Abstract
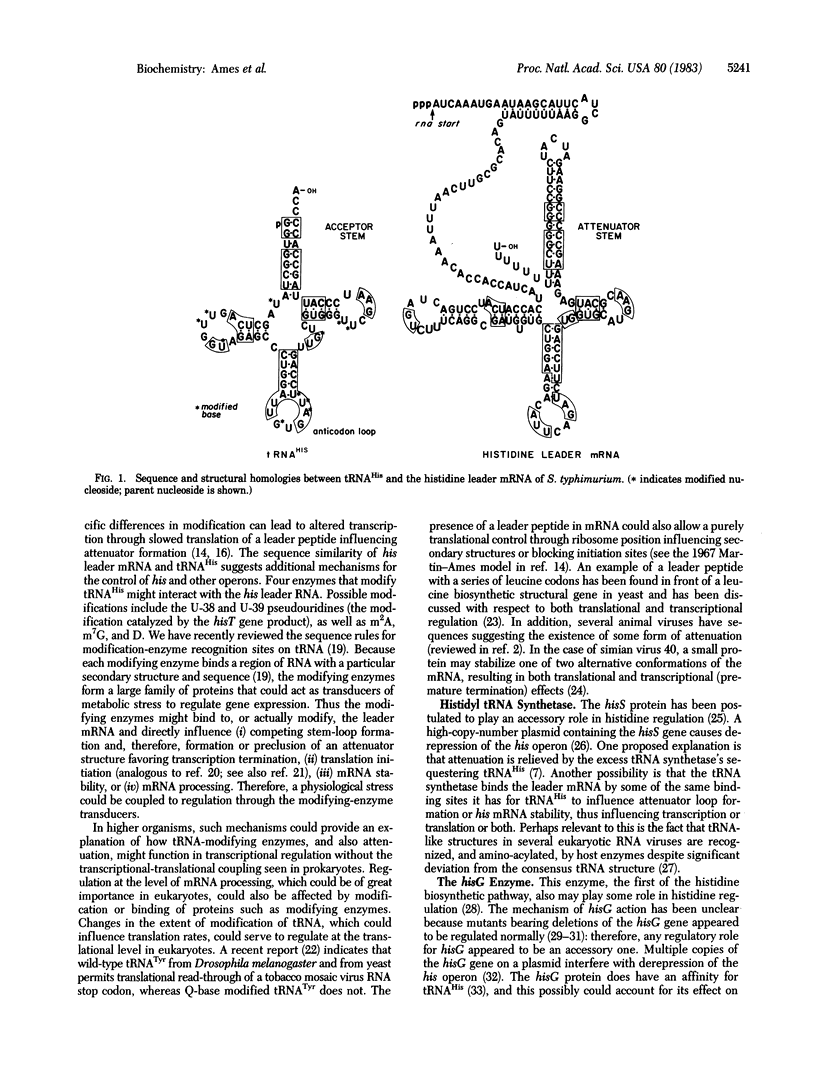
The leader region of the mRNA of the his operon is involved in regulating the frequency of transcription termination through attenuation and therefore expression of the his structural genes. We now report that the his leader mRNA has a remarkable sequence homology with the tRNAHis molecule. Of the 75 nucleotides forming tRNAHis (not counting the -CCA tail), 45 are homologous to nucleotide sequences found in the his leader mRNA. This homology extends to secondary structures which can form in the leader mRNA. The stems and loops of tRNAHis are thus related to those of the his leader mRNA which play a critical role in regulating expression of the his operon through attenuation. Many proteins that bind tRNAHis thus might bind to the similar structures found in the his leader mRNA and influence regulation by favoring the attenuator or anti-attenuator configuration. These include tRNA-modifying enzymes, the histidyl-tRNA synthetase, and the hisG enzyme. The significance of similar structures in other regulatory systems is discussed, particularly in relation to the role of tRNA-modifying enzymes as important regulatory molecules in both prokaryotes and eukaryotes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Andreadis A., Hsu Y. P., Kohlhaw G. B., Schimmel P. Nucleotide sequence of yeast LEU2 shows 5'-noncoding region has sequences cognate to leucine. Cell. 1982 Dec;31(2 Pt 1):319–325. doi: 10.1016/0092-8674(82)90125-8. [DOI] [PubMed] [Google Scholar]
- Barnes W. M. DNA sequence from the histidine operon control region: seven histidine codons in a row. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4281–4285. doi: 10.1073/pnas.75.9.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni C. B., Musti A. M., Frunzio R., Blasi F. Structural and physiological studies of the Escherichia coli histidine operon inserted into plasmid vectors. J Bacteriol. 1980 Apr;142(1):32–42. doi: 10.1128/jb.142.1.32-42.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck M., Connick M., Ames B. N. Complete analysis of tRNA-modified nucleosides by high-performance liquid chromatography: the 29 modified nucleosides of Salmonella typhimurium and Escherichia coli tRNA. Anal Biochem. 1983 Feb 15;129(1):1–13. doi: 10.1016/0003-2697(83)90044-1. [DOI] [PubMed] [Google Scholar]
- Buck M., Griffiths E. Iron mediated methylthiolation of tRNA as a regulator of operon expression in Escherichia coli. Nucleic Acids Res. 1982 Apr 24;10(8):2609–2624. doi: 10.1093/nar/10.8.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese R., Kammen H. O., Spengler S. J., Ames B. N. Biosynthesis of pseudouridine in transfer ribonucleic acid. J Biol Chem. 1974 Feb 25;249(4):1103–1108. [PubMed] [Google Scholar]
