 33.67 (1.83) | 33.97 (1.83) | 33.82 (1.83) |
| Birth length (cm)a | 49.88 (3.74) | 51.01 (3.09) | 50.43 (3.48) |
| Birth weight (g) | 3198 (631) | 3288 (573) | 3243 (604) |
Note. PME = prenatal methamphetamine exposure.
Difference between PME and nonexposed comparisons, p < .01.
Difference between PME and nonexposed comparisons, p <.001.
Measures
PME
PME was indicated in two ways. Mothers either (a) self-reported methamphetamine use during pregnancy and/or (b) methamphetamine metabolites were confirmed present in infant meconium based on a positive immunoassay and gas chromatography-mass spectrometry. In regard to maternal report, 157 self-reported use of methamphetamine during pregnancy. The remaining 5 participants in the PME condition denied use, but infant meconium tested positive for methamphetamine metabolites. Meconium screenings were performed for each infant, and exposure was identified and confirmed for 47 infants.
ND
ND was indexed using several behavioral and cognitive indices. Tarter et al.’s (2003) conceptualization of ND included three dimensions (behavioral control, emotion regulation, and executive cognitive functioning) within a single index of ND. The current manuscript operationalized ND as a two-factor construct, including (a) behavioral and emotional control, and (b) executive function for several reasons. Our preliminary models found the two-factor model provided better fit to the data than a one-factor model. This might be the case in the current study because the measures available in the IDEAL study for behavioral and emotional control were taken at 5 years of age, whereas the battery of measures indexing executive functioning was taken at 6.5 years of age. This temporal precedence of behavioral and emotional control before executive functioning could have impacted the makeup and function of a single factor of ND. Tarter and colleagues’ (2003) sample was also older at baseline (10 to 12 years) than the current sample, which could influence the latent structure of the ND construct. In addition, the current manuscript framed the analyses on Fisher et al. (2011), which employed a two-factor model of ND (i.e., behavioral dysregulation and executive function difficulties). Their cross-lag model found that baseline level of behavioral dysregulation predicted later growth in executive function difficulties.
At 5 years old, examiners read the list of items to mothers who then reported on child behavioral and emotional control using the Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2000). Following scale developers’ recommendations, seven symptom summary scores were derived, including emotionally reactive, anxious/depressed, withdrawn, sleep problems, aggressive, and somatic complaints. All scores were standardized (T scores). At 6.5 years old, children were engaged in a series of objective tasks testing executive function. The Children’s Memory Scale (M. Cohen, 1997) is a standardized test involving an interaction between an examiner and child, designed to assess multiple dimensions of memory, with the current study using standardized indices of attention/concentration, delayed recognition, and general memory. The California Verbal Learning Test (Dells, Kramer, Kaplan, & Ober, 1994) assesses how verbal learning occurs or fails to occur in children as well as the amount of verbal material remembered in a structured interaction with an examiner, with the current study employing the representative standardized indices of long delay, free recall memory and short delay, cued recall memory. Examiners were blind to methamphetamine exposure status, and trained and annually certified by senior staff at the host institution (Brown Alpert Medical School). The Attention Network Task (Rueda et al., 2004) is a computerized flanker task with orienting cues appropriate for use with children to tap inhibitory control. The current study uses child total accuracy score (% correct). For clarity of presentation and congruity with the ND framework, the indicators measured at 6.5 years were multiplied by −1, such that higher scores represent poorer functioning, and the resulting factor was termed “Executive Function Deficits.” Score means and standard deviations, by exposure status and overall, are presented in Table 2.
Table 2.
Neurobehavioral Disinhibition Indicators by Exposure Group and Overall
| PME | Nonexposed | Overall | |
|---|---|---|---|
| Means (SDs) | |||
| Behavioral and emotional control | |||
| Emotionally reactivea | 57.32 (8.38) | 55.36 (6.54) | 56.35 (7.57) |
| Anxious/Depresseda | 56.76 (7.37) | 54.93 (6.30) | 55.86 (6.91) |
| Withdrawn | 57.52 (7.43) | 57.40 (7.74) | 57.46 (7.57) |
| Sleep problems | 56.23 (7.54) | 55.38 (6.25) | 55.81 (6.93) |
| Aggressivea | 58.18 (9.75) | 55.86 (7.49) | 57.03 (8.76) |
| Somatic complaints | 54.56 (6.24) | 54.33 (6.16) | 54.45 (6.19) |
| Executive Functionb | |||
| Attention/Concentration index | 96.33 (14.99) | 97.71 (17.56) | 97.02 (16.32) |
| Delayed recognition index | 95.62 (17.27) | 95.03 (18.16) | 95.32 (17.69) |
| General memory index | 95.48 (14.95) | 98.85 (19.37) | 97.18 (17.37) |
| Long delay (free) | −0.38 (1.10) | −0.30 (1.26) | −0.34 (1.18) |
| Short delay (cued) | −0.57 (1.24) | −0.46 (1.36) | −0.52 (1.30) |
| Accuracy score | 82.22 (15.60) | 83.15 (14.60) | 82.69 (15.09) |
Note. PME = prenatal methamphetamine exposure.
Difference between PME and nonexposed comparisons, p < .05.
Values represent scores before recoded to represent executive function deficits.
Early adversity
A single index score was created to represent early adversity using procedures and indicators similar to those used by Fisher et al. (2011) and Flaherty et al. (2006). Postnatal visits occurred at 1 month, 1 year, 2 years, 2.5 years, and 3 years postbirth, such that cumulative measures of adversity were available. In the current study, the early adversity index was the sum of a set of binary indicators, including (a) any self-reported maternal postnatal substance use through 3 years (i.e., tobacco, alcohol, marijuana, methamphetamine); (b) any extreme poverty experienced between birth and 3 years, as indicated by annual income less than $10,000 (representing approximately 50% of the U.S. Department of Health and Human Services poverty line for families with two to five members during the years data were collected); (c) any primary caregiver changes through 3 years; (d) any reported caregiver sexual or physical abuse through 3 years; (e) any maternal subscale score on the Brief Symptom Inventory above the clinical cut point (Derogatis, 1993) through 3 years; (f) maternal depression one standard deviation or greater above the mean from birth through 3 years as indicated by the Beck Depression Inventory (M = 9.44, SD = 7.03; Beck, Steer, & Brown, 1996); (g) quality of the living environment one standard deviation or greater below the mean at 2.5 years as indicated by the Home Inventory (only available at 2.5 years, M = 37.69, SD = 4.39; Caldwell & Bradley, 2001); (h) community violence one standard deviation or greater above the mean from birth through 3 years as indicated by the Neighborhood Problems section of the Lifestyle Interview (M = 1.70, SD = 1.84); and (i) social position one standard deviation or greater below the sample mean from birth through 3 years as indicated by the Index of Social Position, which represents a weighted average of parental occupational status and educational level (M = 31.32, SD = 8.92; see Hollingshead, 1975; LaGasse et al., 1999). Table 3 describes the proportions of participants who have experienced each indicator of early adversity by group and overall.
