Abstract
The proapoptotic proteins BAX and BAK constitute the mitochondrial apoptotic gateway that executes cellular demise after integrating death signals. The lethal BAK is kept in check by voltage-dependent anion channel 2 (VDAC2), a mammalian-restricted VDAC isoform. Here, we provide evidence showing a critical role for the VADC2-BAK complex in determining thymocyte survival in vivo. Genetic depletion of Vdac2 in the thymus resulted in excessive cell death and hypersensitivity to diverse death stimuli including engagement of the T cell receptor. These phenotypes were completely rescued by the concurrent deletion of Bak but not that of Bax. Thus, the VDAC2-BAK axis provides a mechanism that governs the homeostasis of thymocytes. Our study reveals a sophisticated built-in rheostat that likely fine-tunes immune competence to balance autoimmunity and immunodeficiency.
INTRODUCTION
Proper execution of cell death ensures normal biological processes, and its deregulation causes human illnesses ranging from cancers to neurodegenerative disorders (1, 2). Members of the BCL-2 family of proteins control a critical checkpoint at the mitochondria by either preventing or promoting apoptosis (1, 3–6). The BCL-2 family is divided into three subfamilies on the basis of their regulatory activity as well as of homology shared within the four conserved BCL-2 homology domains (BH1 to BH4). The antiapoptotic members include BCL-2, BCL-XL, and MCL-1. The proapoptotic proteins are further divided into the multidomain members BAX and BAK in addition to a diverse group of “BH3-only” members. The BH3-only molecules (BH3s) promote apoptosis either by activating BAX or BAKor by inactivating BCL-2, BCL-XL, or MCL-1 (7–14). Upon apoptosis, the “activator” BH3s, including truncated BID (tBID), BIM, and PUMA, trigger homo-oligomerization of BAX and BAK to mediate efflux of cytochrome c from the mitochondria, which leads to the activation of caspases (8, 10, 14). Conversely, the antiapoptotic BCL-2, BCL-XL, and MCL-1 proteins sequester activator BH3s into inert complexes, thus preventing the activation of BAX or BAK (10, 14). The remaining BH3-only proteins, including BAD, NOXA, BMF, HRK, and BIK-BLK, do not activate BAX or BAK directly, but instead prevent the anti-apoptotic BCL-2 members from sequestering the activator BH3s (11–14).
Although the multidomain, proapoptotic molecules BAK and BAX are the essential effectors of mitochondrial apoptosis, their activities are kept tightly in check (3, 9, 10, 15). BAX exists in the cytosol as a monomer with its C-terminal α9 helix occupying the dimerization pocket (16). This auto-inhibited BAX monomer may be further stabilized by other associated proteins (5). In contrast, the C-terminal α9 helix of BAK is constitutively inserted into the mitochondrial outer membrane, and BAK is converted from the monomeric form to homo-oligomers upon its activation by activator BH3s (8). Through in situ cross-linking of mitochondrial proteins in conjunction with serial protein chromatography, we previously identified a mammalian-specific voltage-dependent anion channel (VDAC) isoform, VDAC2, that interacts specifically with the inactive conformer of BAK (17). In viable cells, VDAC2 occupies the dimerization pocket of BAK to keep BAK in the monomeric, inactive conformation. In response to death signals, the activator BH3-only molecules tBID, BIM, and PUMA disrupt the interaction between BAK and VDAC2, enabling homo-oligomerization of BAK, which results in apoptosis (14, 17). Although antiapoptotic BCL-2 proteins are also proposed to keep BAK in the inactive form, they appear to bind more tightly to the active conformation of BAK than to the inactive conformation, and these types of interactions have only been shown in solution rather than in mitochondrial outer membranes (18, 19).
VDAC proteins constitute the major gateway for the transport of metabolites across the mitochondrial outer membrane, yet the existence of three mammalian isoforms (VDAC1, VDAC2, and VDAC3) suggests that they may each have distinct physiological roles (20–22). Indeed, cells deficient in Vdac2 are more susceptible to death stimuli than are cells deficient in Vdac1 or Vdac3, (17, 23). Conversely, overexpression of VDAC2 selectively prevents the activation of BAK and the induction of cell death, which is consistent with the observation that VDAC2 interacts with BAK but not with BAX (17). The unique prosurvival function of VDAC2 was further supported by the embryonic lethality of Vdac2 knockout (KO), but not Vdac1 or Vdac3 KO mice (17, 24, 25). Of note, both VDAC2 and VDAC1 are found in all of the mouse tissues that we have examined (fig. S1).
Here, we report that conditional genetic inactivation of Vdac2 in the mouse thymus resulted in an ~50% reduction in the number of thymocytes compared to that in the thymus of wild-type (WT) mice, which was associated with enhanced apoptosis. Vdac2-deficient thymocytes were more sensitive to diverse apoptotic stimuli, including cytokine deprivation, DNA damage, glucocorticoids, and calcium ion flux, than were WT thymocytes. Moreover, Vdac2-deficient thymocytes were extremely sensitive to apoptosis stimulated by engagement of the T cell receptor (TCR)–CD3 complex both ex vivo and in vivo, suggesting a critical role for VDAC2 in the negative selection of thymocytes. Mechanistically, cells deficient in Vdac2 had a lower threshold for apoptosis because BAK in these cells was prone to undergo homo-oligomerization. Consequently, Vdac2-deficient cells exhibited earlier translocation of cytochrome c and activation of caspases in response to death signals than did WT cells. Deletion of Bak not only restored the number of thymocytes but also abrogated the enhanced apoptotic response associated with the deficiency in Vdac2, confirming that the increased apoptosis of Vdac2-deficient thymocytes indeed resulted from the enhanced activation of BAK. In stark contrast, deletion of Bax failed to rescue Vdac2-deficient T cells. Our data thus providegenetic evidence specifying the unique role of VDAC2 in controlling the activation of BAK but not of BAX.
