Abstract
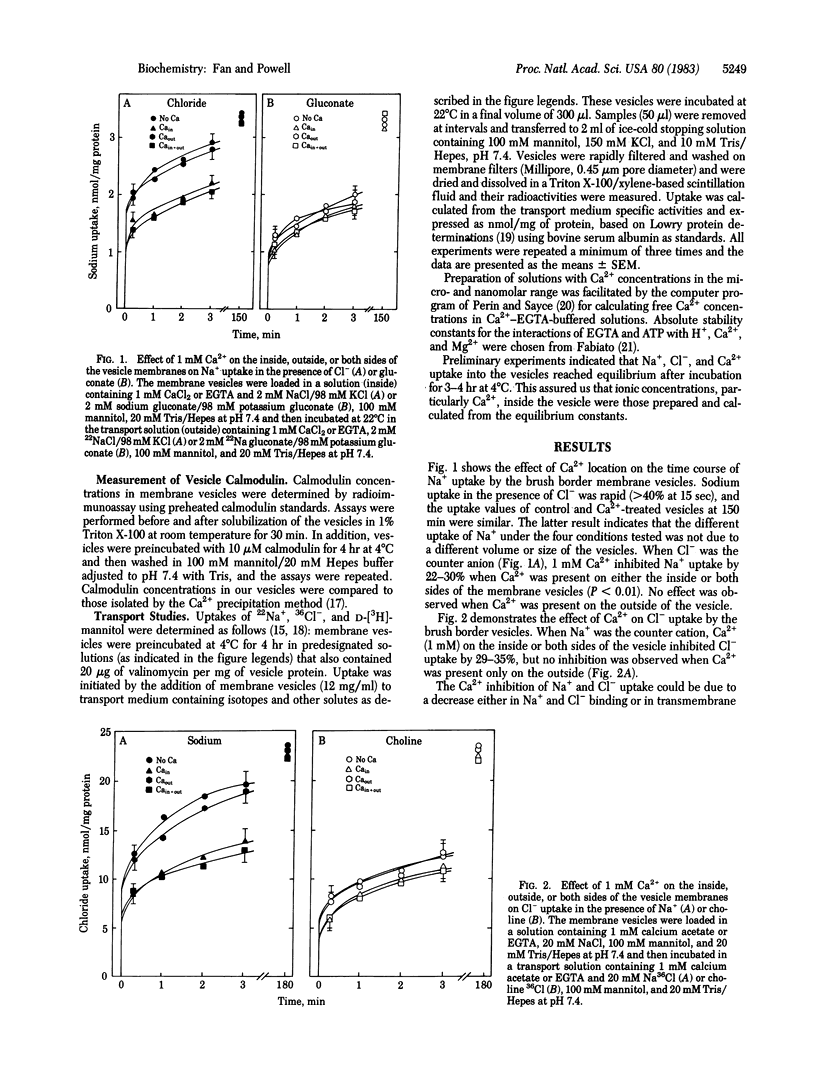
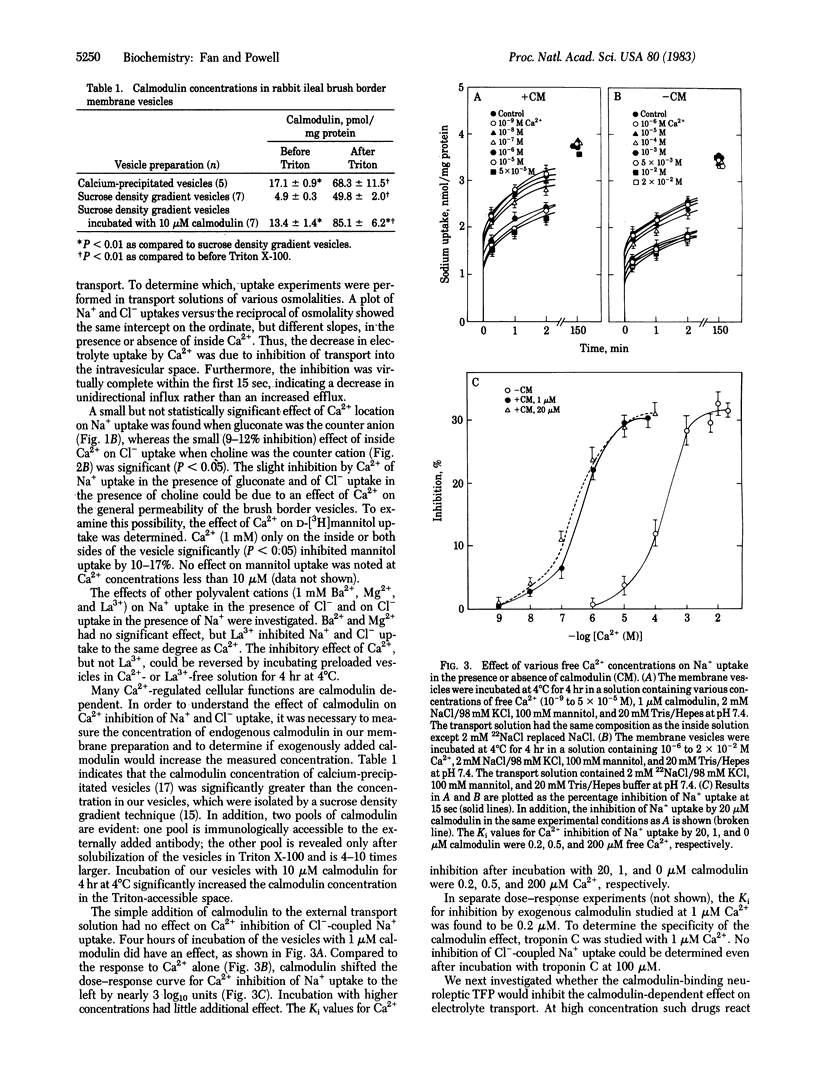
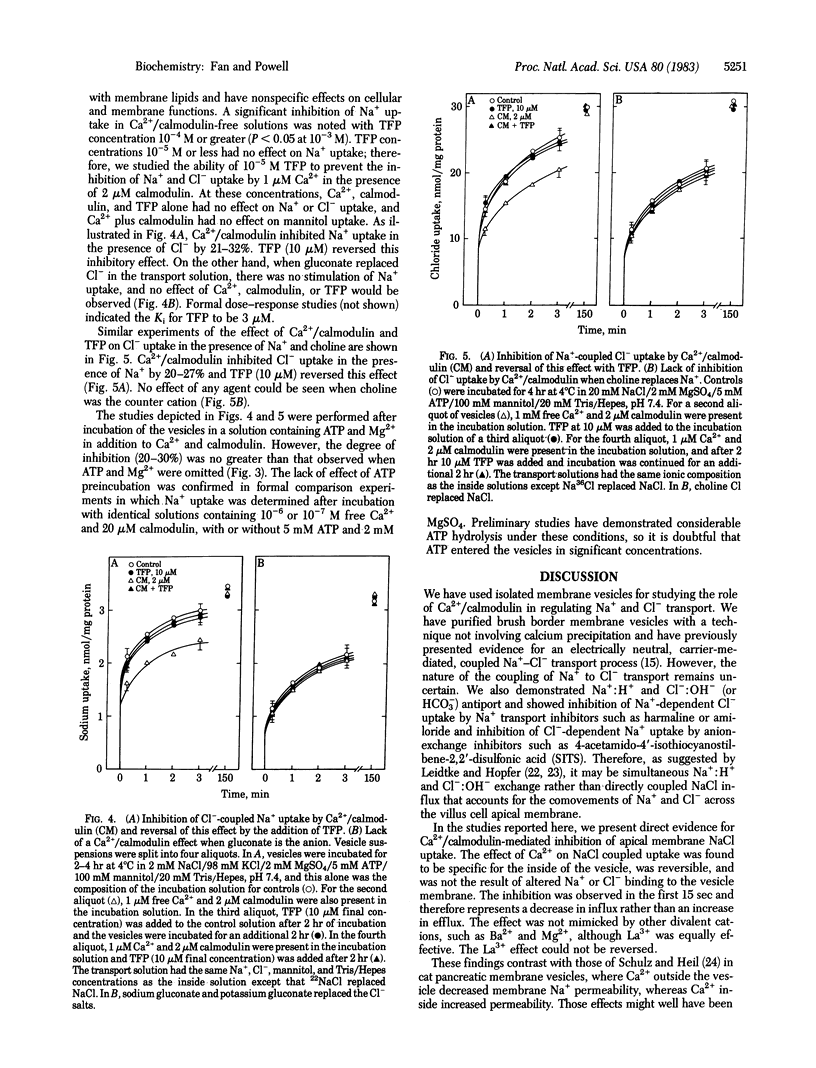
The role of Ca2+ and calmodulin in regulating coupled NaCl transport has been investigated in membrane vesicles from rabbit ileal brush border. Uptake of 22Na+ and 36Cl- was determined by a rapid filtration technique in vesicles isolated with a sucrose density gradient ultracentrifugation method. Ca2+ on the inside of the vesicle inhibited Na+ uptake when Cl- was the anion and Cl- uptake when Na+ was the cation by approximately equal to 30%. Ca2+ on the outside had no effect. When gluconate was the anion or when choline was the cation, Na+ or Cl- uptake was reduced by only 9-12%. A similar inhibition of D-[3H]mannitol uptake (10-17%) suggests this was due to a nonspecific decrease in the membrane permeability. Other cations such as Ba2+ and Mg2+ had no effect, but La3+ inhibited Na+ and Cl- uptake to the same degree as Ca2+. Calmodulin (2 microM) in combination with Ca2+ (1 microM, free concentration) significantly inhibited Na+ uptake when Cl- was the anion by 21-32% and Cl- uptake when Na+ was the cation by 20-27%. This effect was completely reversed by 10 microM trifluoperazine. When gluconate was the anion or when choline was the cation, Na+ or Cl- uptake was unaffected by Ca2+/calmodulin and trifluoperazine. The Ki for Ca2+ inhibition of Cl- -coupled Na+ uptake was reduced from 200 microM to 0.2 microM by incubation with 20 microM calmodulin. The Ki for exogenously added calmodulin studied at 1 microM Ca2+ was 0.2 microM. The Ki for trifluoperazine inhibition of the Ca2+/calmodulin response was 3 microM. These results represent compelling evidence for intracellular Ca2+/calmodulin regulation of coupled NaCl transport across the intestinal microvillus membrane. The exact mechanism of this regulation remains to be delineated.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton J. E., Field M. Ca ionophore-stimulated ion secretion in rabbit ileal mucosa: relation to actions of cyclic 3',5'-AMP and carbamylcholine. J Membr Biol. 1977 Jun 30;35(2):159–173. doi: 10.1007/BF01869947. [DOI] [PubMed] [Google Scholar]