Table 3.
Early Adversity Index Indicator Endorsement Across Exposure Group and Overall
| PME | Nonexposed | Overall | |
|---|---|---|---|
| Frequencies (%) | |||
| Maternal postnatal substance use (n = 300) | 121 (81%) | 109 (73%) | 230 (77%) |
| Any extreme poverty (n = 309)a | 73 (45%) | 29 (18%) | 102 (32%) |
| Any primary caregiver changes (n = 307)a | 79 (52%) | 11 (7%) | 90 (29%) |
| Any reported caregiver sexual or physical abuse (n = 269) | 7 (5%) | 5 (4%) | 12 (5%) |
| Any positive maternal diagnosis of psychological distress (n = 319) | 87 (54%) | 72 (46%) | 159 (50%) |
| Any high maternal depression (n = 319) | 27 (17%) | 19 (12%) | 46 (14%) |
| Poor quality living environment (n = 265) | 24 (19%) | 21 (16%) | 45 (17%) |
| High community violence (n = 309) | 22 (14%) | 28 (18%) | 50 (16%) |
| Any low social position (n = 320)a | 30 (19%) | 9 (6%) | 39 (12%) |
| Means (SDs) | |||
| Overall early adversity indexb | 2.73 (1.44) | 1.81 (1.22) | 2.27 (1.41) |
Note. Frequencies based on available data as indicated. PME = prenatal methamphetamine exposure.
Difference between PME and nonexposed comparisons, p < .001.
Covariates
Covariates were selected based on previous research on prenatal drug exposure and later outcomes (e.g., Eiden, Veira, & Granger, 2009; Chaplin, Freiburger, Mayes, & Sinha, 2010; Zabaneh et al., 2012; see Lester & Lagasse, 2010, for a review). Child sex (0 = female, 1 = male) was included as a covariate, as was site of data collection (dummy coded; reference group = California), gestational age at birth, head circumference at birth, maternal age at birth, and maternal quantity of self-reported use of tobacco, alcohol, and marijuana while pregnant (see Table 1). These covariates represent extraneous factors that were not matched upon at enrollment and were chosen to help isolate the unique associations between PME, ND, and early adversity.
Plan of Analysis
To better understand the latent structure of ND, we first performed confirmatory factor analysis to test the fit of the ND factor structure. As mentioned previously, based on Fisher et al. (2011) and the temporal placements of the ND measures, we hypothesized the behavioral and emotional measures taken at 5 years of age would represent a factor, whereas the executive function indicators from 6.5 years of age would represent a second factor. We then tested a base model where PME predicted later behavioral and emotional problems and executive function deficits. Next, we included early adversity as a mediator of the effect of PME on the two factors representing ND. Both of these structural models were conducted with and without covariates. Covariates were entered as a group and retained in the model if they had an effect on any of the endogenous variables at the level of p ≤ .20. All analyses were performed in Mplus 6.0 (Muthén & Muthén, 1998–2010) using a full information maximum likelihood estimator robust to normality to account for any missing data. Mediation, in the current study, was defined as a statistically significant indirect effect determined by the Sobel test in Mplus (MacKinnon, Lockwood, Hoffman, West, & Sheets, 2002; Sobel, 1982). This framework does not require the initial direct effect of a predictor variable on outcomes to be significant, so long as the associations between the predictor and mediator and between mediator and outcomes are significant and of sufficient magnitude, depending on sample size. Model fit was compared using χ2, the comparative fit index CFI; (Bentler, 1990), and the root mean square error of approximation (RMSEA; Steiger & Lind, 1980).
Results
Preliminary Analyses
Mothers of PME children reported use of methamphetamine on an average of 1.68 days per week throughout pregnancy (SD = 1.74). In regard to participant demographics, PME participants were similar to the nonexposed comparison condition, with the exceptions of maternal substance use, infant gestational age at birth, and length at birth (see Table 1). PME infants were exposed to significantly more tobacco, alcohol, and marijuana in utero than comparison infants. PME infants, on average, were also born approximately three-quarters of a week earlier and, relatedly, were a little more than 1 cm shorter than comparison infants.
Individual indicators of behavioral and emotional problems and executive function deficits were also compared across exposure conditions (see Table 2). Results indicated that, at 5 years of age, PME children were significantly more emotionally reactive, anxious and/or depressed, and aggressive than comparison children. There were no significant differences by exposure condition on the individual indices of executive function.
There were also significant differences on the overall early adversity index and its constituent items (see Table 3). PME children were exposed to significantly more overall early adversity than comparison children, with the largest differences seen on chronic poverty (27% difference), changes in the primary caregiver of the child (45% difference), and low social position (13% difference).
Confirmatory Factor Analysis
Results from the confirmatory factor analysis supported our hypothesized two-factor structure, with the behavioral and emotional factor (Behavioral and Emotional Control) and cognitive factor (Executive Function Deficits) positively associated (r = .25, p < .001). The two-factor model was shown to fit the data well, χ2 (51) = 100.93, p < .001; CFI = 0.95, RMSEA = 0.06. All factor loadings were significant and positively associated with the latent factors (standardized λ ≥ 0.41, p < .001). Two residual covariances (rs ≥ 0.25; ps < 0.05) were estimated between items within the same measure in order to account for method variance.
A one-factor model was also fit including the two correlated residual variances, but this model was rejected due to poor absolute and relative fit to the data, χ2 (52) = 334.03, p < .001; CFI = 0.74, RMSEA = 0.13.
Base Model: PME Associated With Behavioral and Emotional Control and Executive Function Deficits
Given the ages at which data were collected, a model was proposed where PME was associated with later behavioral and emotional control problems, which, in turn, predicted executive function deficits. The direct effect of PME on executive function deficits was also modeled. Results indicated that this model also fit the data well, χ2 (61) = 111.13, p < .001; CFI = 0.95, RMSEA = 0.05. PME was associated with increased behavioral and emotional control problems at 5 years (β = 0.15, p < .05; R2 = .02), which was associated with greater executive function deficits at 6.5 years (β = 0.24, p < .001; overall R2 = .06). The direct effect of PME on executive function deficits was not significant (β = 0.05, p > .05).