- Eisenbeis S. J., Parker J. Strains of Escherichia coli carrying the structural gene for histidyl-tRNA synthetase on a high copy-number plasmid. Mol Gen Genet. 1981;183(1):115–122. doi: 10.1007/BF00270148. [DOI] [PubMed] [Google Scholar]
- Gemmill R. M., Wessler S. R., Keller E. B., Calvo J. M. leu operon of Salmonella typhimurium is controlled by an attenuation mechanism. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4941–4945. doi: 10.1073/pnas.76.10.4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goitein R. K., Parsons S. M. Possible regulation of the Salmonella typhimurium histidine operon by adenosine triphosphate phosphoribosyltransferase: large metabolic effects. J Bacteriol. 1980 Oct;144(1):337–345. doi: 10.1128/jb.144.1.337-345.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberger R. F. Autogenous regulation of gene expression. Science. 1974 Mar 1;183(4127):810–816. doi: 10.1126/science.183.4127.810. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Gabay J., Débarbouillé M., Schwartz M. A role for mRNA secondary structure in the control of translation initiation. Nature. 1982 Feb 18;295(5850):616–618. doi: 10.1038/295616a0. [DOI] [PubMed] [Google Scholar]
- Hay N., Skolnik-David H., Aloni Y. Attenuation in the control of SV40 gene expression. Cell. 1982 May;29(1):183–193. doi: 10.1016/0092-8674(82)90102-7. [DOI] [PubMed] [Google Scholar]
- Hudson L., Rossi J., Landy A. Dual function transcripts specifying tRNA and mRNA. Nature. 1981 Dec 3;294(5840):422–427. doi: 10.1038/294422a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ino I., Hartman P. E., Hartman Z., Yourno J. Deletions fusing the hisG and hisD genes in Salmonella typhimurium. J Bacteriol. 1975 Sep;123(3):1254–1264. doi: 10.1128/jb.123.3.1254-1264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston H. M., Barnes W. M., Chumley F. G., Bossi L., Roth J. R. Model for regulation of the histidine operon of Salmonella. Proc Natl Acad Sci U S A. 1980 Jan;77(1):508–512. doi: 10.1073/pnas.77.1.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston H. M., Roth J. R. DNA sequence changes of mutations altering attenuation control of the histidine operon of Salmonella typhimurium. J Mol Biol. 1981 Feb 5;145(4):735–756. doi: 10.1016/0022-2836(81)90312-0. [DOI] [PubMed] [Google Scholar]
- Johnston H. M., Roth J. R. Genetic analysis of the histidine operon control region of Salmonella typhimurium. J Mol Biol. 1981 Feb 5;145(4):713–734. doi: 10.1016/0022-2836(81)90311-9. [DOI] [PubMed] [Google Scholar]
- Keng T., Webster T. A., Sauer R. T., Schimmel P. Gene for Escherichia coli glycyl-tRNA synthetase has tandem subunit coding regions in the same reading frame. J Biol Chem. 1982 Nov 10;257(21):12503–12508. [PubMed] [Google Scholar]
- Kolter R., Yanofsky C. Attenuation in amino acid biosynthetic operons. Annu Rev Genet. 1982;16:113–134. doi: 10.1146/annurev.ge.16.120182.000553. [DOI] [PubMed] [Google Scholar]