- Campbell K. P., MacLennan D. H. A calmodulin-dependent protein kinase system from skeletal muscle sarcoplasmic reticulum. Phosphorylation of a 60,000-dalton protein. J Biol Chem. 1982 Feb 10;257(3):1238–1246. [PubMed] [Google Scholar]
- Chase H. S., Jr, Al-Awqati Q. Regulation of the sodium permeability of the luminal border of toad bladder by intracellular sodium and calcium: role of sodium-calcium exchange in the basolateral membrane. J Gen Physiol. 1981 Jun;77(6):693–712. doi: 10.1085/jgp.77.6.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Donowitz M., Asarkof N. Calcium dependence of basal electrolyte transport in rabbit ileum. Am J Physiol. 1982 Jul;243(1):G28–G35. doi: 10.1152/ajpgi.1982.243.1.G28. [DOI] [PubMed] [Google Scholar]
- Donowitz M., Asarkof N., Pike G. Calcium dependence of serotonin-induced changes in rabbit ileal electrolyte transport. J Clin Invest. 1980 Aug;66(2):341–352. doi: 10.1172/JCI109862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J Gen Physiol. 1981 Nov;78(5):457–497. doi: 10.1085/jgp.78.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan C. C., Faust R. G., Powell D. W. Coupled sodium-chloride transport by rabbit ileal brush-border membrane vesicles. Am J Physiol. 1983 Apr;244(4):G375–G385. doi: 10.1152/ajpgi.1983.244.4.G375. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A. Active chloride secretion by rabbit colon: calcium-dependent stimulation by ionophore A23187. J Membr Biol. 1977 Jun 30;35(2):175–187. doi: 10.1007/BF01869948. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Dugas M. C., Schultz S. G. Sodium chloride transport by rabbit gallbladder. Direct evidence for a coupled NaCl influx process. J Gen Physiol. 