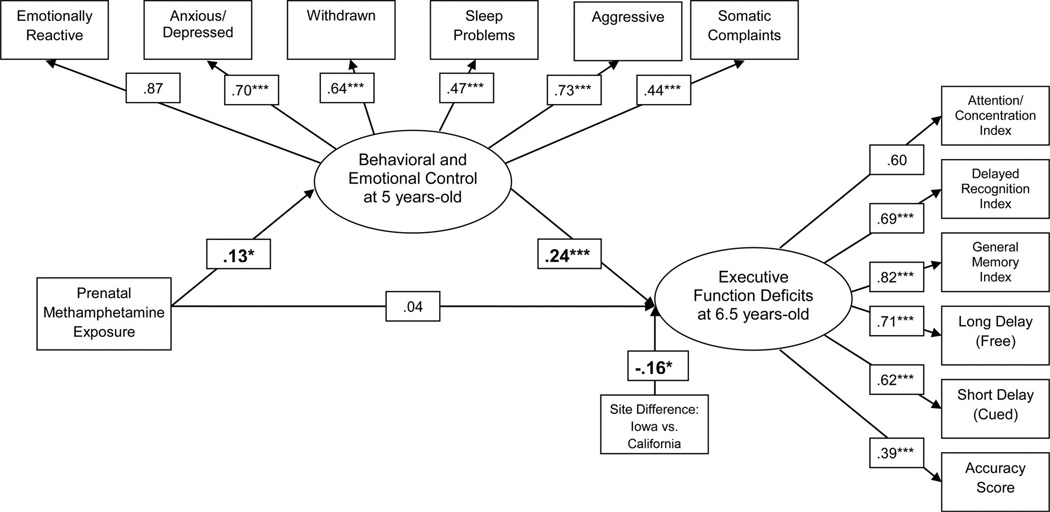
We then included the described set of covariates as additional predictors of the ND constructs. With inclusion of these covariates, the association between PME and behavioral and emotional control remained significant (see Figure 1), as did the relationship between behavioral and emotional control and later executive function deficits. Given the interaction previously observed between PME and prenatal alcohol exposure (Sowell et al., 2010), we included this interaction term, but it did not predict behavioral and emotional control or later executive function deficits (p > .05). The only significant covariate effect observed was a significant site difference between Iowa and California on executive function deficits. The overall model accounted for 6% of the variance in behavioral and emotional control and 11% of the variance in executive function deficits.
Figure 1.
Relations between behavioral and emotional control and executive function deficits with the inclusion of covariates. Beta coefficients reported while controlling for the following retained covariates: prenatal exposure to alcohol, data collection site, and maternal age. All covariate paths were modeled, but paths are only included in the figure if significant at the p < 0.05 level. The substantive paths of interest were unaffected by the elimination of noncontributing covariates. Neurobehavioral disinhibition is represented by both the Behavioral and Emotional Control and Executive Function Deficits latent factors. * p < 0.05. ** p < 0.01. *** p < 0.001.
Early Adversity Index Included as a Mediator
We then conducted a model with early adversity index as a mediator of the effects of PME on the two latent factors representing ND. This model also fit the data well, χ2 (71) = 118.84, p < .001; CFI = 0.96, RMSEA = 0.05. Results indicated that PME was associated with increased early adversity experienced by the child (β = 0.33, p < .001; R2 = .11). High levels of early adversity were associated with higher behavioral and emotional control problems at 5 years (β = 0.33, p < .01), and increased executive function deficits at 6.5 years (β = 0.24, p < .001). The effect of behavioral and emotional control on executive function deficits also remained significant (β = 0.21, p < .01). The direct effects of PME on behavioral and emotional control and executive function deficits were not significant (β = 0.09, p > .05; β = −0.03, p > .05, respectively). The mediated effects of PME on the two latent factors were statistically significant (PME indirect effectBehavioral and Emotional Control = 0.06, p < .05; PME indirect effectExecutive Function Deficits = 0.08, p < .01), such that PME is indirectly associated with greater behavioral and emotional control problems and executive function deficits. The combined effects of PME and early adversity accounted for 5% of the variance in behavioral and emotional control, and 12% of the variance in executive function deficits were accounted for by PME, early adversity, and behavioral and emotional control.
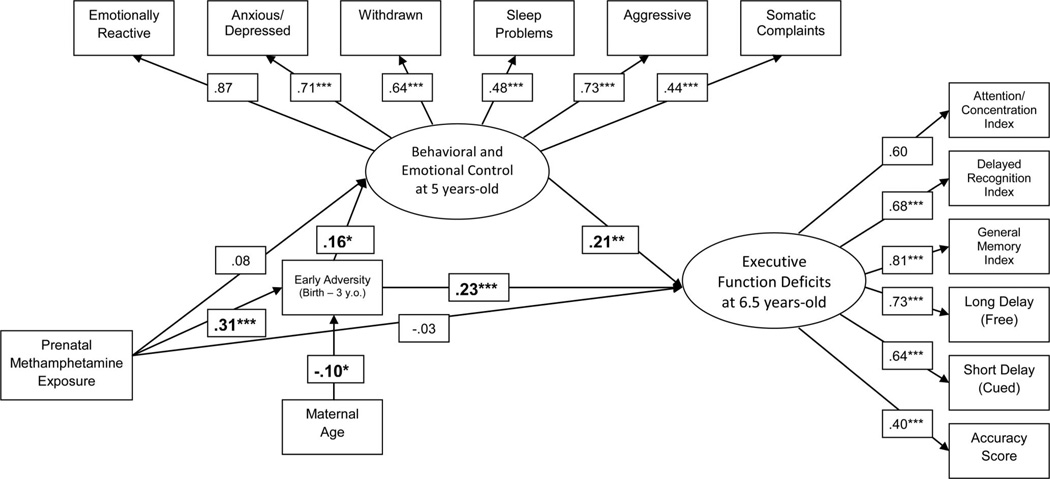
Finally, we ran the mediation model with the added set of covariates. There were no substantive changes in the model pathways of interest (see Figure 2), and the indirect effects of PME on the two latent factors representing ND through early adversity remained significant. The only significant covariate effect was a negative association between maternal age and early adversity. The overall model accounted for 14% of the variance in early adversity, 8% in behavioral and emotional control, and 15% in executive function deficits.
Figure 2.
