Abstract
There remain considerable questions regarding the evidence for population-level handedness in nonhuman primates when compared with humans. One challenge in comparing human and nonhuman primate handedness involves the procedures used to characterize individual handedness. Studies of human handedness use consistency in hand use within and between tasks as a basis for hand preference classification. In contrast, studies of handedness in nonhuman primates use statistical criteria for classifying handedness. In this study, we examined within- and between-task consistency in hand use as a means of characterizing individual handedness in a sample of 300 captive chimpanzees (Pan troglodytes). Chimpanzees showed population-level right-handedness for both within- and between-tasks consistency, though the proportion of right-handed chimpanzees was lower than what has typically been reported for humans. We further found that there were small, but significant, associations in hand use between measures. There were no significant sex or colony effects on the distribution of handedness. The results are discussed in the context of theories on the evolution of handedness in nonhuman primates.
Keywords: handedness, chimpanzees, motor skill, evolution, primates
One of the defining features of the human species is hemispheric specialization. Hemispheric specialization refers to sensory, cognitive, and motor processes that are preferentially performed by the left or right cerebral hemispheres (Hellige, 1993; Hugdahl, 2000; Serrien, Ivry, & Swinnen, 2006). Two of the most pronounced manifestations of functional asymmetries in humans are handedness and left hemisphere lateralization in language. Specifically, though there is some cultural variation, all human populations studied to date show population-level right-handedness (Perelle & Ehrman, 1994; Raymond & Pontier, 2004). Moreover, clinical, experimental, and, more recently, functional imaging studies have consistently reported that the left hemisphere is dominant for language and, in particular, speech (Josse & Tzouio-Mazoyer, 2004; Sommer, Aleman, Somers, Boks, & Kahn, 2008). Additional studies in humans have suggested that there is an inherent link between left hemisphere dominance for language and right-handedness. For example, studies using either the Wada test or Doppler blood flow sonography have reported that a significantly greater majority of right-handed individuals are left hemisphere dominant for language compared with non-right-handed individuals (Knecht et al., 2000, 2001; Rasmussen & Milner, 1977). Still other studies have reported neuroanatomical differences between right- and left-handed individuals in brain regions, such as the motor–hand area of the precentral gyrus, planum temporale, and inferior frontal gyrus (Beaton, 1997; Foundas, Leonard, & Hanna-Pladdy, 2002; Hammond, 2002; Hopkins & Nir, 2010; Kertesz, Polk, Black, & Howell, 1990; Sommer, Ramsey, & Kahn, 2001). In short, there seems to be solid evidence that the functional and anatomical organization of the brain differs between right-handed and non-right-handed individuals, suggesting that handedness may be a potential indicator of hemispheric specialization.
There is considerable historical and contemporary scientific debate over whether nonhuman animals, notably primates, exhibit population-level limb preferences (Ettlinger, 1988; Hopkins, 1999; Hopkins & Cantalupo, 2005; McGrew & Marchant, 1997; Papa-demetriou, Sheu, & Michel, 2005; Warren, 1980). In the past 25 years, there has been a plethora of studies on handedness in a variety of species, with claims of evidence in favor of and against population-level biases in limb use (Frasnelli, Vallortigara, & Rogers, 2012; MacNeilage, Rogers, & Vallortigara, 2009; Vallor-tigara & Rogers, 2005) and hand preference (among primates; Fagot & Vauclair, 1991; Hopkins, 2006; MacNeilage, Studdert-Kennedy, & Lindblom, 1987; McGrew & Marchant, 1993; Ward & Hopkins, 1993). In cases where population-level handedness has been reported in nonhuman primates, some have suggested that these instances represent evidence of “task-specific” hand use that may qualitatively differ from human handedness (Cashmore, 2009; Cashmore, Uomini, & Chapelain, 2008; Diamond & McGrew, 1994; McGrew & Marchant, 1997). Task-specific handedness refers to instances in which the majority of individuals within the sample show the same hand preference, but this bias is only evident for some tasks and not others (Diamond & McGrew, 1994; McGrew & Marchant, 1997). In contrast, it is argued that only humans exhibit “true-handedness,” that is, consistent hand use across multiple measures (i.e., right-hand preferences for multiple tasks). Evidence of so-called true handedness is rare in the non-human primate literature (Diamond & McGrew, 1994; Hopkins & Pearson, 2000), but arguably few investigators have evaluated its presence in their subjects.
An important related, but relatively uninvestigated, issue is consistency in hand use within handedness tasks. In the study of human handedness, researchers typically distinguish between ambiguously handed and ambidextrous subjects. Ambiguously handed individuals are those who show inconsistent hand preferences when tested on the same task. In contrast, ambidextrous individuals are those individuals who show inconsistent hand use across multiple tasks. In the study of handedness in nonhuman primates, very little attention is paid to this distinction in handedness. Indeed, ambiguous and ambidextrous handedness are often treated as synonymous terms and usually describe those individuals who do not show a significant hand preference based on the statistical difference in the frequency of right and left hand use (i.e., z scores).
In the majority of hand preference studies in nonhuman primates, z scores are computed based on the frequency of left and right hand use for any given task. Subjects with z scores >1.95 or <−1.95 are classified as right- or left-handed, whereas individuals with z scores ≥−1.95 or ≤1.95 are classified as ambidextrous or ambiguously handed. One limitation with the use of z scores is that they are not sensitive to variation in consistency in hand use across multiple tests on the same task. For example, one might test a subject twice, and on the first handedness test, they could use the left hand nine times and the right one time. On the second test, the subject might use their left hand three times and their right hand 35 times. The total frequency of left and right hand use would be 12 and 36 responses, respectively, with a resulting z score of 3.46. On the basis of this z score, the subject would be classified as right-handed, even though their hand preferences were inconsistent between the two tests. Additionally, this same problem exists when considering frequencies in right and left hand use across multiple tasks. Unless the number of observations of hand use is consistent across tasks (which is rare in the nonhuman primate handedness literature), any attempts to characterize individual hand preferences on the basis of the total frequency in left and right hand use, when summed across tasks, could bias the characterization of individual hand preferences.
Most importantly, the characterization of individual handedness in nonhuman primates based on z scores is quite different than the methods employed with humans. In human handedness, subjects are typically characterized as right- or left-handed based on consistency in hand use across different handedness tasks or questionnaire items (Annett, 1985; Coren, Porac, & Duncan, 1981; Perelle & Ehrman, 1994). As noted, in studies on nonhuman primate handedness, individual hand preferences are typically based on z scores and not on consistency in hand use within or across hand preference measures. Arguably, this severely limits the extent to which one can make meaningful comparisons of the distribution of handedness between human and nonhuman primates.
The aim of this study was to reconsider the question of population-level handedness in chimpanzees, focusing on consistency, instead of frequency, in hand use as the means by which to characterize individual hand preferences. To accomplish this aim, two studies were conducted in captive chimpanzees. First, in Experiment 1, we sought to assess the consistency in hand use within one handedness task. Specifically, rather than assess individual handedness using z scores based on the frequency of right and left hand use, we tested a group of chimpanzees on the same handedness task on four different occasions. For this study, we used the TUBE task, a handedness measure that requires coordinated bimanual hand use and has previously been shown to reveal population-level right-handedness in chimpanzees when quantified on the basis of frequencies in right and left hand use (Hopkins, Wesley, Izard, Hook, & Schapiro, 2004). Here, individual hand preferences on the TUBE task were made on the basis of their consistency in hand use across the four tests, rather than on total frequency of right and left hand use. Our hypothesis was that if chimpanzees show population-level right-handedness for the TUBE task, then significantly more chimpanzees should exhibit consistent right, compared with left, hand use.
