Abstract
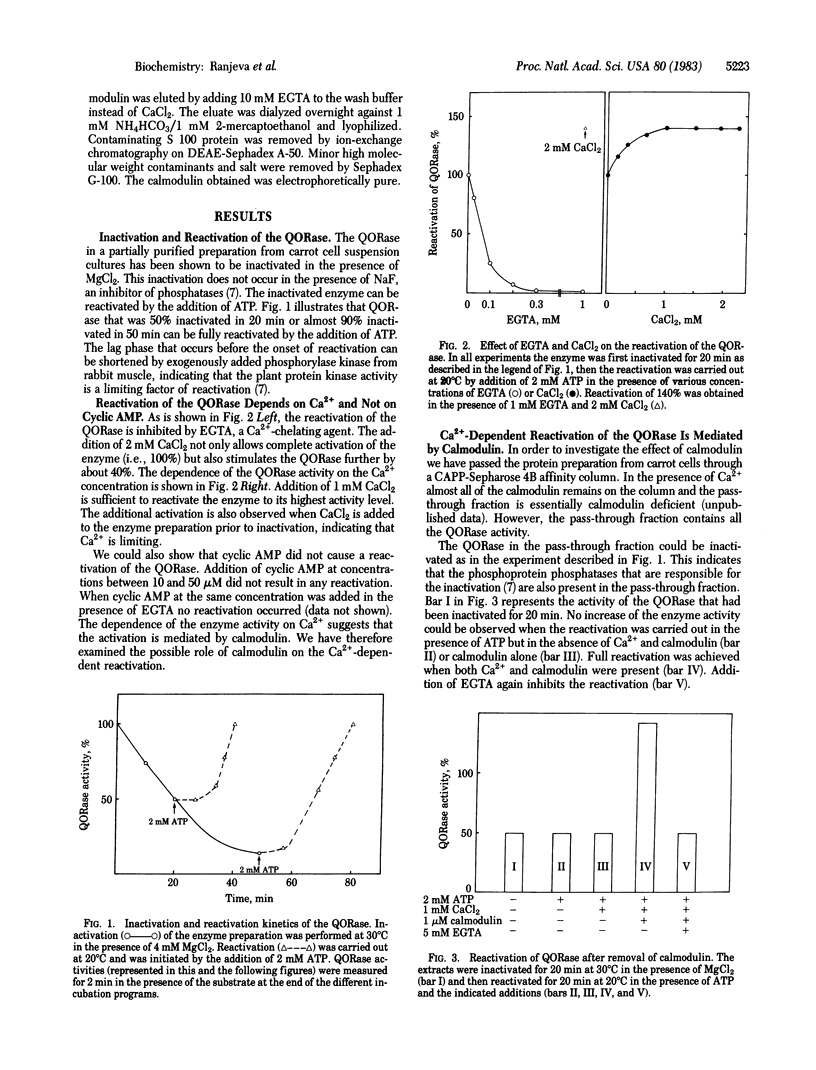
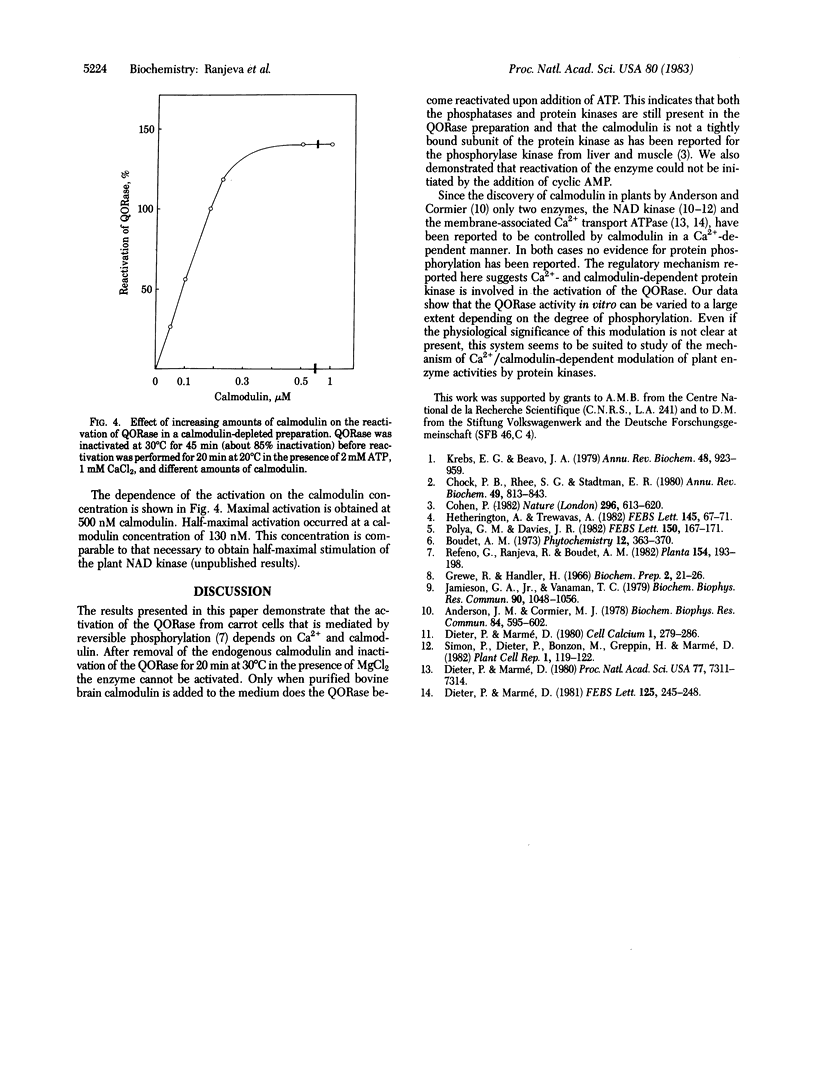
Quinate:NAD+ 3-oxidoreductase (EC 1.1.1.24) from carrot cell suspension cultures has previously been shown to be activated by phosphorylation and inactivated by dephosphorylation. Here it is shown that the reactivation of the inactivated quinate:NAD+ oxidoreductase is an enzyme-mediated process that requires ATP and protein kinase activity. The reactivation is completely inhibited by EGTA and can be restored by the addition of Ca2+. Cyclic AMP at concentrations up to 5 μM did not have any effect on the reactivation either with or without EGTA in the medium. Calmodulin-depleted fractions containing quinate:NAD+ oxidoreductase were obtained by passage of the crude extracts through an affinity column of 2-chloro-10-(3-aminopropyl)phenothiazine coupled to Sepharose 4B. The enzyme in this calmodulin-deficient fraction could be inactivated but not reactivated even in the presence of ATP and Ca2+. However, addition of bovine brain calmodulin completely restored the activity of the enzyme. Half-maximal activation occurred at 130 nM calmodulin. We conclude from these data that the quinate:NAD+ oxidoreductase is activated by a Ca2+ - and calmodulin-dependent plant protein kinase.
Keywords: carrot cells, cyclic AMP, protein kinase, enzyme phosphorylation
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Cormier M. J. Calcium-dependent regulation of NAD kinase. Biochem Biophys Res Commun. 1978 Oct 16;84(3):595–602. doi: 10.1016/0006-291x(78)90747-7. [DOI] [PubMed] [Google Scholar]
- Chock P. B., Rhee S. G., Stadtman E. R. Interconvertible enzyme cascades in cellular regulation. Annu Rev Biochem. 1980;49:813–843. doi: 10.1146/annurev.bi.49.070180.004121. [DOI] [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in neural and hormonal control of cellular activity. Nature. 1982 Apr 15;296(5858):613–620. doi: 10.1038/296613a0. [DOI] [PubMed] [Google Scholar]
- Dieter P., Marmé D. Calmodulin activation of plant microsomal Ca uptake. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7311–7314. doi: 10.1073/pnas.77.12.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson G. A., Jr, Vanaman T. C. Calcium-dependent affinity chromatography of calmodulin on an immobilized phenothiazine. Biochem Biophys Res Commun. 1979 Oct 12;90(3):1048–1056. doi: 10.1016/0006-291x(79)91932-6. [DOI] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]