Relations between PME, early adversity, behavioral and emotional control, and executive function deficits with the inclusion of covariates. Values presented represent standardized coefficients. Beta coefficients reported while controlling for the following retained covariates: prenatal exposure to alcohol and marijuana, data collection site, and maternal age. All covariate paths were modeled, but paths are only included in the figure if significant at the p < 0.05 level. The substantive paths of interest were unaffected by the elimination of noncontributing covariates. Indirect effects of PME on the two latent factors representing ND remained significant with the inclusion of covariates (INDBehavioral and Emotional = 0.05, p < 0.05; INDExecutive Function = 0.07, p < 0.01). * p < 0.05. ** p < 0.01. *** p < 0.001.
Discussion
Methamphetamine use in the United States is a serious public health concern (Substance Abuse and Mental Health Services Administration, 2011), and the use of this drug has been associated with a host of negative consequences (e.g., Chang et al., 2007; Ernst, Chang, Leonido-Yee, & Speck, 2000; Sommers et al., 2006). Methamphetamine use is particularly insidious for pregnant women, as consequences are experienced both by the user and the developing fetus (e.g., Piper et al., 2011; Sowell et al., 2010; Zabaneh et al., 2012). The current study sought to add to a limited body of research on PME in humans by prospectively examining the association between PME and childhood ND and the extent to which early adversity, experienced from birth through 3 years, mediated these relationships. The present study represents the earliest ages at which ND has been defined, to date, in the literature. PME was associated with later behavioral and emotional control, which was associated with later deficits in executive function. Results also indicated that the effects of PME on ND largely functioned through early adversity. These findings are particularly meaningful, given that prenatal exposure to other teratogenic substances (tobacco, alcohol, and marijuana) was controlled for, highlighting a potentially unique association between PME and later child development.
The current study expanded upon previous research through the use of large, multisite sample of PME children and matched controls. The demographic characteristics of the current sample of maternal methamphetamine users during pregnancy was quite similar to those observed by Good, Solt, Acuna, Rotmensch, and Kim (2010) in an independent medical chart review study of maternal users with respect to maternal race, educational level, socioeconomic status, and polydrug use pre- and postnatally. Similar correspondences were observed between the current sample and pregnant methamphetamine users in federal drug treatment centers (Terplan et al., 2009). These similarities speak to the strong generalizability of the current study to the population of PME children and their mothers in the United States.
The current study also added to the literature on prenatal drug exposure effects on child ND. The conceptualization of ND used here departs from the original framework presented by Tarter and colleagues (2003), in that their work employed a single factor of ND consisting of indices of executive cognitive functioning, emotion regulation, and behavioral control, whereas the current study used a two-factor model. As mentioned in the Measures section, the two-factor model may have fit better in the current study due to timing of the measurement of the various manifest indicators of the ND factors. Future work in prenatal exposure effects in childhood should seek to define ND at the same time point(s). It is possible that the original one-factor model may provide the best solution under these conditions.
Given that the IDEAL study represents the first and largest prospective examination of methamphetamine exposure from birth through childhood, there is relatively little literature to directly compare the current findings to regarding ND. Piper and colleagues (2011) found that PME children 7 to 9 years of age were rated by their parents as displaying greater executive function deficits than matched comparisons. Regarding behavioral and emotional control, Billing et al. (1994) found that 8-year-old children exposed to high levels of amphetamine displayed greater aggression than less-exposed children, which is similar to our current findings showing a relationship between PME and the behavioral and emotional control construct. It is important to note that amphetamine and methamphetamine are very similar substances chemically, with the primary differences experienced by users being that methamphetamine is stronger and acts more quickly in the body than amphetamine (Goodwin et al., 2009).
Although there is a paucity of research linking PME and ND, research on prenatal exposure to other illicit drugs of abuse does provide insight into the current findings. The literature on cocaine exposure is an appropriate analog, given both cocaine and methamphetamine impact dopaminergic and serotinergic systems in the developing brain and have vasoconstrictive effects that impact placental blood flow (see Salisbury et al., 2009, for a review). A recent review of 42 studies on prenatal cocaine exposure (Lester & Lagasse, 2010) indicated behavioral problems and executive function deficits as some of the more salient areas of development negatively impacted by prenatal exposure. For example, as mentioned, Fisher and colleagues (2011) showed prenatal exposure to cocaine and/or opiates were associated with behavioral dysregulation across late childhood and early adolescence, and early behavioral dysregulation was associated with increases in executive function difficulties over time. Finally, early adversity was shown to mediate the effects of prenatal exposure on (a) baseline levels of behavioral dysregulation and executive function difficulties, and (b) increases in behavioral dysregulation over time (Fisher et al., 2011). Lester and colleagues (2012) then expanded these findings by linking behavioral dysregulation trajectories with later substance use behaviors, thus paralleling Tarter et al.’s (2003) work in a nonexposed sample of children and adolescents. Similarly, Bada and colleagues (2011) found children prenatally exposed to cocaine displayed higher levels of externalizing behaviors at 7 years than matched comparison children, as reported both by parents and teachers. These behavioral control deficits in prenatal cocaine exposed children have been observed in several independent projects (e.g., Delaney-Black et al., 2000; Dennis, Bendersky, Ramsay, & Lewis, 2006) and closely correspond with the findings observed in the current study regarding PME and childhood ND.
In regard to cognitive effects, mild executive function deficits were also observed in children exposed to cocaine prenatally relative to comparison individuals in a neuroimaging study by Warner and colleagues (2006). Similar subtle effects of prenatal cocaine exposure were observed on attention-based tasks at 10 years of age (Savage, Brodsky, Malmud, Giannetta, & Hurt, 2005), and visuospatial memory decrements have been observed among prenatally exposed 8- to 10-year-old children (Mayes, Snyder, Langlois, & Hunter, 2007). Although similar direct effects of PME on executive function deficits were not observed in the current study, there was an indirect effect through early adversity.