In Experiment 2, we evaluated whether captive chimpanzees show consistent hand preferences across multiple measures of hand use. There is good evidence for task-specific population-level handedness in captive (Hopkins, Taglialatela, Leavens, Russell, & Schapiro, 2010) and, to a lesser extent, wild chimpanzees (Biro et al., 2003; Biro, Sousa, & Matsuzawa, 2006; Boesch, 1991; Humle & Matsuzawa, 2009; Lonsdorf & Hopkins, 2005; Marchant & McGrew, 1996; Marchant & McGrew, 2007; McGrew & Marchant, 2001), but previous studies have not assessed intertask variability in hand use. To test this hypothesis, we measured hand preferences on four different tasks in a sample of chimpanzees. Based on their hand preferences for each task, we quantified the consistency in hand use for each individual chimpanzee using criteria that have been employed to characterize individual handedness in humans (Annett, 1985, 2006; Beaton, 2003). Specifically, we characterized each chimpanzee’s hand preference as right- or left-handed for each task. We then determined how consistent their hand preferences were across the four tasks as a means of characterizing their individual hand preferences. If captive chimpanzees exhibit true population-level right-handedness, as manifested by consistency in hand preference across multiple tasks, then we hypothesized that the number of chimpanzees that preferred the right hand across all four measures would differ significantly from the number of individuals that preferred the left hand for all four tasks. In contrast, if hand preferences are randomly determined across different tasks, then no significant population bias should be evident when considering multiple measures of hand use, and the number of chimpanzees who show consistent left or right hand preferences should not differ within the sample.
Experiment 1
Method
Subjects
Hand preference data were available for 283 captive chimpanzees (Pan troglodytes), including 121 males and 162 females. The chimpanzees ranged from 10 to 51 years of age (M = 21.53, SD = 10.31). The chimpanzees were housed at two research facilities: the Yerkes National Primate Research Center (YNPRC; n = 132) and The University of Texas MD Anderson Cancer Center (UTMDACC; n = 151).
Procedure
Hand use for coordinated bimanual actions was assessed using a measure referred to as the TUBE task. For the TUBE task, peanut butter is smeared on the inside edges of PVC tubes approximately 15 cm in length and 2.5 cm in diameter. Peanut butter is smeared on both ends of the PVC pipe and is placed far enough down the tube such that the subjects cannot lick the contents completely off with their mouths, but rather must use one hand to hold the tube and the other hand to remove the peanut butter. The PVC tubes were handed to the subjects in their home enclosures, and a focal sampling technique was used to collect individual data from each subject. Each subject was tested on four separate occasions that were separated by at least 1 day, and no subjects were tested more than once per day. On average, 7 days separated each test session. During each test, the experimenter recorded right or left hand use for the first 20 responses. Each time the subjects reached into the tube with their finger, extracted peanut butter, and brought it to their mouth, the hand used was recorded as left or right. Eighty responses were obtained from each chimpanzee (20 responses × 4 tests).
Data analysis
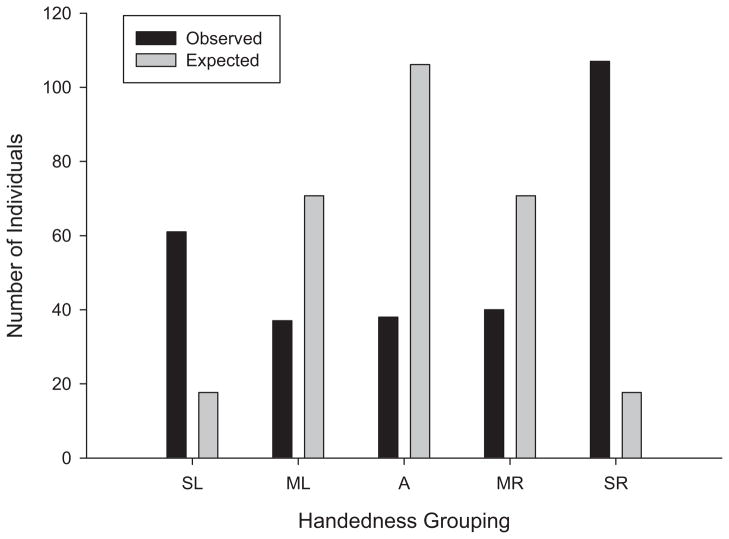
For each of the four tests, we computed a handedness index (HI) following the formula, HI = (R − L)/(R + L), where R and L were the frequencies of left and right hand use. HI values ranged from −1.0 to 1.0, with positive values indicating right hand preferences and negative values indicating left hand preferences. A composite hand preference (CHP) measure was computed for each subject based on the sign of the HI value for each test. The CHP score was computed by assigning a value of 0 or 1, based on the sign of the HI score for each test. Subjects with HI values ≤0 were assigned a value of 0, and those with HI values >0 were assigned a value of 1 for each test. The assigned values were then summed across the four tests, which produced CHP values of 0 (left hand preference for all four tests), 1 (right hand preference for one of the four tests), 2 (right hand preferences for two tests), 3 (right hand preferences for three of the four tests), or 4 (right hand preferences for all four tests). Based on the CHP score, we classified the chimpanzee as strongly left-handed (CHP = 0), moderately left-handed (CHP = 1), ambiguously handed (CHP = 2), moderately right-handed (CHP = 3), or strongly right-handed (CHP = 4). The distribution of handedness based on the CHP scores was then compared between sexes, colonies, and based on rearing history using chi-square tests of independence. Note that, unlike some previous studies that have claimed evidence of true handedness in nonhuman primates (Diamond & McGrew, 1994), the frequencies of left and right hand use were not summed across subjects in this study. Rather, individual handedness was characterized in terms of consistency across the different tests, and therefore each subject’s data contributed equally to the overall distribution of hand preferences. We initially compared the distribution of hand preferences based on the CHP scores using a chi-square goodness-of-fit test. To calculate the expected probabilities, we used the computations and categorization scheme provided in Table 1. The probability of being classified as right- or left-handed on a given test was .5 or 50:50. The probability of any sequence of hand use across the four tests was .0625 or .54. Based on the sequence of the different handedness outcomes, expected probabilities of different hand use combinations across the four tests yields different expected probabilities (see Table 1). We multiplied the summed expected probabilities for each of the five hand preference outcomes by N (or 283) to derive the expected distribution of handedness, under the assumption that hand preference would be randomly distributed across subjects.
Table 1.
Possible Sequences of Hand Use Within Tests and Between Tasks, Associated Probabilities, and Handedness Classification
| Hand use sequence | Expected probability | Handedness classification | |
|---|---|---|---|
| L, L, L, L = |
|
= 6.25% | Strongly left-handed (SL) |
| L, L, L, R = |
|
= 25.0% | Moderately left-handed (ML) |
| L, L, R, L = | |||
| L, R, L, L = | |||
| R, L, L, L = | |||
| R, R, L, L = |
|
= 37.5% | Ambiguously handed or ambidextrous (A) |
| R, L, R, L = | |||
| R, L, L, R = | |||
| L, L, R, R = | |||
| L, R, L, R = | |||
| L, R, R, L = | |||
| R, R, R, L = |
|
= 25.0% | Moderately right-handed (MR) |
| R, R, L, R = | |||
| R, L, R, R = | |||
| L, R, R, R = | |||
| R, R, R, R = |
|
= 6.25% | Strongly right-handed (SR) |
For all analyses, alpha was set at p ≤ .05 and all post hoc tests of inferential statistics were conducted using Tukey’s Honestly Significant Difference (HSD) test.
Results
Descriptive and population-level handedness
Based on the distribution of the CHP scores, chi-square tests of independence failed to reveal significant associations between handedness and either sex, χ2(4, N = 283) = 2.12, ns, or colony χ2(4, N = 283) = 4.27, ns. Therefore, for all subsequent analyses, the data were pooled across subjects. Overall, we found that the hand preference distribution across tests differed significantly from the randomly predicted distribution, χ2 (4, N = 283) = 630.24, p < .001 (see Figure 1). The number of consistently right-handed chimpanzees was significantly higher than the number of moderately right-handed, χ 2(1, N = 147) = 30.54, p < .001, ambiguous, χ2 (1, N = 135) = 32.83, p < .001, moderately left-handed, χ2 (1, N = 144) = 34.03, p < .001, and consistently left-handed, χ2 (1, N = 168) = 12.59, p < .001, chimpanzees. As is traditional in handedness studies, we also calculated one-sample t tests on the HI scores for each test session, as well as the mean HI across test sessions. Significant population-level right hand preferences were found for Test 1 t(282) = 3.228, p < .001, Test 2, t(281) = 3.31, p < .001, Test 3, t(282) = 3.17, p < .001, Test 4, t(282) = 3.70, p < .001, and the average across all tests t(282) = 3.88, p < .001. The mean HI scores for each test, as well as for the average across tests, were .140, .140, .138, .161, and .142, respectively. The Pearson product-moment correlations between the HI scores from the four tests are shown in Table 2. As can be seen, all of the correlations were positive and significant, suggesting consistent individual hand use across the different tests. We next considered the effects of sex and colony on handedness, and no significant results were found.