RESULTS
Conditional deletion of Vdac2 results in loss of thymocytes, which is associated with enhanced apoptosis
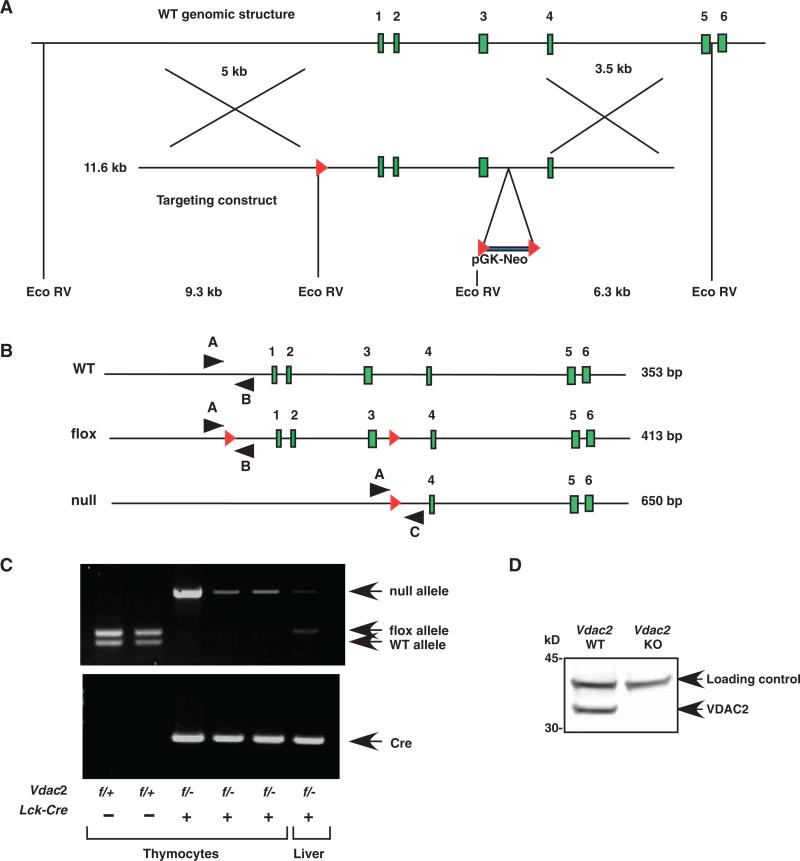
To investigate the functional link between VDAC2 and BAK in vivo, we generated a conditional Vdac2 allele by inserting loxP sites upstream of the promoter and in the intron between exons 3 and 4 (Fig. 1). The Vdac2 flox allele was transmitted through the germ line, and matings of Vdac2f/+ mice yielded viable Vdac2f/f offspring at the expected Mendelian frequency. The abundance of VDAC2 protein in both Vdac2f/+ and Vdac2f/f cells was comparable to that of WT cells (fig. S2). We also generated a Vdac2 null allele by deleting the promoter and exons 1 to 3 (Fig. 1). To restrict the deletion of the Vdac2 flox allele to T cell development, we introduced the Cre transgene under the control of the proximal promoter of the gene encoding the lymphocyte-specific protein tyrosine kinase (Lck) (Lck-Cre) to Vdac2+/– mice (26). Vdac2f/f mice were crossed with Vdac2+/–Lck-Cre+ mice to generate Vdac2f/+Lck-Cre– (Vdac2 “WT”), Vdac2f/–Lck-Cre– (Vdac2 heterozygous), Vdac2f/+Lck-Cre+ (Vdac2 heterozygous), or Vdac2f/–Lck-Cre+ (Vdac2 null) littermates.
Fig. 1.
Strategy for conditional knockout of Vdac2. (A) Schematics of the murine Vdac2 locus (top) and the targeting construct (bottom). The targeted allele was first derived in RW4 ES cells by homologous recombination, followed by transient expression of Cre recombinase to generate the flox or null alleles. Red triangles, LoxP sites. (B) Schematics of Vdac2 alleles. Red triangles, LoxP sites; black arrowheads, PCR primers. Primers A and C amplify only the null allele. (C) Genomic DNA from thymocytes or liver was analyzed by PCR to determine the representation of Vdac2 alleles as well as that of Lck-Cre. (D) Analysis of a Western blot of thymocyte lysates from mice of the indicated genotypes with an antibody against VDAC2. A cross-reactive protein served as a loading control.