The role of environmental adversity in predicting developmental outcomes among children exposed to drugs prenatally has been discussed and/or addressed in several studies (e.g., Chapman et al., 2007; Delaney-Black et al., 2000; Warner et al., 2006). The current study employed a strategy similar to Fisher et al. (2011), where early adversity was placed as a mediator between PME and neurobehavioral outcomes. In terms of temporal precedence, this strategy is intuitive, given that early adversity, as defined here and in other studies, necessarily occurs after birth (therefore, after prenatal exposure). In terms of the effects of parental addiction, it is also intuitive. Methamphetamine is one of the most addictive drugs of abuse in the United States (Barr et al., 2006; Castro, Barrington, Walton, & Rawson, 2000), used in association with a host of social (e.g., Brown & Hohman, 2006; J. B. Cohen et al., 2003), psychosocial (e.g., Darke, Kaye, McKetin, & Duflou, 2008), and socioeconomic effects (e.g., Rawson et al., 2000). As such, infants prenatally exposed to methamphetamine were likely to become children exposed to early adversity. The chaotic and unsafe environments fostered by maternal methamphetamine use, in turn, were strongly associated with behavioral, emotional, and cognitive deficits in childhood (Brown & Hohman, 2006; Wachs et al., 2004). It is important to also consider the potential that early adversity functions as a confounding factor in the relationships between PME and indices of ND. The inability to randomly assign individuals to exposure conditions allows for this explanation of the observed results. However, the unique effect of PME on early adversity experienced by children, after controlling the effects of prenatal exposure to other substances and multiple mother and child characteristics, highlights the strength of this association and a potential target for intervention.
Implications for Prevention
Results of the current study imply the need for both enhanced efforts at preventing maternal use of methamphetamine as well as enhanced efforts to support mothers and infants exposed to substances. Efforts have been made to prevent the onset and escalation of methamphetamine use among adolescents (Anderson, 2010; Erceg-Hurn, 2008; Spoth, Clair, Shin, & Redmond, 2006), with results showing family-based prevention programs to be efficacious at reducing the prevalence of lifetime methamphetamine use over 5 years postintervention (Spoth et al., 2006). Empirical support for more information-based advertising programming aimed at teenagers is much weaker (Anderson, 2010; Erceg-Hurn, 2008). Additional empirically based programs should be designed specifically for the prevention of use among women of reproductive age, as well as among older adolescents and young adults in general.
Postnatal service provision might also provide a mechanism for preventing the negative effects of PME on child development, particularly given the mediating and proximal role that early adversity seems to play in predicting later ND. Ongoing cross-cultural work (LaGasse et al., 2011) is exploring the potential protective influence of this postnatal service provision by comparing outcomes of matched PME children in the United States (where standard service provision to drug-abusing mothers is limited) and in New Zealand (where standard services are more extensive and mitigate early adversity). In the United States, programs that mirror services provided elsewhere, like the Nurse-Family Partnership (e.g., Olds, 2006), might be successful at mitigating the effects of PME by improving the home environment and early parenting practices.
Limitations
There are several limitations to this study that should be addressed. First, whereas exposure groups in the IDEAL study were matched on a set of relevant demographic characteristics, the current study was correlational. As such, causality could not be attributed in the model. Additional quasiexperimental research employing propensity score matching (Peikes, Moreno, & Orzol, 2008) is required to better describe the directionality of observed effects. Second, the measurement of the executive function latent variable did not completely cover the range of the construct, lacking a focus on goal setting/progressive planning. Future studies examining PME should incorporate tasks that tap these facets of executive function, such as the California Tower Test or the Cognitive Estimation Test used in previous work on prenatal exposure to alcohol (e.g., Kopera-Frye, Dehaene, & Streissguth, 1996; Mattson, Goodman, Caine, Delis, & Riley, 1999). Third, maternal self-report was relied upon for several of the primary and secondary measures (e.g., prenatal substance use, child behavioral and emotional control, maternal depression). Future research examining PME should seek to incorporate reports from other parties as well as additional objective measures. Regarding behavioral and emotional control, however, it is important to note that very similar exposure effects have been observed when using either parental or teacher reports in the prenatal cocaine literature (Bada et al., 2011). Finally, the current study presents a model where the association between PME and childhood ND is mediated by early adversity, but it is also possible that early adversity is actually a more distal predictor and contemporaneous adversity is more closely associated with ND. Additional work is required to define the specific contributions of early and later adversity on outcomes, as well as the nature of the relationship between PME and later adversity.
Conclusions
Methamphetamine is currently the most frequently cited primary drug problem among pregnant women who have been admitted to substance use treatment in the United States (Terplan et al., 2009). Although animal research has shown a host of effects of maternal prenatal use on offspring (e.g., Chen et al., 2010; Cui et al., 2006; Bubenikova-Valesova et al., 2009), limited human work has examined the potential relationship between maternal use of methamphetamine during pregnancy and later child neurobehavioral disinhibition. The current study highlights the potential importance of PME on child development, as well as a potential mechanism by which effects are observed.
Acknowledgments
This research was supported by a grant through the National Institute on Drug Abuse (RO1 DA14948; PI Lester).
Contributor Information
Beau Abar, Center for Study of Children at Risk, Brown University Warren Alpert Medical School & Women and Infants Hospital of Rhode Island, Providence, Rhode Island.
Linda L. LaGasse, Center for the Study of Children at Risk, Brown University Warren Alpert Medical School & Women and Infants Hospital of Rhode Island, Providence, Rhode Island
Elana Newman, Department of Psychology, The University of Tulsa.
Lynne M Smith, LABioMed Institute at Harbor-UCLA Medical Center and David Geffen School of Medicine at UCLA, Torrance, California.
Marilyn Huestis, National Institute on Drug Abuse, National Institutes of Health, Bethesda, Maryland.
Charles Neal, John A. Burns School of Medicine, University of Hawaii.
Chris DeRauf, John A. Burns School of Medicine, University of Hawaii.
Rizwan Shah, Blank Hospital Regional Child Protection Center – Iowa Health, Des Moines, Iowa.
Amelia Arria, Center for Substance Abuse Research (CESAR), University of Maryland, College Park, Maryland.
Sheri Della Grotta, Center for the Study of Children at Risk, Brown University Warren Alpert Medical School & Women and Infants Hospital of Rhode Island, Providence, Rhode Island.