1975 Jun;65(6):769–795. doi: 10.1085/jgp.65.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Weber K. Calmodulin-binding proteins of the microfilaments present in isolated brush borders and microvilli of intestinal epithelial cells. J Biol Chem. 1980 Nov 25;255(22):10551–10554. [PubMed] [Google Scholar]
- Grinstein S., Erlij D. Intracellular calcium and the regulation of sodium transport in the frog skin. Proc R Soc Lond B Biol Sci. 1978 Jul 26;202(1148):353–360. doi: 10.1098/rspb.1978.0072. [DOI] [PubMed] [Google Scholar]
- Haase W., Schäfer A., Murer H., Kinne R. Studies on the orientation of brush-border membrane vesicles. Biochem J. 1978 Apr 15;172(1):57–62. doi: 10.1042/bj1720057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer U., Nelson K., Perrotto J., Isselbacher K. J. Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem. 1973 Jan 10;248(1):25–32. [PubMed] [Google Scholar]
- Howe C. L., Mooseker M. S., Graves T. A. Brush-border calmodulin. A major component of the isolated microvillus core. J Cell Biol. 1980 Jun;85(3):916–923. doi: 10.1083/jcb.85.3.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilundain A., Naftalin R. J. Role of Ca(2+)-dependent regulator protein in intestinal secretion. Nature. 1979 May 31;279(5712):446–448. doi: 10.1038/279446a0. [DOI] [PubMed] [Google Scholar]
- Kessler M., Acuto O., Storelli C., Murer H., Müller M., Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978 Jan 4;506(1):136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Richman P. G. Calmodulin. Annu Rev Biochem. 1980;49:489–515. doi: 10.1146/annurev.bi.49.070180.002421. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landt M., McDaniel M. L., Bry C. G., Kotagal N., Colca J. R., Lacy P. E., McDonald J. M. Calmodulin-activated protein kinase activity in rat pancreatic islet cell membranes. Arch Biochem Biophys. 1982 Jan;213(1):148–154. doi: 10.1016/0003-9861(82)90449-0. [DOI] [PubMed] [Google Scholar]