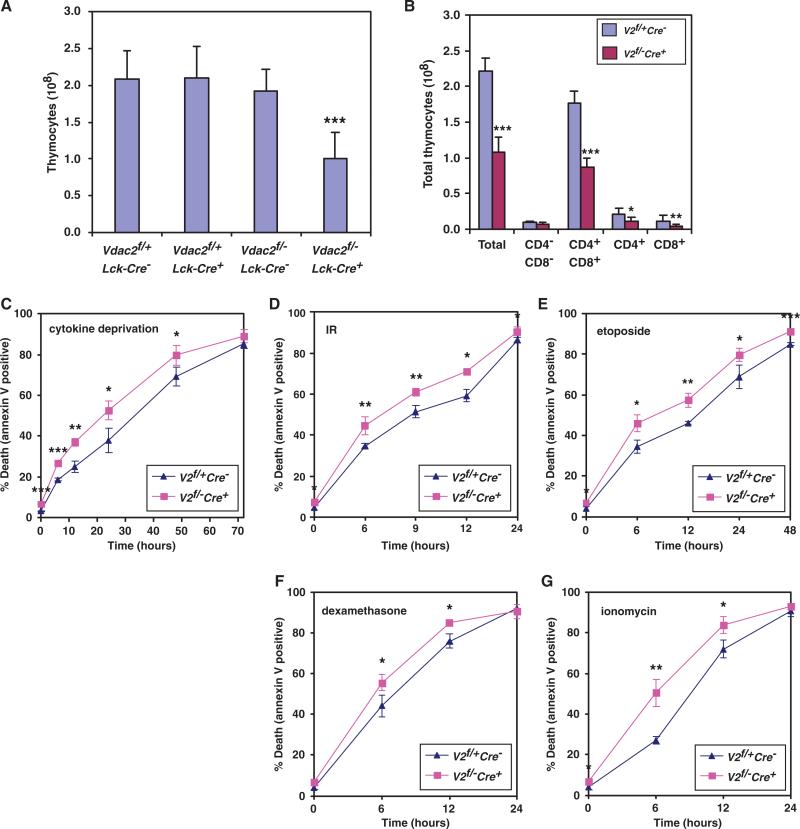
Lck-Cre–mediated deletion of Vdac2 resulted in a twofold reduction in the number of thymocytes compared to that of Vdac2f/+Lck-Cre– mice, whereas haploinsufficiency of Vdac2 did not affect thymocyte numbers (Fig. 2A). Of note, Vdac2f/+Lck-Cre– and Vdac2+/+Lck-Cre+ animals had comparable numbers of thymocytes (fig. S3). Analysis of Western blots confirmed the lack of VDAC2 protein in Vdac2 conditional KO thymocytes, which was not accompanied by compensatory alterations in the abundance of VDAC1 or VDAC3 (Fig. 1D and fig. S4). Although the number of Vdac2 null thymocytes was reduced, the developmental profile within the thymus appeared to be normal (Fig. 2B). The loss of CD4+ or CD8+ cells (single positive, SP) or of CD4+CD8+ (double positive, DP) cells was comparable (Fig. 2B) and was caused by increased apoptosis. Vdac2-deficient thymocytes were more sensitive to diverse stimuli of apoptosis including cytokine withdrawal, DNA damage, glucocorticoids, and calcium ion flux (Fig. 2, C to G, and fig. S5). The enhanced apoptosis associated with Vdac2 deficiency was not likely caused by defects in metabolite homeostasis, coupled respiration, or both, because VDAC1 and VDAC3 are found in thymocytes (fig. S4).
Fig. 2.
Conditional deletion of Vdac2 results in loss of thymocytes, which is associated with enhanced apoptosis. (A) Numbers of total thymocytes (mean ± SD) from sex-matched littermate mice of the indicated genotypes at 6 to 8 weeks of age. n = 19 for Vdac2f/+Lck-Cre– mice, n = 5 for Vdac2f/+Lck-Cre+ mice, n = 5 for Vdac2f/–Lck-Cre– mice, and n = 23 for Vdac2f/–Lck-Cre+ mice). (B) Numbers of total thymocytes, DN, CD4 SP, CD8 SP, and DP T cells from the thymi of Vdac2 WT (Vdac2f/+Lck-Cre–, n = 7) or Vdac2 KO (Vdac2f/–Lck-Cre+, n = 11) sex-matched littermate miceat 6 to 8 weeks of age. Data presented are the mean thymocyte number ± SD. (C to G) Thymocytes from the mice indicated in (B) were cultured under the following conditions: in the absence of cytokine (C), for the indicated time after exposure to 2.5 Gy of γ-radiation (D), in the presence of etoposide (E), in the presence of dexamethasone (F), or in the presence of ionomycin (G), and in each case, cell death was quantified by annexin V staining at the indicated times. Data are the mean percentage of annexin V–positive cells ± SD from three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
Vdac2 null thymocytes are more sensitive to apoptosis because of enhanced activation of BAK
We next investigated why Vdac2-deficient cells died faster than WT cells after exposure to death stimuli. Because our previous studies suggested that VDAC2 acts as a gatekeeper of the activation of BAK, we examined whether BAK was prone to be activated to undergo homo-oligomerization in the absence of VDAC2. Gel filtration was performed to assess homo-oligomerization of BAK. In both WT and Vdac2-deficient cells before exposure to death stimuli, BAK was eluted at an approximate molecular mass of 50 to 60 kD (Fig. 3A). After treatment with ionomycin for 6 hours, BAK formed higher-order oligomers in Vdac2-deficient cells but not in WT cells (Fig. 3A). Consistent with the enhanced oligomerization of BAK observed in Vdac2-deficient cells, these cells also exhibited earlier translocation of cytochrome c and activation of caspases than did WT cells (Fig. 3, B to D). Whereas cytochrome c remained in the mitochondria and exhibited a punctate staining pattern in most WT cells after 6 hours of treatment with ionomycin, cytochrome c had started to translocate from mitochondria to the cytosol in Vdac2 null cells (Fig. 3, B and C). Activation of caspases, as determined by the cleavage of poly(adenosine diphosphate–ribose) polymerase (PARP) and caspase-3, also occurred earlier in Vdac2 null cells than in WT cells (Fig. 3D). The BH3-only member BIM is induced to trigger apoptosis in response to calcium ion flux (27). Indeed, both WT and Vdac2 null cells displayed comparable kinetics of BIM induction (fig. S6), supporting the idea that accelerated homo-oligomerization of BAK was not caused by differences in signal transduction. Collectively, cells deficient for Vdac2 had a lower threshold for apoptosis than did WT cells because BAK in Vdac2-deficient cells was prone to undergo homo-oligomerization.