Lynne M. Dansereau, Center for the Study of Children at Risk, Brown University Warren Alpert Medical School & Women and Infants Hospital of Rhode Island, Providence, Rhode Island
Barry M. Lester, Center for the Study of Children at Risk, Brown University Warren Alpert Medical School & Women and Infants Hospital of Rhode Island, Providence, Rhode Island
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA Preschool Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2000. [Google Scholar]
- Anderson DM. Does information matter? The effect of the Meth Project on meth use among adults. Journal of Health Economics. 2010;29:732–742. doi: 10.1016/j.jhealeco.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arria AM, Derauf C, Lagasse LL, Grant P, Shah R, Smith L, Lester B. Methamphetamine and other substance use during pregnancy: Preliminary estimates from the Infant Development, Environment, and Lifestyle (IDEAL) study. Maternal and Child Health Journal. 2006;10:293–302. doi: 10.1007/s10995-005-0052-0. [DOI] [PubMed] [Google Scholar]
- Bada HS, Bann CM, Bauer CR, Shankaran S, Lester B, LaGasse L, Higgins R. Preadolescent behavior problems after prenatal cocaine exposure: Relationship between teacher and caretaker ratings (Maternal Lifestyle Study) Neurotoxicology and Teratology. 2011;33:78–87. doi: 10.1016/j.ntt.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr AM, Panenka WJ, MacEwan GW, Thornton AE, Lang DJ, Honer WG, Lecomte T. The need for speed: An update on methamphetamine addiction. Journal of Psychiatry & Neuroscience. 2006;31:301–313. [PMC free article] [PubMed] [Google Scholar]
- Beck A, Steer R, Brown G. Beck Depression Inventory–II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Bendersky M, Bennett D, Lewis M. Aggression at age 5 as a function of prenatal exposure to cocaine, gender, and environmental risk. Journal of Pediatric Psychology. 2006;31:71–84. doi: 10.1093/jpepsy/jsj025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentler PM. Comparative fit indices in structural models. Psychological Bulletin. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Berman S, O’Neill J, Fears S, Bartzokis G, London ED. Abuse of amphetamines and structural abnormalities in the brain. Annals of the New York Academy of Sciences. 2008;1141:195–220. doi: 10.1196/annals.1441.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billing L, Eriksson M, Jonsson B, Steneroth G, Zetterström R. The influence of environmental factors on behavioural problems in 8-year-old children exposed to amphetamine during fetal life. Child Abuse & Neglect. 1994;18:3–9. doi: 10.1016/0145-2134(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Billing L, Eriksson M, Larsson G, Zetterström R. Amphetamine addiction and pregnancy. III. One year follow-up of the children. Psychosocial and pediatric aspects. Acta Paediatrica Scandinavica. 1980;69:675–680. doi: 10.1111/j.1651-2227.1980.tb07342.x. [DOI] [PubMed] [Google Scholar]
- Billing L, Eriksson M, Steneroth G, Zetterström R. Preschool children of amphetamine-addicted mothers. I. Somatic and psychomotor development. Acta Paediatrica Scandanavica. 1985;74:179–184. doi: 10.1111/j.1651-2227.1985.tb10946.x. [DOI] [PubMed] [Google Scholar]
- Billing L, Eriksson M, Steneroth G, Zetterström R. Predictive indicators for adjustment in 4-year-old children whose mothers used amphetamine during pregnancy. Child Abuse & Neglect. 1988;12:503–507. doi: 10.1016/0145-2134(88)90067-1. [DOI] [PubMed] [Google Scholar]
- Block RI, Erwin WJ, Ghoneim MM. Chronic drug use and cognitive impairment. Pharmacology, Biochemistry, and Behavior Journal. 2002;73:491–504. doi: 10.1016/s0091-3057(02)00816-x. [DOI] [PubMed] [Google Scholar]
- Brown JA, Hohman M. The impact of methamphetamine use on parenting. Journal of Social Work Practice in the Addictions. 2006;6:63–88. [Google Scholar]
- Bubenikova-Valesova V, Kacer P, Syslova K, Rambousek L, Janovsky M, Schutova B, Slamberova R. Prenatal methamphetamine exposure affects the mesolimbic dopaminergic system and behavior in adult offspring. International Journal of Developmental Neuroscience. 2009;27:525–530. doi: 10.1016/j.ijdevneu.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Caldwell B, Bradley R. Home inventory administration manual. 3rd ed. Little Rock, AR: University of Arkansas at Little Rock; 2001. [Google Scholar]
- Castro FG, Barrington EH, Walton MA, Rawson RA. Cocaine and methamphetamine: Differential addiction rates. Psychology of Addictive Behaviors. 2000;14:390–396. [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102:16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- Chang L, Cloak C, Jiang CS, Farnham S, Tokeshi B, Buchtal S, Ernst T. Altered neurometabolites and motor integration in children exposed to methamphetamine in utero. NeuroImage. 2009;48:391–397. doi: 10.1016/j.neuroimage.2009.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Smith LM, LoPresti C, Yonekura ML, Kuo J, Walot I, Ernst T. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Research. 2004;132:95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Chaplin TM, Freiburger MB, Mayes LC, Sinha R. Prenatal cocaine exposure, gender, and adolescent stress response: A prospective longitudinal study. Neurotoxicology and Teratology. 2010;32:595–604. doi: 10.1016/j.ntt.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman K, Tarter RE, Kirisci L, Cornelius MD. Childhood neurobehavior disinhibition amplifies the risk of substance use disorder: Interaction of parental history and prenatal alcohol exposure. Journal of Developmental and Behavioral Pediatrics. 2007;28:219–224. doi: 10.1097/DBP.0b013e3180327907. [DOI] [PubMed] [Google Scholar]
- Chen JY, Yeh G, Tao P, Kuo C, Chen K, Wen Y. Prenatal exposure to methamphetamine alters the mechanical withdrawal threshold and tonic hyperalgesia in the offspring. Neurotoxicology. 2010;31:432–438. doi: 10.1016/j.neuro.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Cohen JB, Dickow A, Horner K, Zweben JE, Balabis J, Vander-sloot D, Reiber C. Abuse and violence history of men and women in treatment for methamphetamine dependence. American Journal on Addictions. 2003;12:377–385. [PubMed] [Google Scholar]
- Cohen M. Children’s Memory Scale (CMS) San Antonio, TX: Harcourt Assessments; 1997. [Google Scholar]
- Colliver JD, Kroutil LA, Dai L, Gfroerer JC. Misuse of prescription drugs: Data from the 2002, 2003, and 2004 surveys on drug use and health. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2006. [Google Scholar]