Figure 1.
Observed and expected values for within-task variation in handedness. A = ambiguously handed; ML = moderately left-handed; MR = moderately right-handed; SL = strongly left-handed; SR = strongly right-handed.
Table 2.
Person Product Moment Correlations Between Different Handedness Tests
| Test 1 | Test 2 | Test 3 | Test 4 | |
|---|---|---|---|---|
| Test 1 | — | |||
| Test 2 | .660** | — | ||
| Test 3 | .598** | .695** | — | |
| Test 4 | .612** | .663** | .739* | — |
p < .05.
p < .01.
Experiment 2
Method
Subjects
Hand preference data were available for 300 captive chimpanzees (Pan troglodytes), including 130 males and 170 females. The chimpanzees ranged from 3 to 51 years of age (M = 21.53, SD = 10.31). The chimpanzees were housed at two research facilities: the Yerkes National Primate Research Center (YNPRC, n = 126) and the University of Texas MD Anderson Cancer Center (UTMDACC, n = 174).
Procedure
Handedness measurement
Handedness was assessed on four measures of hand use previously described in these subjects, including manual gestures, simple reaching, tool use, and a task measuring coordinated bimanual actions, referred to as the TUBE task (Hopkins, 1995a; Hopkins, Russell, Freeman, et al., 2005; Hopkins, Russell, Hook, Braccini, & Schapiro, 2005; Hopkins, Russell, Schaeffer, Gardner, & Schapiro, 2009). These four measures were selected to derive a composite measure of handedness because (a) they each elicit consistent hand preferences in the chimpanzees, and (b) these measures were available in the largest cohort of subjects. A brief description of each measure is provided in the following sections.
Simple reaching
On each trial, a raisin was thrown into the subject’s home enclosure. The raisin was thrown by the experimenter to a location at least 3 m from the focal subject, such that the chimpanzees had to locomote to the location of the raisin, pick up the raisin, and bring it to their mouths for consumption. When the chimpanzee acquired the raisin, the experimenter recorded the hand used as left or right. One, and only one, reaching response was recorded each trial to assure independence of data points. Thus, raisins were not randomly scattered in home enclosures; rather, an individual raisin was thrown into the enclosure and subjects retrieved the raisin before another was thrown in. Subjects were required to locomote at least three strides between reaching responses to allow for postural readjustment between trials. Fifty responses were collected from each subject.
Tool use
Testing was conducted using a device consisting of three PVC pipes (15 cm long, 4 cm diameter) that were glued at 45° angles into three holes (4 cm diameter). The holes were placed horizontally, 15 cm apart, on a rectangular plastic board (50 cm long by 20 cm wide). The end of each glued tube was open to allow access to food at the other end (bottom) of the tube. The bottom end of the tube was comprised of a removable PVC cap. During testing, each PVC tube in the apparatus was first filled with a preferred food that had some adhesive qualities (honey or applesauce) to about one third of the length of the tube. This made it impossible for the subject to reach the food directly with its fingers. After placing the device on the cage, a stick or a bamboo skewer was handed directly to the subjects. The chimpanzees had to insert the small stick/skewer (~.5 cm diameter) into the hole to extract the hidden food. Each time the chimpanzees inserted the stick, a left or right hand response was recorded. A minimum of 50 dipping responses, across at least two test sessions, was recorded from each subject.
TUBE
Hand use for coordinated bimanual actions was assessed using a measure referred to as the TUBE task, as described in Study 1. Each chimpanzee received two tests and a minimum of 30 responses was obtained from each chimpanzee.
Manual gestures
At the onset of each trial, an experimenter would approach the chimpanzee’s home cage and center themselves in front of the chimpanzee at a distance of approximately 1.0 to 1.5 m. If the chimpanzee was not already positioned in front of the experimenter at the onset of the trial, the chimpanzee would immediately move toward the front of the cage when the experimenter arrived with the food. The experimenter then called the chimpanzee’s name and offered a piece of food until the chimpanzee produced a manual gesture. Only responses in which the chimpanzee unimanually extended the digit(s) through the cage mesh to request the food were considered a response. Other possible manual responses such as cage banging or clapping were not counted as a gesture. Two-handed gestures, although rare, were not scored, nor were gestures that were produced by the chimpanzee prior to the experimenter arriving in front of the chimpanzee’s home cage. Thirty responses were obtained from each chimpanzee.
Characterizing hand preference between tasks
For each measure, we computed a handedness index for each subject and measure following the formula, HI = (R − L)/R + L), where R and L indicated the frequencies in right and left hand use. HI values ranged from −1.0 to 1.0, with positive values indicating right hand preferences and negative values indicating left hand preferences. A summary handedness preference (SHP) measure was computed for each subject based on the sign of the HI value for each task. Similar to Experiment 1, the SHP score was computed by assigning a value of 0 or 1, based on the sign of the HI score for each hand preference measure. Subjects with HI values ≤ 0 were assigned a value of 0 and those with HI values >0 were assigned a value of 1 for each task. The assigned values were then summed across the four measures, producing values ranging from included 0 (right hand preference for none of the measures) to 4 (right hand preferences for all four measures), which corresponded to strongly left-handed, moderately left-handed, ambidextrous, moderately right-handed, and strongly right-handed, respectively. The distribution of handedness based on the SHP scores was then compared across sexes, colonies, and rearing histories. Note that, unlike in some previous studies that have claimed evidence of true handedness in nonhuman primates (Diamond & McGrew, 1994), the frequencies in left and right hand use were not summed across subjects in this study. Rather, individual handedness was characterized in terms of consistency across the different tasks, and therefore each subject’s data contributed equally to the overall distribution of hand preferences. For all analyses, alpha was set at p ≤ .05 and all post hoc tests of inferential statistics were conducted using Tukey’s HSD test.
Results
Summary handedness
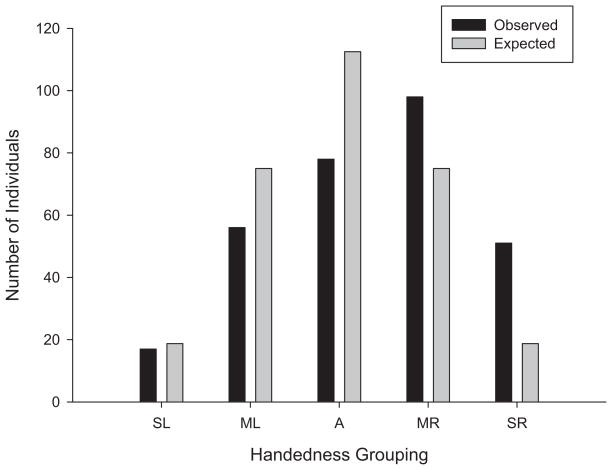
Expected values for each handedness group were derived following the same probabilities and assumptions of random distribution described in Table 1. A chi-square goodness-of-fit test revealed that the distribution of the SHP handedness scores differed significantly from a randomly predicted distribution, χ2(4, N = 300) = 78.08, p < .001 (see Figure 2). In general, there was a higher proportion of right- compared with left-handed and ambidextrous chimpanzees, with the modal pattern being moderately right-handed. Subsequent chi-square goodness-of-fit tests showed that the number of strongly right-handed apes was significantly higher than the number of strongly left-handed individuals, χ2(1, N = 68) = 17.00, p < .001. Similarly, the number of moderately right-handed subjects was significantly higher than the number of moderately left-handed, χ2(1, N = 154) = 11.45, p < .001. We next compared the SHP distribution as a function of sex and colony using chi-square tests of independence. None of the associations between sex, χ2(4, N = 300) = 4.65, ns, colony χ2(4, N = 300) = 3.39, ns, and SHP classification were significant.