Fig. 3.
Vdac2 null thymocytes display early homo-oligomerization of BAK, translocation of cytochrome c, and activation of caspases upon apoptosis. (A) Thymocytes from Vdac2 WT (Vdac2f/+Lck-Cre–) or Vdac2 KO (Vdac2f/–Lck-Cre+) littermate mice were left untreated or were treated with ionomycin for 6 hours. Protein lysates were subjected to Superdex 200 (HR10/30) gel-filtration chromatography. Fractions were analyzed by Western blotting with an antibody against BAK. Data shown are representative of two experiments. (B) Fluorescence microscopy of Vdac2 WT (Vdac2f/+Lck-Cre–) or Vdac2 KO (Vdac2f/–Lck-Cre+) thymocytes treated with ionomycin for 6 hours. Red represents cytochrome c immunostaining and blue is Hoechst staining. Arrowheads denote cells displayingdiffuse cytosolicstaining for cytochrome c or loss of cytochrome c staining, which is indicative of translocation of cytochrome c. Images shown are representative of three independent experiments. (C) Analyses of multiple fields from experiments shown in (B) summarize the percentage of cells displaying translocation of cytochrome c. **P < 0.05. (D) Cell lysates from Vdac2 WT (Vdac2f/+Lck-Cre–) or Vdac2 KO (Vdac2f/–Lck-Cre+) thymocytes treated with ionomycin for the indicated times were assessed by Western blotting with antibodies specific for PARP, cleaved PARP, cleaved caspase-3, or actin. Data shown are representative of two experiments.
Deficiency in Bak, but not Bax, rescues the apoptotic phenotype associated with the deletion of Vdac2
To address whether the increased apoptosis in Vdac2 null cells was indeed caused by the enhanced activation of BAK, we deleted both Vdac2 and Bak in T cells. Deletion of Bak corrected the reduction in thymocyte number and thymus size incurred by Vdac2 deficiency (Fig. 4, A and B, and fig. S7A). In stark contrast, deletion of Bax failed to rescue Vdac2 null T cells (Fig. 4, C and D, and fig. S8A), consistent with the inability of BAX to bind to Vdac2 (17). Moreover, deficiency in Bak, but not Bax, abrogated the increased apoptosis that resulted from the deficiency in Vdac2 (Fig. 4, E and F, and figs. S7, B to E, and S8, B to E). Conditional deletion of Bax was used to facilitate breeding because a deficiency in Bax affects male fertility (28, 29). Lck-Cre–mediated deletion of Bax was confirmed by Western blotting analysis with an antibody against BAX (fig. S8F). Furthermore, thymocytes derived from Bak–/–Baxf/fLck-Cre+ were completely resistant to apoptosis, recapitulating the phenotypes reported for Bax–/–Bak–/– double knockout (DKO) animals (15, 30). Either BAX or BAK alone can efficiently serve as the death effector that controls mitochondrial apoptosis such that Bax or Bak single KO thymocytes have minimal apoptotic defects (15, 30). Thus, thymocytes deficient in both Vdac2 and Bak would be expected to respond to apoptotic stimuli similarly to WT cells, because mitochondrial apoptosis could still operate through BAX. Of note, Vdac2, Bak DKO mice had comparable numbers of thymocyte to those of Bak KO animals (Fig. 4G and fig. S9, A and B). Indeed, thymocytes derived from these two genotypes displayed similar responses to various apoptotic stimuli (Fig. 4H and fig. S9, C to F). These data provide genetic evidence that the VDAC2-BAK axis regulates the homeostasis and survival of thymocytes.
Fig. 4.
Deficiency in Bak but not Bax rescues the apoptotic phenotype associated with deletion of Vdac2. (A) Number of total thymocytes (represented as the mean ± SD) from sex-matched littermate mice of the indicated genotypes at 6 to 8 weeks of age (n = 8 for Vdac2f/–Bak+/+Lck-Cre+ mice and n = 5 for Vdac2f/–Bak–/–Lck-Cre+ mice). (B) A representative photograph of thymi from sex-matched littermate mice of the indicated genotypes. (C) Numbers of total thymocytes (represented as the mean ± SD) from sex-matched littermate mice of the indicated genotypes at 6 to 8 weeks of age (n = 8 for Vdac2f/–Bax+/+Lck-Cre+ mice and n = 9 for Vdac2f/–Baxf/–Lck-Cre+ mice). (D) A representative photograph of thymi from sex-matched littermate mice of the indicated genotypes. (E) Vdac2 KO or Vdac2, Bak DKO thymocytes were treated with ionomycin and cell death was quantified by staining with annexin V at the indicated times. Data shown are the mean percentage of annexin V–positive cells ± SD from n = 3 experiments. (F) Vdac2 KO or Vdac2, Bax DKO thymocytes were treated with ionomycin and cell death was quantified by staining with annexin V at the indicated times. Data shown are the mean percentage of annexin V–positive cells ± SD from n = 3 experiments. (G) Number of total thymocytes (mean ± SD) from sex-matched littermate mice of the indicated genotypes at 6 to 8 weeks of age (n = 5 for Vdac2f/+Bak–/–Lck-Cre– mice and n = 4 for Vdac2f/–Bak–/–Lck-Cre+ mice). (H) Bak KO or Vdac2, Bak DKO thymocytes were treated with ionomycin and cell death was quantified by staining with annexin V at the indicated times. Data shown are the mean ± SD. from three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
To further characterize Vdac2 null thymocytes and explore whether a deficiency in Vdac2 affects BCL-2 family proteins, we examined the abundance of various BCL-2 family proteins, including BAX, BAK, BCL-2, BID, BIM, and PUMA. The only protein whose abundance was substantially altered in Vdac2 null thymocytes was BAK (fig. S4). Indeed, BAK was decreased in abundance by about 50% in the absence of Vdac2 compared to that in WT cells. Because BAK is prone to be activated in Vdac2 null thymocytes, the surviving Vdac2 null thymocytes apparently evade apoptosis by decreasing the abundance of BAK. This finding further supports the link between VDAC2 and BAK in regulating survival and death.