- Le Peuch C. J., Haiech J., Demaille J. G. Concerted regulation of cardiac sarcoplasmic reticulum calcium transport by cyclic adenosine monophosphate dependent and calcium--calmodulin-dependent phosphorylations. Biochemistry. 1979 Nov 13;18(23):5150–5157. doi: 10.1021/bi00590a019. [DOI] [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Binding of trifluoperazine to the calcium-dependent activator of cyclic nucleotide phosphodiesterase. Mol Pharmacol. 1977 Jul;13(4):690–697. [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Mechanism by which psychotropic drugs inhibit adenosine cyclic 3',5'-monophosphate phosphodiesterase of brain. Mol Pharmacol. 1976 Jul;12(4):581–589. [PubMed] [Google Scholar]
- Liedtke C. M., Hopfer U. Mechanism of Cl- translocation across small intestinal brush-border membrane. I. Absence of Na+-Cl- cotransport. Am J Physiol. 1982 Mar;242(3):G263–G271. doi: 10.1152/ajpgi.1982.242.3.G263. [DOI] [PubMed] [Google Scholar]
- Liedtke C. M., Hopfer U. Mechanism of Cl- translocation across small intestinal brush-border membrane. II. Demonstration of Cl--OH- exchange and Cl- conductance. Am J Physiol. 1982 Mar;242(3):G272–G280. doi: 10.1152/ajpgi.1982.242.3.G272. [DOI] [PubMed] [Google Scholar]
- Merritt J. E., Tomlinson S., Brown B. L. Phenothiazines inhibit prolactin secretion in vitro. A possible role for calmodulin in stimulus--secretion coupling in the pituitary. FEBS Lett. 1981 Nov 30;135(1):107–110. doi: 10.1016/0014-5793(81)80954-4. [DOI] [PubMed] [Google Scholar]
- Nellans H. N., Frizzell R. A., Schultz S. G. Coupled sodium-chloride influx across the brush border of rabbit ileum. Am J Physiol. 1973 Aug;225(2):467–475. doi: 10.1152/ajplegacy.1973.225.2.467. [DOI] [PubMed] [Google Scholar]
- Nellans H. N., Popovitch J. E. Calmodulin-regulated, ATP-driven calcium transport by basolateral membranes of rat small intestine. J Biol Chem. 1981 Oct 10;256(19):9932–9936. [PubMed] [Google Scholar]