Figure 2.
Observed and expected values for between-task variation in handedness. A = ambidextrous; ML = moderately left-handed; MR = moderately right-handed; SL = strongly left-handed; SR = strongly right-handed.
Task-specific comparisons in hand preferences
The mean HI scores, as well as the distribution of right- and left-handed subjects for each task, based on the sign of their HI scores, are shown in Table 3. We initially compared the HI measures for each task as a function of sex and colony using a repeated measures ANOVA. HI scores for each task were the within subjects measure, and sex and colony were the between-group variables. A significant main effect for task was found, F(3, 894) = 20.624, p < .001. Post hoc analysis indicated that the mean HI scores for gestures were significantly higher than all other measures. We further found that HI scores for the TUBE task were significantly higher than simple reaching and the tool use tasks. No significant differences in HI scores were found between the reaching and tool use tasks. No other significant main effects or interactions were found.
Table 3.
Mean HI and Distribution of Handedness for Each Measure
| # L | # R | Mean HI | SE | |
|---|---|---|---|---|
| Tool use | 146 | 154 | .013 | .031 |
| Simple reaching | 137 | 167 | .037 | .023 |
| Tube task | 120 | 180 | .128 | .032 |
| Manual gestures | 86 | 214 | .268 | .027 |
Note. Handedness classification was based on the sign of the HI score. Subjects with positive HI scores were classified as right and subjects with negative HI scores or a score of 0 (this was rare) were classified as left. HI = handedness index; SE = standard error.
After adjusting alpha for multiple tests, one-sample t tests on the HI scores for each measure revealed significant population-level right-handedness for the tube, t(299) = 3.95, p < .001, and gesture, t(299) = 9.60, p < .001, behaviors. Similarly, chi-square goodness-of-fit tests comparing the handedness distributions of the apes, based on the sign of their HI scores, indicated that there were significantly more right- than left-handed individuals for the tube, χ2(1, N = 300) = 12.00, p < .001, and gesture, χ2(1, N = 300) = 54.61, p < .001, behaviors. Finally, we correlated the HI measures for each task across subjects and these results are shown in Table 4. In general, the HI scores for a majority of the tasks were significantly and positively correlated with each other, though the associations were relatively weak.
Table 4.
Pearson Product Moment Correlations Between Hand Preference Measures
| Tool use | Reaching | Tube | Gesture | |
|---|---|---|---|---|
| Tool use | — | |||
| Reaching | .233a | — | ||
| Tube | .179a | .249a | — | |
| Manual gesture | .219a | .211a | .053 | — |
r values significant at p < .001.
Discussion
The results of this study are straightforward. Captive chimpanzees show population-level right-handedness when considering consistency in hand use within and between different tasks. Considering handedness for the TUBE task from Study 1, the findings reported here are consistent with previous studies in chimpanzees for this measure that have used frequency in right and left hand use as the basis for characterizing individual hand preference (Hopkins et al., 2004; Llorente et al., 2010). Thus, the TUBE task seems to be a very sensitive measure of hand preference in chimpanzees, and other nonhuman primates (Hopkins et al., 2011; Meguerditchian, Donnot, Molesti, Francioly, & Vauclair, 2012; Vauclair, Meguerditchian, & Hopkins, 2005; Zhao, Hopkins, & Li, 2012). When considering hand preferences across multiple measures of hand use, a significant majority of the chimpanzees also show right- compared with left-handedness. These findings directly challenge claims that a fundamental distinction between human and nonhuman primate handedness is the existence of true compared with task-specific handedness. That is, chimpanzees show “true handedness” at least as reflected by a significant majority of chimpanzees showing consistent right hand use across multiple measures.
The interpretation of our results is predicated on the assumption that “true” handedness is a human-specific trait that is distinguishable from that observed in nonhuman primates. However, we would question this very assumption as it applies to human handedness. A myriad of studies in human subjects, using both questionnaire data and observational measures, have shown that the degree of handedness varies considerably across measures, with some tasks eliciting a stronger degree of left- or right-handedness compared with other tasks. For example, Bryden (1977) measured handedness in 620 men and 487 women with two questionnaires, one comprised of 14 items (Crovitz-Zener test) and one comprised of 10 items (Oldfield test). When considering the responses for each item within each questionnaire, the percentage of individuals who identified themselves as “always right” or “mostly right” varied from 86% (writing) to as low as 25% (upper hand on a broom). This type of between-item variation is not unusual in the study of human handedness and, in fact, reflects task-specific handedness (i.e., some measures are better than others in eliciting consistent hand preferences across subjects; see also Salmaso & Longoni, 1985). Similar evidence of task-specific handedness has also been reported in observational studies of hand use in traditional human societies. Marchant, McGrew, and Eibl-Eibesfeldt (1995) observed hand use in three different traditional studies for a variety of motor actions and found that, overall, there was weak right-handedness in these populations. Importantly, some measures, such as tool use, elicited significantly more robust asymmetries in hand use than other actions, which is indicative of task-specific handedness. Thus, the underlying assumption that human handedness is consistent across all measures of hand use is not supported by the published literature.
We are not suggesting that human and chimpanzee handedness does not differ; in fact, the results from this study are consistent with the view that captive chimpanzees show population-level right-handedness, but their consistency in hand use across tasks is not as robust as has been reported in humans. Putting aside, for the moment, subjects classified as ambidextrous, the ratio of right-(number of MR + SR subjects) to left-handed (number of ML + SL subjects) apes (R:L ratio) is 2.11:1, which is substantially lower than the often-cited 8:1 to 9:1 ratio reported in humans (Beaton, 2003). Our results suggest that both humans and chimpanzees show task-specific handedness, but humans appear to exhibit more consistent right hand use across multiple measures than do chimpanzees. The greater consistency in right hand use in human’s results in a significantly higher majority being classified as right-compared with left-handed.
An important issue in the nonhuman primate handedness literature is whether the evidence of population-level handedness is restricted to captive populations of primates or whether the data generalize to individuals living in the wild. The present study was conducted on chimpanzees living in captivity and does not directly address this important matter. Nonetheless, it is of note that when population-level handedness has been found in wild chimpanzees, for tasks such as leaf sponging, ant dipping, or termite fishing, a similar 2:1 ratio of right-to-left-handed individuals has been found in captive chimpanzees (or left-to-right, in the case of termite fishing; Hopkins et al., 2010); thus, what we are reporting here in captive chimpanzees does not seem to be an anomaly or an artifact of testing captive apes. Indeed, in one of the only studies that has reported hand use for multiple measures in wild chimpanzees, for tasks that induce significant hand preference measures at the individual level (Humle & Matsuzawa, 2009), the results are not all that different from the findings reported here. Specifically, Humle and Matsuzawa (2009) reported individual hand preferences for five measures in the wild chimpanzees from Bossou, including ant dipping, nut cracking, algae dipping, pestle pounding, and reaching. Based on the sign of the HI values for each of these measures reported by Humle and Matsuzawa (2009), we assigned weighted scores of 1 or 0 and then computed the proportion of subjects that were SR, MR, A, ML, and SL. Within the sample of 23 chimpanzees, there were 10 SR, 4 MR, 4 A, 4 ML, and 1 SL individuals. Thus, though the sample size is relatively small, the distribution is clearly skewed rightward in a manner not unlike the findings presented here. It should be noted that several studies on spontaneous hand use in wild chimpanzees have examined multiple measures of handedness and have claimed that apes fail to show population-level asymmetries (Marchant & McGrew, 1996; McGrew & Marchant, 2001); however, none of the behaviors of interest in these studies actually elicited significant hand preferences at the individual level of analysis. Thus, we believe the validity of these findings is questionable, due to the simple fact that the behaviors were very poor at actually measuring individual hand preference. In short, if one wants to measure handedness in chimpanzees (and, indeed, all primates), it is absolutely paramount that the behaviors or task to be quantified result in subjects exhibiting consistent hand preferences.