The VDAC2-BAK axis regulates negative selection of thymocytes
Programmed cell death or apoptosis is crucial for normal lymphocyte development and function (31). More than 90% of thymocytes undergo apoptosis during their development. Most thymocytes die because they are not positively selected and do not receive a survival signal, whereas a minority of thymocytes undergo TCR-mediated apoptosis, a process known as negative selection. Negative selection deletes potentially self-reactive thymocytes, thereby generating a repertoire of peripheral T cells that is largely self-tolerant. Calcium flux triggered by TCR engagementis believed to initiate the deletion of autoreactive thymocytes (32). The observation that Vdac2 null thymocytes were extremely sensitive to ionomycin prompted us to investigate whether Vdac2 null T cells were more susceptible than WT cells to TCR activation-induced apoptosis.
One widely used experimental model of negative selection is the exposure of thymocytes to antibody against CD3 in vivo or in vitro, which aggregates the TCR-CD3 complex and thereby kills the vulnerable CD4+CD8+ DP population (31). Indeed, Vdac2 null thymocytes cultured in the presence of antibody against CD3 (anti-CD3) died faster than did WT cells (Fig. 5A). Enhanced oligomerization of BAK was detected in Vdac2 null thymocytes in comparison with that in WT cells after TCR stimulation (Fig. 5B). Of note, deficiency in Bak corrected the enhanced apoptotic response caused by deficiency in Vdac2 (Fig. 5A). Consistent with previous reports, more than 90% of the WT DP thymocytes were killed after the injection of the anti-CD3, which resulted in shrinkage of the thymus (Figs. 5, C and E, and 6). Thymi from Vdac2 conditional KO animals were even more markedly reduced in size after injection of the anti-CD3 (Fig. 5C). The DP cell population was reduced from ~80% to ~25% of total thymocytes in WT mice in response to engagement of the TCR (Fig. 6). This reduction was more substantial in Vdac2 null mice because less than 5% of the DP population was left in Vdac2 null mice (Fig. 6). Whereas only a twofold difference in the numbers of the DP population was observed between WT and Vdac2 conditional KO animals, administration of anti-CD3 led to a 12-fold difference in this population (Figs. 2B, 5E, and 6). These findings highlight the extreme sensitivity to TCR stimulation caused by the deficiency in Vdac2. Strikingly, deficiency in Bak fully rescued the enhanced sensitivity of Vdac2 null thymocytes to TCR stimulation in vivo (Figs. 5, C and E, and 6). On the contrary, Vdac2, Bax DKO thymocytes were as sensitive as Vdac2 null thymocytes to activation of the TCR (Figs. 5, D and E, and 6). Although in vivo administration of anti-CD3 mainly depleted the DP cells, a decrease in other populations was also observed, but to a lesser degree (fig. S10). The increased sensitivity to TCR activation–induced apoptosis conferred by the deficiency in Vdac2 was most prominent for the DP cell population even though a comparable deletion of the Vdac2 allele was detected in SP cells (Fig. 5E and figs. S10 and S11). Of note, deletion of Vdac2 was incomplete in the CD4–CD8– double-negative (DN) population (fig. S11), which is a common feature of the proximal promoter Lck-Cre transgene (26). Taken together, these data suggest that VDAC2 may have a unique role in regulating apoptosis in the DP population. Alternatively, the abundance of BAK may be differentially regulated during thymocyte differentiation. Nevertheless, our data provide a functional link between VDAC2 and BAK and suggest the presence of a critical VDAC2-BAK axis that regulates the negative selection of thymocytes.
Fig. 5.
Vdac2 null thymocytes display an enhanced apoptotic response to stimulation of the TCR, which is rescued by a deficiency in Bak but not Bax. (A) Thymocytes from Vdac2 WT (Vdac2f/+Lck-Cre–), Vdac2 KO (Vdac2f/–Lck-Cre+) or Vdac2, Bak DKO (Vdac2f/–Bak–/–Lck-Cre+) littermate mice at 6 to 10 weeks of age were cultured in plates coated with anti-CD3ε. Cell death was quantified by staining with annexin V at the indicated times. Data shown are the mean ± SD from three independent experiments. (B) Vdac2 null thymocytes display early homo-oligomerization of BAK in response to stimulation of the TCR. Thymocytes from Vdac2 WT (Vdac2f/+Lck-Cre–) or Vdac2 KO (Vdac2f/–Lck-Cre+) littermate mice were cultured in plates coated with anti-CD3ε for 14 hours. Protein lysates were subjected to Superdex 200 (HR10/30) gel-filtration chromatography. Fractions were analyzed by Western blotting with an antibody against BAK. (C) A representative photograph of thymi from sex-matched littermate mice of the indicated genotypes at 48 hours after intraperitoneal injection of anti-CD3e antibody or PBS as a negative control. (D) A representative photograph of thymi from sex-matched littermate mice of the indicated genotypes at 48 hours after intraperitoneal injection of anti-CD3ε or PBS (negative control). (E) Numbers of DP thymocytes that remain viable from Vdac2 WT (Vdac2f/+Lck-Cre–), Vdac2 KO (Vdac2f/–Lck-Cre+), Vdac2, Bak DKO (Vdac2f/–Bak–/–Lck-Cre+), Vdac2, Bax DKO (Vdac2f/–Baxf/–Lck-Cre+), or Bak KO (Bak–/–) mice (6 to 10 weeks of age) 48 hours after intraperitoneal injection with anti-CD3ε. Data shown are the mean ± SD from three independent experiments.
