Abstract
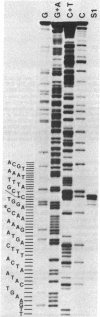
The nucleotide sequence of a cloned section of the Escherichia coli chromosome containing the tonB gene has been determined. Transcription initiation and termination sites for tonB RNA have been determined by S1 nuclease mapping. The tonB promoter and terminator resemble other E. coli promoters and terminators; the sequence of the tonB terminator region suggests that it may function bidirectionally. The DNA sequence specifies an open translation reading frame between the 5' and 3' RNA termini whose location is consistent with the position of previously isolated tonB::IS1 mutations. The DNA sequence predicts a proline-rich protein with a calculated size of 26.1-26.6 kilodaltons (239-244 amino acids), depending on which of three potential initiation codons is utilized. The predicted NH2 terminus of tonB protein resembles the proteolytically cleaved signal sequences of E. coli periplasmic and outer membrane proteins; the overall hydrophilic character of the protein sequence suggests that the bulk of the tonB protein is not embedded within the inner or outer membrane. A significant discrepancy exists between the calculated size of tonB protein and the apparent size of 36 kilodaltons determined by sodium dodecyl sulfate/polyacrylamide gel electrophoresis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. H. Growth Requirements of Virus-Resistant Mutants of Escherichia Coli Strain "B". Proc Natl Acad Sci U S A. 1946 May;32(5):120–128. doi: 10.1073/pnas.32.5.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassford P. J., Jr, Bradbeer C., Kadner R. J., Schnaitman C. A. Transport of vitamin B12 in tonB mutants of Escherichia coli. J Bacteriol. 1976 Oct;128(1):242–247. doi: 10.1128/jb.128.1.242-247.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassford P. J., Jr, Kadner R. J., Schnaitman C. A. Biosynthesis of the outer membrane receptor for vitamin B12, E colicins, and bacteriophage BF23 by Escherichia coli: kinetics of phenotypic expression after the introduction of bfe+ and bfe alleles. J Bacteriol. 1977 Jan;129(1):265–275. doi: 10.1128/jb.129.1.265-275.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Burkhardt R., Schneider R., Zimmermann L. Chromosomal genes for ColV plasmid-determined iron(III)-aerobactin transport in Escherichia coli. J Bacteriol. 1982 Aug;151(2):553–559. doi: 10.1128/jb.151.2.553-559.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Di Girolamo P. M., Kadner R. J., Bradbeer C. Isolation of vitamin B 12 transport mutants of Escherichia coli. J Bacteriol. 1971 Jun;106(3):751–757. doi: 10.1128/jb.106.3.751-757.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Frost G. E., Rosenberg H. Relationship between the tonB locus and iron transport in Escherichia coli. J Bacteriol. 1975 Nov;124(2):704–712. doi: 10.1128/jb.124.2.704-712.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L., Pribnow D., Schneider T., Shinedling S., Singer B. S., Stormo G. Translational initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- Gratia J. P. Studies on defective lysogeny due to chromosomal deletion in Escherichia coli. I. Single lysogens. Biken J. 1966 Jun;9(2):77–87. [PubMed] [Google Scholar]
- Hancock R. W., Braun V. Nature of the energy requirement for the irreversible adsorption of bacteriophages T1 and phi80 to Escherichia coli. J Bacteriol. 1976 Feb;125(2):409–415. doi: 10.1128/jb.125.2.409-415.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. Membrane receptor dependent iron transport in Escherichia coli. FEBS Lett. 1975 Jan 1;49(3):301–305. doi: 10.1016/0014-5793(75)80771-x. [DOI] [PubMed] [Google Scholar]
- Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet. 1981;182(2):288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes: a proposal for a synonymous codon choice that is optimal for the E. coli translational system. J Mol Biol. 1981 Sep 25;151(3):389–409. doi: 10.1016/0022-2836(81)90003-6. [DOI] [PubMed] [Google Scholar]
- Kiss A., Sain B., Csordás-Tòth E., Venetianer P. A new sequence-specific endonuclease (Bsp) from Bacillus sphaericus. Gene. 1977 Jul;1(5-6):323–329. doi: 10.1016/0378-1119(77)90037-3. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- MATSUSHIRO A. Specialized transduction of tryptophan markers in Escherichia coli K12 by bacteriophage phi-80. Virology. 1963 Apr;19:475–482. doi: 10.1016/0042-6822(63)90041-2. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Michaelis S., Beckwith J. Mechanism of incorporation of cell envelope proteins in Escherichia coli. Annu Rev Microbiol. 1982;36:435–465. doi: 10.1146/annurev.mi.36.100182.002251. [DOI] [PubMed] [Google Scholar]
- Plastow G. S., Holland I. B. Identification of an Escherichia coli inner membrane polypeptide specified by a lambda-tonB transducing. Biochem Biophys Res Commun. 1979 Oct 12;90(3):1007–1014. doi: 10.1016/0006-291x(79)91927-2. [DOI] [PubMed] [Google Scholar]
- Postle K., Reznikoff W. S. HindII and HindIII restriction maps of the attphi80-tonB-trp region of the Escherichia coli genome, and location of the tonB gene. J Bacteriol. 1978 Dec;136(3):1165–1173. doi: 10.1128/jb.136.3.1165-1173.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle K., Reznikoff W. S. Identification of the Escherichia coli tonB gene product in minicells containing tonB hybrid plasmids. J Mol Biol. 1979 Jul 5;131(3):619–636. doi: 10.1016/0022-2836(79)90011-1. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Reeves P. Iron uptake in colicin B-resistant mutants of Escherichia coli K-12. J Bacteriol. 1976 Jun;126(3):1052–1062. doi: 10.1128/jb.126.3.1052-1062.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Staden R. A new computer method for the storage and manipulation of DNA gel reading data. Nucleic Acids Res. 1980 Aug 25;8(16):3673–3694. doi: 10.1093/nar/8.16.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. T., Norrell S. A., Hanna M. L. Uptake of cyanocobalamin by Escherichia coli B: some characteristics and evidence for a binding protein. Arch Biochem Biophys. 1972 Feb;148(2):366–381. doi: 10.1016/0003-9861(72)90154-3. [DOI] [PubMed] [Google Scholar]
- Treisman R., Kamen R. Structure of polyoma virus late nuclear RNA. J Mol Biol. 1981 May 25;148(3):273–301. doi: 10.1016/0022-2836(81)90539-8. [DOI] [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Limited proteolysis of the penicillin-sensitive D-alanine carboxypeptidase purified from Bacillus subtilis membranes. Active water-soluble fragments generated by cleavage of a COOH-terminal membrane anchor. J Biol Chem. 1981 Feb 25;256(4):2059–2066. [PubMed] [Google Scholar]
- Weaver C. A., Konisky J. tonB-independent ferrichrome-mediated iron transport in Escherichia coli spheroplasts. J Bacteriol. 1980 Sep;143(3):1513–1518. doi: 10.1128/jb.143.3.1513-1518.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wookey P., Rosenberg H. Involvement of inner and outer membrane components in the transport of iron and in colicin B action in Escherichia coli. J Bacteriol. 1978 Feb;133(2):661–666. doi: 10.1128/jb.133.2.661-666.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wookey P. The tonB gene product in Escherichia coli. Energy-coupling or molecular processing of permeases? FEBS Lett. 1982 Mar 22;139(2):145–154. doi: 10.1016/0014-5793(82)80838-7. [DOI] [PubMed] [Google Scholar]