Why the discrepancy in ratio of right-to-left handedness exists between humans and chimpanzees is unclear, but there are several possible explanations to be considered. First, the differences in the R:L ratio might be attributable to measurement discrepancies. Adult human handedness is typically measured by self-report questionnaire, whereas chimpanzee handedness (and, indeed, all primate handedness) is measured by observing the subjects’ behavior. The simple fact that one species is being measured by self-report for a trait that is clearly influenced by sociological and cultural factors, whereas the other species is not, introduces an element of measurement error and creates difficult challenges for direct comparison between data sets. Moreover, the manner in which handedness is quantified varies dramatically, in part, because the measurements of handedness in the two species are on different scales. This, in turn, necessitates or results in the application of different statistical procedures and approaches for the quantification and classification of individuals’ handedness (Hopkins, 1999). There are some exceptions to the assessment of human handedness (or other forms of laterality) that utilize self-report questionnaires, notably, studies of hand preference in young, developing children. With respect to studies in developing children, preschool individuals typically show less pronounced manifestations of right-handedness than is typically reported in adult humans (Coren et al., 1981; Curt, Maccario, & Dellatolas, 1992). For example, Fagard and Marks (2000) measured handedness for grasping, unimanual manipulation, and bimanual manipulation in a sample of 40 infants ranging from 18 to 35 months of age. Though there were trends toward right-handedness within the sample, based on the hand preference classification criteria used in this study, for no measure did the proportion of right-handed individuals exceed 65%, a value far lower than is typically reported in adult humans. Similarly, Coren, Porac, and Duncan (1981) measured handedness in 384 preschool children for four measures: throwing a ball, pointing, drawing with a crayon, and touching one’s nose. Within this sample, 68% used their right hand for all four measures compared with 81% in a sample of young adults. Differences in the magnitude of expression of right-handedness between adults and children are often attributed to maturational factors. This is certainly possible and cannot be ruled out; however, just as plausible are methodological differences in the assessment and quantification of handedness between the age groups.
Another possibility is that the 2:1 right-to-left handedness ratio found in chimpanzees, and in young human children, reflects the biological expression of handedness, whereas the data from adult humans reflects the additive effects of sociological and cultural conformity to this biological expression. In other words, what is manifest in adult humans by way of handedness is shifted more strongly to the right hand due to sociological (or reinforcement) or cultural conformity. Similar types of explanations have been proposed to explain differences in both the direction and strength of handedness between human adults and children (McManus, Sik, Cole, & Mellon, 1988). The idea is that infants begin to use their hands for various functions early in life and their differential use is either intrinsically or extrinsically reinforced, which subsequently leads to increased preferential use of the hand over time. In the case of humans, the assumption is that there is an intrinsically small, but nonetheless statistically significant, preference for right-handedness early in life and this gets reinforced (in very broad terms), resulting in an increased strength in the use of the right hand over time. Adding to this effect would be sociocultural norms toward preferential use of the hands when developing children enter institutions that may or may not impose some constraints on preferential hand use, such as on the hand used for writing. For chimpanzees, they likewise may show preferential use of the right hand early in life (Fagot & Bard, 1995; Hopkins, 1995b; Hopkins & Bard, 1993, 1995, 2000), which may or may not be reinforced during development. What would be absent in the chimpanzees is the sociocultural or institutional conformity toward preferential use of one hand over the other. Thus, chimpanzees never dramatically shift toward preferential use of the right hand due to the lack of societal rules governing conformity toward preferential use of one hand or another. The role of sociocultural traditions in the expression of handedness has been proposed as one of the major variables influencing handedness in different human societies (Perelle & Ehrman, 1994; Raymond & Pontier, 2004).
Third, it could be there have been some genetic changes in the human brain that have resulted in the robust expression of right-handedness relative to chimpanzees (Annett, 2006; Corballis, 1997). For example, Williams, Close, Giouzeli, and Crow (2006) suggested that brain asymmetries in humans evolved as a consequence of a duplication of the Protocadherin11X/Y gene, which is found only on the X chromosome of apes but is represented on both the X and Y chromosomes of humans. They argue that this duplication is necessary for the development of language and the associated brain asymmetries found in human, but not in nonhuman primate, brains (Williams et al., 2006). One problem with this view is that it is saltational; that is, it implies that behavioral and brain asymmetries evolved de novo in humans after the split with the common ancestor with chimpanzees. The results reported here are not consistent with this viewpoint. Alternatively, Annett (2006) proposed that both humans and chimpanzees exhibit a right-shift (RS) in handedness that may be genetically determined, but the shift is much more robust in humans. Specifically, Annett (2006) has estimated that RS in humans is approximately −1.0 z score away from a normal distribution prediction of zero, whereas she estimated the RS in chimpanzees to be −0.32, based on the previously published data on the TUBE task (Hopkins, Cantalupo, et al., 2005). Using the same formula used by Annett (2006), we estimated the RS for the CHP data reported here and found it to be −0.30, which is similar to her original analyses of previously published chimpanzee data. These findings are consistent with the claim that chimpanzees are right-handed, but to a lesser degree than humans. If genetic mechanisms explain the differences between human and chimpanzee handedness (and brain asymmetries), it is not clear which genes are involved and whether those genes determine the direction or strength of asymmetry (Francks et al., 2002; Klar, 1999; Sun & Walsh, 2006). It is also possible that different genetic or nongenetic mechanisms underlie different dimensions of functional and anatomical asymmetries (Corballis, Badzakova-Trajkov, & Haberling, 2012; Liu, Stufflebeam, Sepukre, Hedden, & Buckner, 2009) We would also caution that there are reports of limb preferences in animals that rival the typical 8:1 or 9:1 ratio reported in humans (Rogers & Andrew, 2002), and these findings are difficult to reconcile with this interpretation.
Fourth, it is possible that unique human adaptations that occurred after the split from the common ancestor with chimpanzees had facilitative effects on the expression of right-handedness in modern humans. Two possibilities have been discussed and include language and bipedalism (Bradshaw & Rogers, 1993). With respect to language, increasing selection for motor control of the mouth and peripheral articulatory muscles needed for speech in humans had a facilitative effect on the expression of right-handedness in humans relative to chimpanzees. It has been well established that preferential use of the right hand for manual gestures is associated with speech activity in adult humans (Kimura, 1993) and developing children (Cochet & Vauclair, 2010a, 2010b; Iverson & Thelen, 1999). In this theory, a small but significant degree of right-handedness was evident in the common ancestor of humans and chimpanzees. After the split, there was increasing selection for motor control of speech in humans, which capitalized on the inherent left hemisphere specialization for manual motor control. The increased selection in motor control needed for speech resulted in subsequent increasing manual motor skill, both of which were controlled by the left hemisphere (MacNeilage, 2008). Alternatively, when humans evolved bipedal locomotion, the hands became completely free of their locomotor functions and thereby became increasingly specialized for manipulative (Forrester, Leavens, Quaresmini, & Vallortigara, 2011) and, potentially, communicative functions (see Bradshaw & Rogers, 1993).
Finally, we would suggest that the types of measures used to assess hand preferences in human and nonhuman primates differ in their sensitivity to detecting individual hand preferences, and this may have some impact on the characterization of hand preference when considered across multiple measures. For instance, as shown in Table 3, hand preferences for manual gestures and the TUBE task elicit stronger right hand use than simple reaching and the tool use task (as revealed by the higher AQ values). Between-task differences are also manifest in patterns of the distribution of handedness. To illustrate this point, we have plotted frequency distributions in handedness in Figure 3 based on the range of AQ scores for each of the four tasks. As can be seen, for manual gestures and the TUBE task, the frequency distribution is more skewed, with the mode preference being subjects with AQ scores > .80. In other words, the most frequent hand preferences were on the far end of the positive AQ distribution. In contrast, for simple reaching and the tool use task, the patterns are more randomly distributed, with the modal patterns being at the +.20 and −.20 cut points, respectively. In other words, the majority of individuals were weakly right- and left-handed on these two measures. Arguably, simple reaching and tool use do not elicit strong hand preferences in most individuals, and if these two measures were replaced with measures that were more sensitive, then the distribution of hand preference when computed across multiple measures might look quite different from those shown in Figure 2 and often reported in humans. In our case, the measures were selected on the basis of convenience (easy to obtain), and we had the largest sample of possible subjects on these tasks. The results would perhaps be different if tasks were selected on the basis of their sensitivity to detecting individual hand preferences.