- Perrin D. D., Sayce I. G. Computer calculation of equilibrium concentrations in mixtures of metal ions and complexing species. Talanta. 1967 Jul;14(7):833–842. doi: 10.1016/0039-9140(67)80105-x. [DOI] [PubMed] [Google Scholar]
- Pershadsingh H. A., Landt M., McDonald J. M. Calmodulin-sensitive ATP-dependent Ca2+ transport across adipocyte plasma membranes. J Biol Chem. 1980 Oct 10;255(19):8983–8986. [PubMed] [Google Scholar]
- Schulz I., Heil K. Ca2+ control of electrolyte permeability in plasma membrane vesicles from cat pancreas. J Membr Biol. 1979 Apr 12;46(1):41–70. doi: 10.1007/BF01959974. [DOI] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Smith P. L., Field M. In vitro antisecretory effects of trifluoperazine and other neuroleptics in rabbit and human small intestine. Gastroenterology. 1980 Jun;78(6):1545–1553. [PubMed] [Google Scholar]
- Taylor A., Windhager E. E. Possible role of cytosolic calcium and Na-Ca exchange in regulation of transepithelial sodium transport. Am J Physiol. 1979 Jun;236(6):F505–F512. doi: 10.1152/ajprenal.1979.236.6.F505. [DOI] [PubMed] [Google Scholar]
- Taylor L., Guerina V. J., Donowitz M., Cohen M., Sharp G. W. Calcium and calmodulin-dependent protein phosphorylation in rabbit ileum. FEBS Lett. 1981 Aug 31;131(2):322–324. doi: 10.1016/0014-5793(81)80395-x. [DOI] [PubMed] [Google Scholar]
- Waisman D. M., Gimble J. M., Goodman D. B., Rasmussen H. Studies of the Ca2+ transport mechanism of human erythrocyte inside-out plasma membrane vesicles. I. Regulation of the Ca2+ pump by calmodulin. J Biol Chem. 1981 Jan 10;256(1):409–414. [PubMed] [Google Scholar]
- Zimmerman T. W., Dobbins J. W., Binder H. J. Role of calcium in the regulation of colonic secretion in the rat. Am J Physiol. 1983 May;244(5):G552–G560. doi: 10.1152/ajpgi.1983.244.5.G552. [DOI] [PubMed] [Google Scholar]