Fig. 6.
FACS (fluorescence-activated cell sorting) analyses of thymocytes after stimulation of the TCR in vivo. Total thymocytes were isolated from Vdac2 WT (Vdac2f/+Lck-Cre–), Vdac2 KO (Vdac2f/–Lck-Cre+), Vdac2, Bak DKO (Vdac2f/–Bak–/–Lck-Cre+), Vdac2, Bax DKO (Vdac2f/–Baxf/–Lck-Cre+), or Bak KO (Bak–/–) mice (6 to 10 weeks of age) 48 hours after intraperitoneal injection with anti-CD3ε or PBS (control). Cells were stained with PE-conjugated anti-CD4 and FITC-conjugated anti-CD8 and analyzed by flow cytometry. The percentages of the different thymocyte subpopulations in gated live cells are shown within the quadrants. Data shown are representative of three independent experiments.
DISCUSSION
VDAC2 is unique among VDAC family members in that it exhibits a dynamic interaction with BAK (17). Here, we defined a distinct physiological role for VDAC2 in vivo as a specific inhibitor of BAK-dependent mitochondrial apoptosis. Our study uncovered a critical VDAC2-BAK axis involved in regulating the survival and negative selection of thymocytes. These findings raise the possibility that the relative abundance of VDAC2 and BAK may serve as a rheostat that determines the susceptibility of mammals to immunodeficiency or autoimmune disease. Because both VDAC2 and BAK are found in a wide range of tissues, it is conceivable that the VDAC2-BAK axis may regulate the balance between survival and death in other cell types in addition to T cells.
We previously showed that to activate BAK, activator BH3s must disrupt the VDAC2-BAK complex (14, 17). Because deficiency in Vdac2 only reduced the threshold required for the activation of BAK, full activation of BAK still relied on activator BH3s (Fig. 3A). Together, these data suggest that VDAC2 functions as a brake that controls the BAK-centered death machinery, whereas activator BH3-only proteins provide the driving force. BIM is probably the best candidate to release the VDAC2 brake against the oligomerization of BAK because BIM is an essential initiator of apoptosis during the negative selection of thymocytes (33). Accordingly, deficiency in Bim might rescue the enhanced apoptotic response observed in Vdac2 null thymocytes, which waits to be tested in future experiments.
The mitochondrial permeability transition pore (PTP)—a protein complex that spans both the outer and the inner mitochondrial membranes—is proposed to mediate necrotic cell death induced by calcium overload, hypoxia, and oxidative stress (20–22). Although VDACs were originally considered to be the key components of the mitochondrial PTP, ablation of all three VDAC isoforms by knocking down Vdac2 in Vdac1–/–Vdac3–/– DKO cells does not inactivate the PTP (23), excluding the pro-death roles played by VDACs. In contrast, VDAC2 appears to promote survival because Vdac2-deficient mouse embryonic fibroblasts (MEFs) are more sensitive to intrinsic death signals and hydrogen peroxide than are WT MEFs (17, 23). Here, we provide evidence that VDAC2 has an important prosurvival function in the maintenance of thymocyte homeostasis in vivo. Consistent with our findings, it was reported that VDAC2 is required for the survival of Ras-transformed cells and that it can serve as an anticancer target (34). Similarly, manipulation of the VDAC2-BAK axis may offer a new strategy to trigger apoptosis in T cell leukemia or lymphoma. The biochemical and functional link between VDAC2 and BAK provides a paradigm to coregulate mitochondrial metabolism and apoptosis. It remains to be determined whether BAK has a nonapoptotic role in regulating mitochondrial physiology through VDAC2.
MATERIALS AND METHODS
Targeting the Vdac2 genomic locus
The Vdac2 gene locus was targeted by placing a loxP site upstream of the promoter (Bam HI site). In addition, a loxP-flanked pPGKneo cassette was inserted into the intron between exons 3 and 4 (Bgl II site) of Vdac2. Gene targeting was performed as described previously (35). Successfully targeted RW4 embryonic stem (ES) cells (129Svj) were determined by Southern blot analyses followed by transient transfection with CMV-Cre to generate Vdac2f/+ ES clones with a deletion of pPGKneo, as well as Vdac2+/– ES cloneswith adeletion ofthe region from the promoter toexon 3 and pPGKneo. ES cells in which successful recombination had occurred were injected into C57Bl/6 blastocysts. The chimeric mice were bred for germ-line transmission to obtain Vdac2f/+ or Vdac2+/– mice. Vdac2+/– micewere crossed with Lck-Cre transgenic mice (26). Vdac2f/f mice were crossed with Vdac2+/–Lck-Cre+ mice to generate Vdac2f/+Lck-Cre–, Vdac2f/–Lck-Cre–, Vdac2f/+Lck-Cre+, or Vdac2f/–Lck-Cre+ littermate mice. To compare the effects of KO of Vdac2 with those of DKO of Vdac2 and Bak, Vdac2f/fBak+/– mice were crossed with Vdac2+/–Bak+/–Lck-Cre+ mice. To compare the effects of KO of Vdac2 with those of DKO of Vdac2 and Bax, Vdac2f/fBaxf/+ mice were crossed with Vdac2+/–Bax+/–Lck-Cre+ mice. All of the mice were on mixed 129Sv and C57Bl/6 backgrounds. The primers for genotyping mice for Vdac2 are as follows: A, 5′-CACCAAGAAGACCACCCCCT; B, 5′-GATGGTGACTCCTTCCCATC;C, 5′-AATCCCAAAGTCCGGGACAG. Animal experiments were performed according to institutional guidelines.