Figure 3.
Frequency distribution of hand preference based on the handedness index (HI) scores binned in intervals of .20 units. Negative values indicate left hand preferences, whereas positive values indicate right hand preferences.
The question of task selectively is by no means trivial in the context of comparative studies of handedness between human and nonhuman primates. In the human neuropsychological literature, there has been considerable research effort to develop instruments, usually in the form of questionnaires that presumably quantify the underling trait we call handedness. For example, in the Edinburgh handedness inventory originally developed by Oldfield (1971), there were 20 items, but since that time, and even within the very same study, the number of items was reduced to 10. Item reduction was not just randomly determined but was based on cultural, psychometric, and heuristic criteria based on the pattern of handedness for a specific item.
In the nonhuman primate literature, scientists have paid very little attention to this problem, and we would argue that this has created some confusion and inconsistencies in the literature. This is a particular problem for studies that have focused on measuring hand use for daily or spontaneous activities, where there is little control over situational or positional factors that might influence hand use (Harrison & Nystrom, 2008, 2010; Marchant & McGrew, 1996; McGrew & Marchant, 2001; Mittra, Fuentes, & McGrew, 1997). Most studies that have measured hand use for spontaneous or daily behaviors have reported nonsignificant findings; that is, these authors typically find that a statistical majority of subjects fail to show a significant right or left hand preference (based on z scores), but rather, most are classified as nonpreferent. From these findings, the authors subsequently (and rightfully) conclude that their particular species does not show evidence of population-level handedness. Certainly, a lack of individual hand preferences as a trait is a possible and feasible outcome for any species, and this should not be ignored; however, there is also the alternative interpretation that the measures of hand use in these studies are not sensitive enough to detect individual preferences or the handedness trait. From a psychometric perspective, it could be argued that if a series of measures fails to elicit individual hand preferences, then they are probably not measuring the presumed trait.
In summary, the results reported suggest both convergent and divergent patterns of handedness in chimpanzees compared with humans. One the one hand, chimpanzees show population-level handedness when considered across multiple measures of hand use, a trait some believe is unique to humans. On the other hand, the magnitude of right-handedness in chimpanzees is far less than that typically reported in the human literature. What remains unclear is whether these differences are an artifact of the methods used to assess handedness in humans and chimpanzees (and other nonhuman primates) or whether they reflect inherent biological differences between the species.
Acknowledgments
This research was supported in part by NIH Grants NS-42867, NS-73134, HD-60563, and HD-56232, as well as NIH cooperative agreement U42 RR15090 to UTMDACC. American Psychological Association guidelines for the ethical treatment of animals were adhered to during all aspects of this study. We thank two anonymous reviewers and Michael Corballis for helpful comments on this article.
Contributor Information
William D. Hopkins, Division of Developmental and Cognitive Neuroscience, Yerkes National Primate Research Center, Atlanta, Georgia, and Neuroscience Institute and Language Research Center, Georgia State University
Molly Gardner, Department of Veterinary Sciences, The University of Texas MD Anderson Cancer Center, Bastrop, Texas.
Morgan Mingle, Department of Veterinary Sciences, The University of Texas MD Anderson Cancer Center, Bastrop, Texas.
Lisa Reamer, Department of Veterinary Sciences, The University of Texas MD Anderson Cancer Center, Bastrop, Texas.
Steven J. Schapiro, Department of Veterinary Sciences, The University of Texas MD Anderson Cancer Center, Bastrop, Texas, and Department of Experimental Medicine, University of Copenhagen, Copenhagen, Denmark
References
- Annett M. Left, right, hand, and brain: The right-shift theory. London, UK: Lawrence Erlbaum; 1985. [Google Scholar]
- Annett M. The distribution of handedness in chimpanzees: Estimating right shift from the Hopkins’ sample. Laterality: Asymmetries of Body, Brain and Cognition. 2006;11:101–109. doi: 10.1080/13576500500376500. 10.1080/ 13576500500376500. [DOI] [PubMed] [Google Scholar]
- Beaton AA. The relation of planum temporale asymmetry and morphology of the corpus callosum to handedness, gender and dyslexia: A review of the evidence. Brain and Language. 1997;60:255–322. doi: 10.1006/brln.1997.1825. [DOI] [PubMed] [Google Scholar]
- Beaton AA. The nature and determination of handedness. In: Hugdahl K, Davidson RJ, editors. The asymmetrical brain. Cambridge, MA: MIT Press; 2003. pp. 105–158. [Google Scholar]
- Biro D, Inoue-Nakamura N, Tonooka R, Yamakoshi G, Sousa C, Matsuzawa T. Cultural innovation and transmission of tool use in wild chimpanzees: Evidence from field experiments. Animal Cognition. 2003;6:213–223. doi: 10.1007/s10071-003-0183-x. [DOI] [PubMed] [Google Scholar]
- Biro D, Sousa C, Matsuzawa T. Ontogeny and cultural propagation of tool use by wild chimpanzees at Bossou, Guinea: Case studies in nut cracking and leaf folding. In: Matsuzawa T, Tomonaga T, Tanaka M, editors. Cognitive development of chimpanzees. New York, NY: Springer; 2006. pp. 476–508. [DOI] [Google Scholar]
- Boesch C. Handedness in wild chimpanzees. International Journal of Primatology. 1991;12:541–558. doi: 10.1007/BF02547669. [DOI] [Google Scholar]
- Bradshaw JL, Rogers LJ. The evolution of lateral asymmetries, language, tool use, and intellect. San Diego, CA: Academic Press; 1993. [Google Scholar]
- Bryden MP. Measuring handedness with questionnaires. Neuropsychologia. 1977;15:617–624. doi: 10.1016/0028-3932(77)90067-7. [DOI] [PubMed] [Google Scholar]
- Cashmore L. Can hominin “handedness” be accurately assessed? Annals of Human Biology. 2009;36:624–641. doi: 10.1080/03014460902956733. 10.1080/ 03014460902956733. [DOI] [PubMed] [Google Scholar]
- Cashmore L, Uomini N, Chapelain A. The evolution of handedness in humans and great apes: A review and current issues. Journal of Anthropological Sciences. 2008;86:7–35. [PubMed] [Google Scholar]
- Cochet H, Vauclair J. Features of spontaneous pointing gestures in toddlers. Gesture. 2010a;10:86–107. doi: 10.1075/gest.10.1.05coc. [DOI] [Google Scholar]
- Cochet H, Vauclair J. Pointing gestures produced by toddlers from 15 to 30 months: Different functions, hand shapes and laterality patterns. Infant Behavior & Development. 2010b;33:431–441. doi: 10.1016/j.infbeh.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Corballis MC. The genetics and evolution of handedness. Psychological Review. 1997;104:714–727. doi: 10.1037/0033-295X.104.4.714. [DOI] [PubMed] [Google Scholar]
- Corballis MC, Badzakova-Trajkov G, Haberling IS. Right hand, left brain: Genetic and evolutionary basis of cerebral asymmetries for language and manual action. WIREs Cognitive Science. 2012;3:1–17. doi: 10.1002/wcs.158. [DOI] [PubMed] [Google Scholar]
- Coren S, Porac C, Duncan P. Lateral preference behaviors in preschool children and young adults. Child Development. 1981;52:443–450. doi: 10.2307/1129160. [DOI] [Google Scholar]
- Curt F, Maccario J, Dellatolas G. Distributions of hand preference and hand skill asymmetry in preschool children: Theoretical implications. Neuropsychologia. 1992;30:27–34. doi: 10.1016/0028-3932(92)90011-A. [DOI] [PubMed] [Google Scholar]