- Connell AM, Goodman SH. The association between psychopathology in fathers versus mothers and children’s internalizing and externalizing behavior problems: A meta-analysis. Psychological Bulletin. 2002;128:746–773. doi: 10.1037/0033-2909.128.5.746. [DOI] [PubMed] [Google Scholar]
- Cui C, Sakata-Haga H, Ohta K, Nishida M, Yashiki M, Sawada K, Fukui Y. Histological brain alterations following prenatal methamphetamine exposure in rats. Congenital Anomalies. 2006;46:180–187. doi: 10.1111/j.1741-4520.2006.00126.x. [DOI] [PubMed] [Google Scholar]
- Darke S, Kaye S, McKetin R, Duflou J. Major physical and psychological harms of methamphetamine use. Drug and Alcohol Review. 2008;27:253–262. doi: 10.1080/09595230801923702. [DOI] [PubMed] [Google Scholar]
- Delaney-Black V, Covington C, Templin T, Ager J, Nordstron-Klee B, Martier S, Sokol RJ. Teacher-assessed behavior of children prenatally exposed to cocaine. Pediatrics. 2000;106:782–791. doi: 10.1542/peds.106.4.782. [DOI] [PubMed] [Google Scholar]
- Dells D, Kramer J, Kaplan E, Ober B. California Verbal Learning Test-C (CVLT-C) San Antonio, TX: Harcourt Assessments; 1994. [Google Scholar]
- Dennis T, Bendersky M, Ramsay D, Lewis M. Reactivity and regulation in children prenatally exposed to cocaine. Developmental Psychology. 2006;42:688–697. doi: 10.1037/0012-1649.42.4.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derauf C, Lagasse LL, Smith LM, Newman E, Shah R, Neal CR, Lester BM. Prenatal methamphetamine exposure and inhibitory control among young school-age children. Journal of Pediatrics. 2012;161:452–459. doi: 10.1016/j.jpeds.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis L. BSI Brief Symptom Inventory: Administration, scoring, and procedure manual. 4th ed. Minneapolis, MN: National Computer Systems; 1993. [Google Scholar]
- Eiden RD. Exposure to violence and behavior problems during early childhood. Journal of Interpersonal Violence. 1999;14:1299–1313. [Google Scholar]
- Eiden RD, Foote A, Schuetze P. Maternal cocaine use and caregiving status: Group differences in caregiver and infant risk variables. Addictive Behaviors. 2007;32:465–476. doi: 10.1016/j.addbeh.2006.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden RD, Veira Y, Granger DA. Prenatal cocaine exposure and infant cortisol reactivity. Child Development. 2009;80:528–543. doi: 10.1111/j.1467-8624.2009.01277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erceg-Hurn DM. Drugs, money, and graphic ads: A critical review of the Montana Meth Project. Prevention Science. 2008;9:256–263. doi: 10.1007/s11121-008-0098-5. [DOI] [PubMed] [Google Scholar]
- Eriksson M, Zetterström R. Amphetamine addiction during pregnancy: 10-year follow-up. Acta paediatrica (Oslo, Norway: 1992). Supplement. 1994;404:27–31. doi: 10.1111/j.1651-2227.1994.tb13380.x. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L, Leonido-Yee M, Speck O. Evidence for long-term neurotoxicity associated with methamphetamine abuse: A 1H MRS study. Neurology. 2000;54:1344–1349. doi: 10.1212/wnl.54.6.1344. [DOI] [PubMed] [Google Scholar]
- Evans GW, Maxwell LE, Hart B. Parental language and verbal responsiveness to children in crowed homes. Developmental Psychology. 1999;35:1020–1023. doi: 10.1037//0012-1649.35.4.1020. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Lester BM, DeGarmo DS, Lagasse LL, Lin H, Shankaran S, Higgins R. The combined effects of prenatal drug exposure and early adversity on neurobehavioral disinhibition in childhood and adolescence. Development and Psychopathology. 2011;23:777–788. doi: 10.1017/S0954579411000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty EG, Thompson R, Litrownik AJ, Theodore A, English DJ, Black MM, Dubowitz H. Effect of early childhood adversity on child health. Archives of Pediatric and Adolescent Medicine. 2006;160:1232–1238. doi: 10.1001/archpedi.160.12.1232. [DOI] [PubMed] [Google Scholar]
- Good MM, Solt I, Acuna JG, Rotmensch S, Kim MJ. Methamphetamine use during pregnancy: Maternal and neonatal implications. Obstetrics & Gynecology. 2010;116:330–334. doi: 10.1097/AOG.0b013e3181e67094. [DOI] [PubMed] [Google Scholar]
- Goodwin JS, Larson GA, Swant J, Sen N, Javitch NR, De FeliceL J, Khoshbouei H. Amphetamine and methamphetamine differentially affect dopamine transporters in vitro and in vivo. Journal of Biological Chemistry. 2009;284:2978–2989. doi: 10.1074/jbc.M805298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead A. Four Factor Index of Social Status. New Haven, CT: Department of Sociology, Yale University; 1975. [Google Scholar]
- Kopera-Frye K, Dehaene S, Streissguth AP. Impairments of number processing induced by prenatal alcohol exposure. Neuropsychologia. 1996;34:1187–1196. doi: 10.1016/0028-3932(96)00043-7. [DOI] [PubMed] [Google Scholar]
- LaGasse LL, Seifer R, Wright LL, Lester BM, Tronick EZ, Bauer CR, Smeriglio V. The Maternal Lifestyle Study: The caretaking environment of infants exposed to cocaine/opiates. Pediatric Research. 1999;45:247A. [Google Scholar]
- LaGasse LL, Wouldes T, Newman E, Smith LM, Shah RZ, Derauf C, Lester BM. Prenatal methamphetamine exposure and neonatal neurobehavioral outcome in the USA and New Zealand. Neurotoxicology and Teratology. 2011;33:166–175. doi: 10.1016/j.ntt.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester BM, Lagasse LL. Children of addicted women. Journal of Addictive Diseases. 2010;29:259–276. doi: 10.1080/10550881003684921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester BM, Lin H, DeGarmo DS, Fisher PA, LaGasse LL, Levine TP, Higgins RD. Neurobehavioral disinhibition predicts initiation of substance use in children with prenatal cocaine exposure. Drug and Alcohol Dependence. 2012 doi: 10.1016/j.drugalcdep.2012.04.014. Epub ahead of print retrieved from http://dx.doi.org/10.1016/j.drugalcdep.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LH, Johnson A, O’Hare ED, Bookheimer SY, Smith LM, O’Connor MJ, Sowell ER. Effects of prenatal methamphetamine exposure on verbal member revealed with fMRI. Journal of Developmental and Behavioral Pediatrics. 2009;30:185–192. doi: 10.1097/DBP.0b013e3181a7ee6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test the significance of the mediated effect. Psychological Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Goodman AM, Caine C, Delis DC, Riley EP. Executive functioning in children with heavy prenatal alcohol exposure. Alcoholism: Clinical and Experimental Research. 1999;23:1808–1815. [PubMed] [Google Scholar]