Flow cytometry and cell death assays
Thymocytes were isolated from sex-matched littermate mice at 6 to 8 weeks of age. Single-cell suspensions of thymocytes were prepared and cell numbers were determined by HEMAVET (Drew Scientific). Flow cytometry was performed with a FACSCalibur flow cytometer (BD Biosciences), and data were analyzed with CellQuest Pro software (BD Biosciences) or FloJo software. Monoclonal antibodies used in this study included fluorescein isothiocyanate (FITC)-conjugated antibody against CD8 and phycoerythrin (PE)-conjugated antibody against CD4 (BD Biosciences). Thymocytes were cultured in complete RPMI 1640 with 10% fetal bovine serum (Invitrogen). Apoptosis was induced by 10 nM dexamethasone, ionomycin (1 μg/ml), 1 μM etoposide (Sigma), or 2.5 Gy of γ-irradiation. Cell death was quantified by staining with annexin V (BioVision) followed by flow cytometric analyses. Thymocyte sorting was performed with the FACSAria II high-speed cell sorter (BD Biosciences) by the flow cytometry core facility at the Department of Pathology and Immunology, Washington University in St Louis. P values for statistical analyseswere obtained with the Student's t test.
Treatment of cells with antibody against CD3
Thymocytes were cultured in plates precoated with antibody (10 μg/ml) against CD3ε (145-2C11, BD Biosciences). Cell death was quantified by staining with annexin V (BioVision) at the indicated times. Mice at 6 to 10 weeks of age were injected intraperitoneally with 10 μg of antibody against CD3ε in phosphate-buffered saline (PBS) or, as a negative control, with PBS only. Thymocytes were prepared from these mice after 48 hours and cell numberswere determined by HEMAVET SYSTEM. Flow cytometric analyses were performed as described above.
Gel-filtration chromatography
The chromatographic step of Superdex 200 (HR 10/30, GE-Amersham) was performed on an automatic fast protein liquid chromatography device (AKTApurifier, GE-Amersham). The column was equilibrated with 2% CHAPS buffer [2% CHAPS, 300 mM NaCl, 0.2 mM dithiothreitol, 20 mM Hepes (pH 7.5)] and calibrated with thyroglobulin (669 kD, GE-Amersham), ferritin (440 kD, GE-Amersham), catalase (232 kD, GE-Amersham), aldolase (158 kD, GE-Amersham), bovine serum albumin (66 kD, Calbiochem), and cytochrome c (14 kD, Sigma). Thymocytes were lysed in 2% CHAPS buffer supplemented with complete protease inhibitor (Roche). Protein concentrations were determined with the bicinchoninic acid (BCA) kit (Pierce). Lysates (200 μl, 2 mg/ml) were loaded onto the column and eluted at a flow rate of 0.3 ml/min. Fractions of 0.6 ml were collected, precipitated by trichloroacetic acid, and analyzed by 8 to 16% SDS–polyacrylamide gel electrophoresis (PAGE) (Bio-Rad) and Western blotting with antibodies against BAK, as described below.
Western blotting and indirect immunofluorescence microscopy
Thymocytes were lysed in PBS containing 1% Triton X-100. Protein concentrations were determined with the BCA kit. Samples (25 to 50 μg of protein) were resolved by 10% NuPAGE (Invitrogen) and transferred onto polyvinylidine difluoride membranes (Immobilon-P, Millipore). Antibody detection was accomplished with the enhanced chemiluminescence method (Western Lightning, PerkinElmer) and the LAS-3000 Imaging system (FUJIFILM). Antibodies used for the analysis of Western blots were as follows: antibodies against BAK (Upstate Biotechnology), against cleaved caspase-3 (Cell Signaling Technology), against PARP (Cell Signaling Technology), against BIM (Calbiochem), and against actin (Chemicon). The antibody against VDAC2 was generated by immunizing rabbits with a peptide corresponding to amino acid residues 212 to 228 of VDAC2. Cells that had been fixed in 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 were sequentially incubated with antibody against cytochrome c (BD Biosciences), Alexa Fluor 568–conjugated goat secondary antibody against mouse (Invitrogen), and Hoechst 33342 (Invitrogen). Images were acquired with a SPOT camera (Diagnostics Instruments) mounted on an Olympus IX51 microscope (Olympus).
Supplementary Material
Acknowledgments
We thank H.-F. Chen for technical assistance. This work was supported by grants to E. Cheng from the National Cancer Institute–NIH (K01CA98320 and R01CA125562) and the Searle Scholars Program.