- Kovach J. S., Phang J. M., Blasi F., Barton R. W., Ballesteros-Olmo A., Goldberger R. F. Interaction between histidyl transfer ribonucleic acid and the first enzyme for histidine biosynthesis of Salmonella typhimurium. J Bacteriol. 1970 Nov;104(2):787–792. doi: 10.1128/jb.104.2.787-792.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther R. P., Hatfield G. W. Multivalent translational control of transcription termination at attenuator of ilvGEDA operon of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1862–1866. doi: 10.1073/pnas.77.4.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. A., Ames B. N. Histidine regulation in Salmonella typhimurium. XI. The percentage of transfer RNA His charged in vivo and its relation to the repression of the histidine operon. J Mol Biol. 1972 Apr 28;66(1):131–142. doi: 10.1016/s0022-2836(72)80011-1. [DOI] [PubMed] [Google Scholar]
- Meyers M., Blasi F., Bruni C. B., Deeley R. G., Kovach J. S., Levinthal M., Mullinix K. P., Vogel T., Goldberger R. F. Specific binding of the first enzyme for histidine biosynthesis to the DNA of histidine operon. Nucleic Acids Res. 1975 Nov;2(11):2021–2036. doi: 10.1093/nar/2.11.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya J., Ojala D., Attardi G. Distinctive features of the 5'-terminal sequences of the human mitochondrial mRNAs. Nature. 1981 Apr 9;290(5806):465–470. doi: 10.1038/290465a0. [DOI] [PubMed] [Google Scholar]
- Scott J. F., Roth J. R., Artz S. W. Regulation of histidine operon does not require hisG enzyme. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5021–5025. doi: 10.1073/pnas.72.12.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer C. E., Smith G. R., Cortese R., Ames B. N. [Mutant tRNA His ineffective in repression and lacking two pseudouridine modifications]. Nat New Biol. 1972 Jul 19;238(81):72–74. doi: 10.1038/newbio238072a0. [DOI] [PubMed] [Google Scholar]
- Turnbough C. L., Jr, Neill R. J., Landsberg R., Ames B. N. Pseudouridylation of tRNAs and its role in regulation in Salmonella typhimurium. J Biol Chem. 1979 Jun 25;254(12):5111–5119. [PubMed] [Google Scholar]
- Wyche J. H., Ely B., Cebula T. A., Snead M. C., Hartman P. E. Histidyl-transfer ribonucleic acid synthetase in positive control of the histidine operon in Salmonella typhimurium. J Bacteriol. 1974 Feb;117(2):708–716. doi: 10.1128/jb.117.2.708-716.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981 Feb 26;289(5800):751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Brown K., Killingly D., Yanofsky C. Nucleotide sequence of the leader region of the phenylalanine operon of Escherichia coli. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4271–4275. doi: 10.1073/pnas.75.9.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]