- Mayes L, Snyder PJ, Langlois E, Hunter N. Visuospatial working memory in school-aged children exposed in utero to cocaine. Child Neuropsychology. 2007;13:205–218. doi: 10.1080/09297040600888753. [DOI] [PubMed] [Google Scholar]
- McNamee RL, Dunfee KL, Luna B, Clark DB, Eddy WF, Tarter RE. Brain activation, response, inhibition, and increased risk for substance use disorder. Alcoholism: Clinical and Experimental Research. 2008;32:405–413. doi: 10.1111/j.1530-0277.2007.00604.x. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 6th ed. Los Angeles, CA: Author; 1998–2010. [Google Scholar]
- Newton TF, De La Garza R, Kalechstein AD, Nestor L. Cocaine and methamphetamine produce different patterns of subjective and cardiovascular effects. Pharmacology, Biochemistry and Behavior. 2005;82:90–97. doi: 10.1016/j.pbb.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Olds DL. The nurse-family partnership: An evidence-based preventive intervention. Infant Mental Health Journal. 2006;27:5–25. doi: 10.1002/imhj.20077. [DOI] [PubMed] [Google Scholar]
- Peikes DN, Moreno L, Orzol SM. Propensity score matching. The American Statistician. 2008;62:222–231. [Google Scholar]
- Piper BJ, Acevedo SF, Kolchugina GK, Butler RW, Corbett SM, Honeycutt EB, Raber J. Abnormalities in parentally rated executive function in methamphetamine/polysubstance exposed children. Pharmacology, Biochemistry and Behavior. 2011;98:432–439. doi: 10.1016/j.pbb.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson R, Huber A, Brethen P, Obert J, Gulati V, Shoptaw S, Ling W. Methamphetamine and cocaine users: Differences in characteristics and treatment retention. Journal of Psychoactive Drugs. 2000;32:233–238. doi: 10.1080/02791072.2000.10400234. [DOI] [PubMed] [Google Scholar]
- Rueda MR, Fan J, McCandliss BD, Halparin JD, Gruber DB, Lercari LP, Posner MI. Development of attentional networks in childhood. Neuropsychologia. 2004;42:1029–1040. doi: 10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Salisbury AL, Ponder KL, Padbury JF, Lester BM. Fetal effects of psychoactive drugs. Clinical Perinatology. 2009;36:595–619. doi: 10.1016/j.clp.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage J, Brodsky N, Malmud E, Giannetta JM, Hurt H. Attentional functioning and impulse control in cocaine-exposed and control children at age ten years. Journal of Developmental and Behavioral Pediatrics. 2005;26:42–47. [PubMed] [Google Scholar]
- Smith DK, Johnson AB, Pears KC, Fisher PA, DeGarmo DS. Child maltreatment and foster care: Unpacking the effects of prenatal and postnatal parental substance use. Child Maltreatment. 2007;12:150–160. doi: 10.1177/1077559507300129. [DOI] [PubMed] [Google Scholar]
- Smith LM, LaGasse LL, Derouf C, Grant P, Shah R, Arria A, Lester BM. Prenatal methamphetamine use and neonatal neurobehavioral outcome. Neurotoxicology and Teratology. 2008;30:20–28. doi: 10.1016/j.ntt.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LM, LaGasse LL, Derouf C, Newman E, Shah R, Haning W, Lester BM. Motor and cognitive outcomes through three years of age in children exposed to prenatal methamphetamine. Neurotoxicology and Teratology. 2011;33:176–184. doi: 10.1016/j.ntt.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. In: Leinhardt S, editor. Sociological Methodology. Washington, DC: American Sociological Association; 1982. pp. 290–312. [Google Scholar]
- Sommers I, Baskin A, Baskin-Sommers A. Methamphetamine use among young adults: Health and social consequences. Addictive Behaviors. 2006;31:1469–1476. doi: 10.1016/j.addbeh.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Leow AD, Bookheimer SY, Smith LM, O’Connor MJ, Kan E, Thompson PM. Differentiating prenatal exposure to methamphetamine and alcohol versus alcohol and not methamphetamine using tensor-based brain morphometry and discriminant analysis. The Journal of Neuroscience. 2010;30:3876–3885. doi: 10.1523/JNEUROSCI.4967-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoth RL, Clair S, Shin C, Redmond C. Long-term effects of universal preventive intervention on methamphetamine use among adolescents. Archives of Pediatric and Adolescent Medicine. 2006;160:876–882. doi: 10.1001/archpedi.160.9.876. [DOI] [PubMed] [Google Scholar]
- Steiger JH, Lind J. Statistically based tests for the number of common factors. Iowa City, IA: Paper presented at the Annual Spring Meeting of the Psychometric Society; 1980. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: Summary of national findings. Rockville, MD: Author; 2011. HHS Publication No. (SMA) 11–4658. [Google Scholar]
- Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, Clark D. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. The American Journal of Psychiatry. 2003;160:1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- Terplan M, Smith EJ, Kozloski MJ, Pollack HA. Methamphetamine use among pregnant women. Obstetrics & Gynecology. 2009;113:1285–1291. doi: 10.1097/AOG.0b013e3181a5ec6f. [DOI] [PubMed] [Google Scholar]
- Tronick EZ, Beeghly M. Prenatal cocaine exposure, child development, and the compromising effects of cumulative risk. Clinics in Perinatology. 1999;26:151–171. [PubMed] [Google Scholar]
- Wachs TD, Gurkas P, Kontos S. Predictors of preschool children’s compliance behavior in early childhood classroom settings. Journal of Applied Developmental Psychology. 2004;25:439–457. [Google Scholar]
- Warner TD, Behnke M, Eyler FD, Padgett K, Loenard C, Hou W, Blackband SJ. Diffusion tensor imaging of frontal white matter and executive functioning in cocaine-exposed children. Pediatrics. 2006;118:2014–2024. doi: 10.1542/peds.2006-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook TR, Harden BJ. Pathways among exposure to violence, maternal depression, family structure, and child outcomes through parenting: A multigroup analysis. American Journal of Orthopsychiatry. 2010;80:386–400. doi: 10.1111/j.1939-0025.2010.01042.x. [DOI] [PubMed] [Google Scholar]
- Wouldes T, LaGasse L, Sheridan J, Lester B. Maternal methamphetamine use during pregnancy and child outcome: What do we know? The New Zealand Medical Journal. 2004;117:U1180. [PubMed] [Google Scholar]
- Zabaneh R, Smith LM, LaGasse LL, Derauf C, Newman E, Shah R, Lester BM. The effects of prenatal methamphetamine exposure on childhood growth patterns from birth to 3 years of age. American Journal of Perinatology. 2012;29:203–210. doi: 10.1055/s-0031-1285094. [DOI] [PMC free article] [PubMed] [Google Scholar]