Abstract
Objectives
We examined the presence and frequency of alcohol consumption among older primary care patients with generalized anxiety disorder (GAD) and their relation to demographic variables, insomnia, worry, and anxiety. We expected alcohol-use distribution to be similar to previous reports and alcohol use to be associated with higher anxiety and insomnia. A third aim was to examine the moderating role of alcohol use on the relation between anxiety and insomnia. We expected alcohol use to worsen the relation between anxiety and insomnia.
Design
Baseline data from a randomized controlled trial
Sample
223 patients, age 60 and older, with DSM-IV GAD diagnoses
Setting
Patients were recruited through internal medicine, family practice, and geriatric clinics at 2 diverse healthcare settings: Michael E. DeBakey Veterans Administration Medical Center and Baylor College of Medicine.
Measurements
Measures addressed alcohol use (presence and frequency); insomnia (Insomnia Severity Index); self-reported worry severity (Penn State Worry Questionnaire − Abbreviated); clinician-rated worry severity (Generalized Anxiety Disorder Severity Scale); self-reported anxiety severity (State-Trait Anxiety Inventory - Trait); and clinician-rated anxiety (Structured Interview Guidelines for the Hamilton Anxiety Rating Scale).
Results
Most patients endorsed alcohol use in the past month, but overall weekly frequency was low. Presence and frequency of use among patients with GAD were greater than in prior reports of primary care samples. Alcohol use among patients with GAD was associated with higher education and female gender. Higher education also was associated with more drinks per week, and Caucasians reported more drinks per week than African Americans. Alcohol use was associated with less severe insomnia, lower self-reported anxiety, and less clinician-rated worry and anxiety. More drinks per week were associated with lower clinician-rated anxiety. Moderation analyses revealed lower relations between worry/anxiety and insomnia for those who reported drinking than for those who reported not drinking. Frequency of drinks per week moderated the association between PSWQ-A and insomnia, such that the positive association between self-reported worry and insomnia was lower with higher frequency of drinking.
Conclusions
Older adults with GAD appear to use alcohol at an increased rate, but mild-to-moderate drinkers do not appear to experience sleep difficulties. In fact, use of a modest amount of alcohol may be beneficial for minimizing the association between anxiety/worry and insomnia among older adults with GAD.
Keywords: alcohol use, generalized anxiety disorder, insomnia
Introduction
Anxiety and insomnia are common in geriatric patients (1), and they share substantial overlap (2−4). Generalized anxiety disorder (GAD) is 1 of the most common anxiety disorders in older adults, with community prevalence from 1.2% to 7.3% (5,6), and even higher rates in primary care (7,8). A significant number of older people with GAD (44% to 52%) have insomnia (2,9).
Among adult patients whose insomnia is chronic and untreated, alcohol is frequently used as a sedative (1,10). Alcohol use among patients with anxiety may exacerbate anxiety and associated sleep problems by leading to fragmented, nonrestorative sleep. Among older adults in primary care settings, 70.0% do not consume alcohol, 21.5% drink moderately (1−7 drinks a week), 4.1 % are at risk drinkers (8−14 drinks a week) and 4.5% are heavy drinkers or bingers (over 14 drinks a week) (11). Although one study has examined the relation between alcohol use and insomnia among older adults (12), the study had limitations and did not examine the impact of alcohol on those with anxiety, who are at greater risk of experiencing insomnia (1).
Although acute alcohol use may promote sleep, tolerance to alcohol’s sleep-enhancing effects develops within 3−9 nights of daily use, and chronic alcohol use leads to disruption of the normal sleep pattern (13). There are overlapping changes in the sleep of the elderly (14), patients with anxiety (15), and patients with alcohol use. The combination of alcohol and anxiety may have an additive negative influence on sleep. Slow-wave sleep is considered to be the most restorative aspect of sleep (16,17). However, aging, chronic alcohol use, and anxiety have a negative impact on slow-wave sleep.
The current study examined the presence and frequency of alcohol consumption among older primary care patients with GAD and the relation of these variables to demographic variables (age, gender, race, ethnicity, and education), insomnia symptoms, worry, and anxiety. We expected alcohol - use distribution in our sample to be similar to that of previous reports with older adults in primary care (11); and we expected alcohol use to be associated with higher anxiety and insomnia, given prior literature demonstrating similar relations.(11,13). A third aim of the study was to examine the moderating role of alcohol use on the relation between anxiety and insomnia. We expected that alcohol use would worsen the relation between anxiety and insomnia, given that alcohol leads to more nocturnal awakenings (18,19), which present increased opportunities for worry that likely delay return to sleep.