- Diamond AC, McGrew WC. True handedness in the cotton-top tamarin (Saguinus oedipus)? Primates. 1994;35:69–77. doi: 10.1007/BF02381487. [DOI] [Google Scholar]
- Ettlinger G. Hand preference, ability and hemispheric specialization. How far are these factors related in the monkey? Cortex: A Journal Devoted to the Study of the Nervous System and Behavior. 1988;24:389–398. doi: 10.1016/s0010-9452(88)80002-9. [DOI] [PubMed] [Google Scholar]
- Fagard J, Marks A. Unimanual and bimanual tasks and the assessment of handedness in toddlers. Developmental Science. 2000;3:137–147. doi: 10.1111/1467-7687.00107. [DOI] [Google Scholar]
- Fagot J, Bard KA. Asymmetric grasping response in neonate chimpanzees (Pan troglodytes) Infant Behavior & Development. 1995;18:253–255. doi: 10.1016/0163-6383(95)90054-3. [DOI] [Google Scholar]
- Fagot J, Vauclair J. Manual laterality in nonhuman primates: A distinction between handedness and manual specialization. Psychological Bulletin. 1991;109:76–89. doi: 10.1037/0033-2909.109.1.76. [DOI] [PubMed] [Google Scholar]
- Forrester GS, Leavens DA, Quaresmini C, Vallortigara G. Target animacy influences gorilla handedness. Animal Cognition. 2011;14:903–907. doi: 10.1007/s10071-011-0413-6. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Leonard CM, Hanna-Pladdy B. Variability in the anatomy of the planum temporale and posterior ascending ramus: Do right- and left handers differ? Brain and Language. 2002;83:403–424. doi: 10.1016/S0093-934X(02)00509-6. [DOI] [PubMed] [Google Scholar]
- Francks C, Fisher SE, MacPhie L, Richardson AJ, Marlow AJ, Stein JF. A genomewide linkage screen for relative hand skill in sibling pairs. American Journal of Human Genetics. 2002;70:800–805. doi: 10.1086/339249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasnelli E, Vallortigara G, Rogers LJ. Left-right asymmetries of behaviour and nervous system in invertebrates. Neuroscience and Biobehavioral Reviews. 2012;36:1273–1291. doi: 10.1016/j.neubiorev.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Hammond G. Correlates of human handedness in primary motor cortex: A review and hypothesis. Neuroscience and Biobehavioral Reviews. 2002;26:285–292. doi: 10.1016/S0149-7634(02)00003-9. [DOI] [PubMed] [Google Scholar]
- Harrison RM, Nystrom P. Handedness in captive bonobos (Pan paniscus) Folia Primatologica; International Journal of Primatology. 2008;79:253–268. doi: 10.1159/000113539. [DOI] [PubMed] [Google Scholar]
- Harrison RM, Nystrom P. Handedness in captive gorillas (Gorilla gorilla) Primates. 2010;51:251–261. doi: 10.1007/s10329-010-0191-9. [DOI] [PubMed] [Google Scholar]
- Hellige JB. Hemispheric asymmetry: What’s right and what’s left? Cambridge, MA: Harvard University Press; 1993. [Google Scholar]
- Hopkins WD. Hand preferences for a coordinated bimanual task in 110 chimpanzees: Cross-sectional analysis. Journal of Comparative Psychology. 1995a;109:291–297. doi: 10.1037/0735-7036.109.3.291. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Hand preferences in juvenile chimpanzees: Continuity in development. Developmental Psychology. 1995b;31:619–625. doi: 10.1037/0012-1649.31.4.619. [DOI] [Google Scholar]
- Hopkins WD. On the other hand: Statistical issues in the assessment and interpretation of hand preference data in non-human primates. International Journal of Primatology. 1999;20:851–866. 10.1023/A: 1020822401195. [Google Scholar]
- Hopkins WD. Comparative and familial analysis of handedness in great apes. Psychological Bulletin. 2006;132:538–559. doi: 10.1037/0033-2909.132.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Bard KA. Hemispheric specialization in infant chimpanzees (Pan troglodytes): Evidence for a relation with gender and arousal. Developmental Psychobiology. 1993;26:219–235. doi: 10.1002/dev.420260405. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Bard KA. Evidence of asymmetries in spontaneous head turning in infant chimpanzees (Pan troglodytes) Behavioral Neuroscience. 1995;109:808–812. doi: 10.1037/0735-7044.109.4.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Bard KA. A longitudinal study of hand preference in chimpanzees (Pan troglodytes) Developmental Psychobiology. 2000;36:292–300. 10.1002/(SICI)1098-2302(200005)36:4<292:: AID-DEV4>3.0.CO;2-T. [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Cantalupo C. Individual and setting differences in the hand preferences of chimpanzees (Pan troglodytes): A critical analysis and some alternative explanations. Laterality: Asymmetries of Body, Brain and Cognition. 2005;10:65–80. doi: 10.1080/13576500342000301. 10.1080/ 13576500342000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Cantalupo C, Freeman H, Russell J, Kachin M, Nelson E. Chimpanzees are right-handed when recording bouts of hand use. Laterality: Asymmetries of Body, Brain and Cognition. 2005;10:121–130. doi: 10.1080/13576500342000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Nir T. Planum temporale surface area and grey matter asymmetries in chimpanzees (Pan troglodytes): The effect of handedness and comparison within findings in humans. Behavioural Brain Research. 2010;208:436–443. doi: 10.1016/j.bbr.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Pearson K. Chimpanzee (Pan troglodytes) handedness: Variability across multiple measures of hand use. Journal of Comparative Psychology. 2000;114:126–135. doi: 10.1037/0735-7036.114.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Phillips KA, Bania A, Calcutt SE, Gardner M, Russell JL, Schapiro SJ. Hand preferences for coordinated bimanual actions in 777 great apes: Implications for the evolution of handedness in hominins. Journal of Human Evolution. 2011;60:605–611. doi: 10.1016/j.jhevol.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Russell J, Freeman H, Buehler N, Reynolds E, Schapiro S. The distribution and development of handedness for manual gestures in captive chimpanzees (Pan troglodytes) Psychological Science. 2005;16:487–493. doi: 10.1111/j.0956-7976.2005.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Russell J, Hook M, Braccini S, Schapiro S. Simple reaching is not so simple: Association between hand use and grip preferences in captive chimpanzees. International Journal of Primatology. 2005;26:259–277. doi: 10.1007/s10764-005-2924-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Russell JL, Schaeffer JA, Gardner M, Schapiro SJ. Handedness for tool use in captive chimpanzees (Pan troglodytes): Sex differences, performance, heritability and comparison to the wild. Behaviour. 2009;146:1463–1483. doi: 10.1163/156853909X441005. 10.1163/ 156853909X441005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Taglialatela J, Leavens DA, Russell JL, Schapiro SJ. Behavioral and brain asymmetries in chimpanzees. In: Lonsdorf EV, Ross SR, Matsuzawa T, editors. The mind of the chimpanzee. Chicago, IL: University of Chicago Press; 2010. pp. 60–74. [Google Scholar]
- Hopkins WD, Wesley MJ, Izard MK, Hook M, Schapiro SJ. Chimpanzees are predominantly right-handed: Replication in three colonies of apes. Behavioral Neuroscience. 2004;118:659–663. doi: 10.1037/0735-7044.118.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugdahl K. Lateralization of cognitive processes in the brain. Acta Psychologica. 2000;105:211–235. doi: 10.1016/S0001-6918(00)00062-7. [DOI] [PubMed] [Google Scholar]
- Humle T, Matsuzawa T. Laterality in hand use across four tool use behaviors among the wild chimpanzees of Bossou, Guinea, West Africa. American Journal of Primatology. 2009;71:40–48. doi: 10.1002/ajp.20616. [DOI] [PubMed] [Google Scholar]
- Iverson JM, Thelen E. Hand, mouth and brain. Journal of Consciousness Studies. 1999;6:19–40. [Google Scholar]
- Josse G, Tzouio-Mazoyer N. Hemispheric specialization for language. Brain Research Reviews. 2004;44:1–12. doi: 10.1016/j.brainresrev.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Polk M, Black SE, Howell J. Sex, handedness and the morphometry of cerebral hemispheres on magnetic resonance imaging. Brain Research. 1990;530:40–48. doi: 10.1016/0006-8993(90)90655-U. [DOI] [PubMed] [Google Scholar]