Footnotes
Citation: D. Ren, H. Kim, H.-C. Tu, T. D. Westergard, J. K. Fisher, J. A. Rubens, S. J. Korsmeyer, J. J.-D. Hsieh, E. H.-Y. Cheng, The VDAC2-BAK rheostat controls thymocyte survival. Sci. Signal. 2, ra48 (2009).
REFERENCES AND NOTES
- 1.Danial NN, Korsmeyer SJ. Cell death: Critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 2.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 3.Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000;7:1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- 4.Walensky LD. BCL-2 in the crosshairs: Tipping the balance of life and death. Cell Death Differ. 2006;13:1339–1350. doi: 10.1038/sj.cdd.4401992. [DOI] [PubMed] [Google Scholar]
- 5.Reed JC. Proapoptotic multidomain Bcl-2/Bax-family proteins: Mechanisms, physiological roles, and therapeutic opportunities. Cell Death Differ. 2006;13:1378–1386. doi: 10.1038/sj.cdd.4401975. [DOI] [PubMed] [Google Scholar]
- 6.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelekar A, Thompson CB. Bcl-2-family proteins: The role of the BH3 domain in apoptosis. Trends Cell Biol. 1998;8:324–330. doi: 10.1016/s0962-8924(98)01321-x. [DOI] [PubMed] [Google Scholar]
- 8.Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, Thompson CB, Korsmeyer SJ. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- 9.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol. Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 11.Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 12.Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol. Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, Letai A. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 14.Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat. Cell Biol. 2006;8:1348–1358. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- 15.Lindsten T, Ross AJ, King A, Zong W-X, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR, Thompson CB. The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol. Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki M, Youle RJ, Tjandra N. Structure of Bax: Coregulation of dimer formation and intracellular localization. Cell. 2000;103:645–654. doi: 10.1016/s0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
- 17.Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- 18.Cuconati A, Mukherjee C, Perez D, White E. DNA damage response and MCL-1 destruction initiate apoptosis in adenovirus-infected cells. Genes Dev. 2003;17:2922–2932. doi: 10.1101/gad.1156903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM, Huang DC. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 21.Rasola A, Bernardi P. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis. 2007;12:815–833. doi: 10.1007/s10495-007-0723-y. [DOI] [PubMed] [Google Scholar]
- 22.Leung AW, Halestrap AP. Recent progress in elucidating the molecular mechanism of the mitochondrial permeability transition pore. Biochim. Biophys. Acta. 2008;1777:946–952. doi: 10.1016/j.bbabio.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat. Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anflous K, Armstrong DD, Craigen WJ. Altered mitochondrial sensitivity for ADP and maintenance of creatine-stimulated respiration in oxidative striated muscles from VDAC1-deficient mice. J. Biol. Chem. 2001;276:1954–1960. doi: 10.1074/jbc.M006587200. [DOI] [PubMed] [Google Scholar]
- 25.Sampson MJ, Decker WK, Beaudet AL, Ruitenbeek W, Armstrong D, Hicks MJ, Craigen WJ. Immotile sperm and infertility in mice lacking mitochondrial voltage-dependent anion channel type 3. J. Biol. Chem. 2001;276:39206–39212. doi: 10.1074/jbc.M104724200. [DOI] [PubMed] [Google Scholar]
- 26.Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Perez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, Cherry SR, Tsai JH, Tucker SM, Weaver WM, Kelso A, Jaenisch R, Wilson CB. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 27.Canté-Barrett K, Gallo EM, Winslow MM, Crabtree GR. Thymocyte negative selection is mediated by protein kinase C- and Ca2+-dependent transcriptional induction of Bim. J. Immunol. 2006;176:2299–2306. doi: 10.4049/jimmunol.176.4.2299. [DOI] [PubMed] [Google Scholar]
- 28.Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- 29.Takeuchi O, Fisher J, Suh H, Harada H, Malynn BA, Korsmeyer SJ. Essential role of BAX,BAK in B cell homeostasis and prevention of autoimmune disease. Proc. Natl. Acad. Sci. U.S.A. 2005;102:11272–11277. doi: 10.1073/pnas.0504783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rathmell JC, Lindsten T, Zong WX, Cinalli RM, Thompson CB. Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nat. Immunol. 2002;3:932–939. doi: 10.1038/ni834. [DOI] [PubMed] [Google Scholar]
- 31.Opferman JT, Korsmeyer SJ. Apoptosis in the development and maintenance of the immune system. Nat. Immunol. 2003;4:410–415. doi: 10.1038/ni0503-410. [DOI] [PubMed] [Google Scholar]
- 32.Nakayama T, Ueda Y, Yamada H, Shores EW, Singer A, June CH. In vivo calcium elevations in thymocytes with T cell receptors that are specific for self ligands. Science. 1992;257:96–99. doi: 10.1126/science.1621102. [DOI] [PubMed] [Google Scholar]
- 33.Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, Pellegrini M, Cory S, Adams JM, Strasser A. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 34.Yagoda N, von Rechenberg M, Zaganjor E, Bauer AJ, Yang WS, Fridman DJ, Wolpaw AJ, Smukste I, Peltier JM, Boniface JJ, Smith R, Lessnick SL, Sahasrabudhe S, Stockwell BR. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447:864–868. doi: 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takeda S, Chen DY, Westergard TD, Fisher JK, Rubens JA, Sasagawa S, Kan JT, Korsmeyer SJ, Cheng EH, Hsieh JJ. Proteolysis of MLL family proteins is essential for taspase1-orchestrated cell cycle progression. Genes Dev. 2006;20:2397–2409. doi: 10.1101/gad.1449406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.