Method
Participants
We drew the sample from a randomized, controlled trial of cognitive behavioral therapy among older primary care patients with GAD and used only baseline data from an ongoing clinical trial. From October 2008 to April 2012, 223 patients, age 60 and older, with Diagnostic & Statistical Manual, Fourth Edition (DSM-IV) GAD diagnoses (20) were recruited through internal medicine, family practice, and geriatric clinics at 2diverse healthcare settings: Michael E. DeBakey Veterans Administration Medical Center and Baylor College of Medicine. Potential patients were identified in collaboration with primary care providers (PCP) through the electronic medical record (EMR) and by self-referral. We targeted patients with a documented EMR diagnosis of GAD or Anxiety Not Otherwise Specified for recruitment, as well as patients with anxiety symptoms noted on the problem list, or those having a prescription for anti-anxiety or antidepressant medication. With PCP approval, identified patients received a letter of invitation from the PCP and the senior author (MS) to participate in the study. A telephone call followed the letters to invite patients to participate in the study unless patients called to decline participation. We also recruited patients through educational brochures in waiting and examination rooms.
We asked patients who expressed interest in participating 2 anxiety screening questions from the PRIME-MD (21) and scheduled those responding affirmatively to at least 1 of the 2 questions for an in-person visit to review the consent form. At the consent appointment, they responded again to the 2 anxiety screening questions and completed a demographic questionnaire. At either this meeting or a subsequent appointment, trained study staff members administered the 6-item Screener (22) and the full Structured Clinical Interview for DSM-IV Disorders (SCID) (23). We audio taped all SCID interviews to allow rating of a random 20% by a second clinician to assess inter-rater agreement. Kappa coefficients indicated adequate agreement for GAD (.68), depression (major depression or dysthymia; .91), and other anxiety disorders (.75).
Included patients had a principal or co-principal DSM-IV diagnosis of GAD of at least moderate severity (4 on 0−8 scale) according to the SCID. The principal diagnosis was the disorder with the highest severity rating. When 2 diagnoses met these criteria, we categorized them as coprincipal diagnoses. Patients with any coexistent anxiety, affective, and somatization-disorder diagnoses were included, as well as those with coexistent medical conditions. We also allowed previous and current psychological or pharmacological treatments, but psychotropic medication needed to be stable over the prior month. Medication use was assessed with patient self-report questions about the type and frequency of medications used over the prior 3 months. Patients had to be able to speak English, although English did not need to be their first language. We excluded patients for conditions that threatened their safety or precluded participation (e.g., active suicidal intent, current psychosis or bipolar disorder, substance abuse within the past month, cognitive impairment according to a Mini Mental State Exam score of 23 or lower, and patients with a DSM-IV alcohol-use disorder. We also excluded patients who had participated in a prior trial of cognitive behavioral therapy for late-life anxiety. We offered excluded patients appropriate referrals.
Measures
Measures addressed alcohol use, insomnia, worry, and anxiety. Measures of worry and anxiety included both self-report and clinician-rated instruments to allow for a multi-trait, multi-method approach.
Alcohol use
We assessed alcohol use during the prior month with questions derived from prior studies of alcohol use in older adults (11). Specifically, we asked patients 1) whether they had consumed any alcohol in the past month and, if so, 2) what number of drinks they had consumed in an average week over the past month. In line with prior reports (11), moderate drinking was defined as ≤ 7 drinks a week, at-risk drinking was 8−14 drinks a week, and heavy drinking was greater than 14 drinks a week.
Insomnia
We assessed insomnia with the Insomnia Severity Index (ISI); (24). The ISI is a 7-item, self-report measure of sleep difficulties and interference, based on DSM-IV criteria for insomnia. Items assess severity of problems with sleep onset, sleep maintenance, and early-morning awakening; dissatisfaction with sleep; interference with daily functioning; impact on quality of life; and worry about sleep problems. The measure has good internal consistency, concurrent validity, and sensitivity to change among younger and older adults (2,25,26). Internal consistency of the ISI in the current sample was .90.
Worry
We rated self-reported worry severity with the abbreviated Penn State Worry Questionnaire (PSWQ-A), an 8-item inventory derived from the original 16-item PSWQ (27). Among older adults, the PSWQ-A has strong internal consistency, adequate test-retest reliability, unidimensional factor structure, and significant correlation with the full PSWQ (28,29), used widely in clinical trials of late-life GAD (26,30−32). The PSWQ-A is useful for identifying GAD in older primary care patients (33) and demonstrates change following treatment (34). Internal consistency of the PSWQ-A in the current sample was .89.