- Kimura D. Neuromotor mechanisms in human communication. New York, NY: Oxford University Press; 1993. 10. 1093/acprof:oso/ 9780195054927. 001. 0001. [Google Scholar]
- Klar AJ. Genetic models of handedness, brain lateralization, schizophrenia, and manic-depression. Schizophrenia Research. 1999;39:207–218. doi: 10.1016/S0920-9964(99)00075-4. [DOI] [PubMed] [Google Scholar]
- Knecht S, Drager B, Deppe M, Bobe L, Lohmann H, Floel A, Henningsen H. Handedness and hemispheric language dominance in healthy humans. Brain: A Journal of Neurology. 2000;123:2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- Knecht S, Drager B, Floel A, Lohmann H, Breitenstein C, Deppe M, Ringelstein EB. Behavioural relevance of atypical language lateralization in healthy subjects. Brain: A Journal of Neurology. 2001;124:1657–1665. doi: 10.1093/brain/124.8.1657. [DOI] [PubMed] [Google Scholar]
- Liu H, Stufflebeam SM, Sepukre J, Hedden T, Buckner RL. Evidence from intrinsic activity that asymmetry of the human brain is controlled by multiple factors. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20499–20503. doi: 10.1073/pnas.0908073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente A, Palou L, Carrasco L, Riba D, Mosquera M, Colell M, Feliu O. Population-level right handedness for a coordinated bimanual task in naturalistic housed chimpanzees: Replication and extension in 114 animals from Zambia and Spain. American Journal of Primatology. 2010;73:1–10. doi: 10.1002/ajp.20895. [DOI] [PubMed] [Google Scholar]
- Lonsdorf EV, Hopkins WD. Wild chimpanzees show population level handedness for tool use. Proceedings of the National Academy of Sciences. 2005;102:12634–12638. doi: 10.1073/pnas.0505806102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeilage PF. The origin of speech. Oxford, UK: Oxford University Press; 2008. [Google Scholar]
- MacNeilage PF, Rogers LJ, Vallortigara G. Evolutionary origins of your right and left brain. Scientific American. 2009;301:60–67. doi: 10.1038/scientificamerican0709-60. [DOI] [PubMed] [Google Scholar]
- MacNeilage PF, Studdert-Kennedy MG, Lindblom B. Primate handedness reconsidered. Behavioral and Brain Sciences. 1987;10:247–303. doi: 10.1017/S0140525X00047695. [DOI] [Google Scholar]
- Marchant LF, McGrew WC. Laterality of limb function in wild chimpanzees of Gombe National Park: Comprehensive study of spontaneous activities. Journal of Human Evolution. 1996;30:427–443. doi: 10.1006/jhev.1996.0036. [DOI] [Google Scholar]
- Marchant LF, McGrew WC. Ant fishing by wild chimpanzees is not lateralised. Primates. 2007;48:22–26. doi: 10.1007/s10329-006-0020-3. [DOI] [PubMed] [Google Scholar]
- Marchant LF, McGrew WC, Eibl-Eibesfeldt I. In human handedness universal? Ethological analyses from three traditional cultures. Ethology. 1995;101:239–258. doi: 10.1111/j.1439-0310.1995.tb00362.x. [DOI] [Google Scholar]
- McGrew WC, Marchant LF. Are gorillas right-handed or not? Human Evolution. 1993;8:17–23. doi: 10.1007/BF02436462. [DOI] [Google Scholar]
- McGrew WC, Marchant LF. On the other hand: Current issues in and meta-analysis of the behavioral laterality of hand function in non-human primates. Yearbook of Physical Anthropology. 1997;104:201–232. doi: 10.1002/(SICI)1096-8644(1997)25+<201::AID-AJPA8>3.0.CO;2-6. [DOI] [Google Scholar]
- McGrew WC, Marchant LF. Ethological study of manual laterality in the chimpanzees of the Mahale Mountains, Tanzania. Behaviour. 2001;138:329–358. doi: 10.1163/15685390152032497. [DOI] [Google Scholar]
- McManus C, Sik G, Cole DR, Mellon AF. The development of handedness in children. British Journal of Developmental Psychology. 1988;6:257–273. doi: 10.1111/j.2044-835X.1988.tb01099.x. [DOI] [Google Scholar]
- Meguerditchian A, Donnot J, Molesti S, Francioly R, Vauclair J. Sex difference in squirrel monkeys’ handedness for unimanual and bimanual tasks. Animal Behaviour. 2012;83:635–643. doi: 10.1016/j.anbehav.2011.12.005. [DOI] [Google Scholar]
- Mittra ES, Fuentes A, McGrew WC. Lack of hand preference in wild hanuman langurs (Presbytis entellus) American Journal of Physical Anthropology. 1997;103:455–461. doi: 10.1002/(SICI)1096-8644(199708)103:4<455::AID-AJPA3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Papademetriou E, Sheu CF, Michel GF. A meta-analysis of primate hand preferences for reaching and other hand-use measures. Journal of Comparative Psychology. 2005;119:33–48. doi: 10.1037/0735-7036.119.1.33. [DOI] [PubMed] [Google Scholar]
- Perelle IB, Ehrman L. An international study of human handedness: The data. Behavior Genetics. 1994;24:217–227. doi: 10.1007/BF01067189. 10.1007/ BF01067189. [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Milner B. The role of early left-brain injury in determining lateralization of cerebral speech function. Annals of the New York Academy of Sciences. 1977;299:355–369. doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- Raymond M, Pontier D. Is there geographical variation in human handedness? Laterality: Asymmetries of Body, Brain and Cognition. 2004;9:35–51. doi: 10.1080/13576500244000274. [DOI] [PubMed] [Google Scholar]
- Rogers LJ, Andrew JR, editors. Comparative vertebrate lateralization. Cambridge, UK: Cambridge University Press; 2002. [DOI] [Google Scholar]
- Salmaso D, Longoni AM. Problems in the assessment of handedness. Cortex: A Journal Devoted to the Study of the Nervous System and Behavior. 1985;21:533–549. doi: 10.1016/s0010-9452(58)80003-9. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Ivry RB, Swinnen SP. Dynamics of hemispheric specialization and integration in the context of motor control. Nature Reviews Neuroscience. 2006;7:160–166. doi: 10.1038/nrn1849. [DOI] [PubMed] [Google Scholar]
- Sommer I, Ramsey N, Kahn R. Handedness, language later-alisation and anatomical asymmetry in schizophrenia: Meta-analysis. The British Journal of Psychiatry. 2001;178:344–351. doi: 10.1192/bjp.178.4.344. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Aleman A, Somers M, Boks M, Kahn RS. Sex differences in handedness, asymmetry of the planum temporale and functional language lateralization. Brain Research. 2008;1206:76–88. doi: 10.1016/j.brainres.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Sun T, Walsh CA. Molecular approaches to brain asymmetry and handedness. Nature Reviews Neuroscience. 2006;7:655–662. doi: 10.1038/nrn1930. [DOI] [PubMed] [Google Scholar]
- Vallortigara G, Rogers LJ. Survival with an asymmetrical brain: Advantages and disadvantages of cerebral lateralization. Behavioral and Brain Sciences. 2005;28:575–589. doi: 10.1017/S0140525X05000105. 10.1017/ S0140525X05000105. [DOI] [PubMed] [Google Scholar]
- Vauclair J, Meguerditchian A, Hopkins WD. Hand preferences for unimanual and coordinated bimanual tasks in baboons (Papio anubis) Cognitive Brain Research. 2005;25:210–216. doi: 10.1016/j.cogbrainres.2005.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JP, Hopkins WD. Primate laterality: Current behavioral evidence of primate asymmetries. New York, NY: Springer-Verlag; 1993. [Google Scholar]
- Warren JM. Handedness and laterality in humans and other animals. Physiological Psychology. 1980;8:351–359. [Google Scholar]
- Williams NA, Close JP, Giouzeli M, Crow TJ. Accelerated evolution of Protocadherin 11X/Y: A candidate gene-pair for cerebral asymmetry and language. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2006;141B:623–633. doi: 10.1002/ajmg.b.30357. [DOI] [PubMed] [Google Scholar]
- Zhao D, Hopkins WD, Li B. Handedness in nature: First evidence of manual laterality on bimanual coordinated tube task in wild primates. American Journal of Physical Anthropology. 2012;148:36–44. doi: 10.1002/ajpa.22038. [DOI] [PMC free article] [PubMed] [Google Scholar]