Clinician-rated worry severity was assessed with the Generalized Anxiety Disorder Severity Scale (GADSS), which includes 6 items that assess DSM-IV criteria. Initial psychometric data suggested high internal consistency, good convergent and discriminative validity, and sensitivity to change among younger primary care patients (35). Data with older adults indicate good internal consistency and interrater agreement, unidimensional factor structure, and adequate convergent validity (36,37). GADSS scores differ significantly between patients with and without GAD, and distress/interference items reliably predicted diagnoses (37). Internal consistency of the GADSS in the current sample was .84.
Anxiety
We evaluated self-reported anxiety severity with the trait subscale of the State-Trait Anxiety Inventory (STAI-T). The STAI-T is a 20-item self-report measure of general anxiety symptoms (38) that is appropriate for older adults, given its lack of focus on physiological symptoms. The STAI-T has good internal consistency and adequate convergent validity among older adults, as well as sensitivity to change following treatment (26,30,39,40). Internal consistency of the STAI-T in the current sample was .85.
We assessed clinician-rated anxiety with the Structured Interview Guidelines for the Hamilton Anxiety Rating Scale (SIGH-A) that was developed to increase the reliability of the Hamilton Anxiety Rating Scale (41), a well-known measure used routinely in psychosocial and pharmacological clinical trials of late-life GAD (30,32,42). Shear and colleagues (43) reported good test-retest and inter-rater agreement for both the SIGH-A and Hamilton Anxiety Rating Scale but more consistently high inter-rater agreement for the SIGH-A. The SIGH-A also has adequate inter-rater agreement among older adults (44). Internal consistency of the SIGH-A in the current sample was .85.
Procedure
Following participant inclusion into the study, a Master’s-level independent evaluator (IE) administered the baseline measures via telephone; IEs were not involved in any other way with the project and had no other contact with study participants. The fifth author (JC) held regular calibration meetings with the IEs, and we audio taped all interviews so that a second clinician could review at least a random 10% of each IE’s interviews to estimate inter-rater agreement. Intraclass correlation coefficients indicated excellent agreement for both the GADSS (.98) and the SIGH-A (.96).
Data Analysis
We gathered descriptive statistics to describe the presence and weekly frequency of alcohol use, examining associations of these variables with age, gender, race, ethnicity, education, and use of hypnotic/sleep medications (yes/no) by means of independent samples t-tests, zero-order correlations, and chi-square analyses. Correlational analyses examined the relations between monthly alcohol use (point biserial correlations) and weekly frequency (Pearson zero-order correlations) and 1) insomnia, 2) worry, and 3) anxiety. Further analyses tested presence (yes/no) and frequency of alcohol use (average number of drinks per week) as separate moderators of the relations between worry/anxiety and insomnia. We conducted 8 main-effects models (Step 1) and 8 interaction models (Step 2), each predicting insomnia. For each of the 4 anxiety/worry measures (i.e., PSWQ-A, GADSS, STAI, and SIGHA), we conducted 4 models to examine 1) the main effects of the presence of alcohol use and the worry/anxiety measure, 2) the interaction between presence of alcohol use and the worry/anxiety measure, 3) the main effects of weekly frequency of alcohol use and the worry/anxiety measure, and 4) the interaction between weekly frequency of alcohol use and the worry/anxiety measure. We controlled significant associations between demographics and alcohol use in all models. To examine the direction of any significant 2-way interactions between the presence (or weekly frequency) of alcohol use and each of the anxiety or worry measures, we derived equations for the simple slopes of the relationship between the anxiety or worry measure and insomnia (45). We depicted significant slopes at 1 standard deviation above and 1 standard deviation below the mean for each anxiety/worry measure for either 1) those who drink and those who do not drink (for presence of drinking analyses) or 2) those who are lower weekly drinkers (1 standard deviation below the mean) and those who are greater weekly drinkers (1 standard deviation above the mean) (for weekly frequency of drinking analyses). We conducted all analyses with SAS version 9.2 (SAS Institute, Cary, North Carolina, USA).
Results
Descriptive Data
Patient characteristics
A total of 3115 potential participants were referred by letters, direct referrals by PCPs, and self-referrals. Of the 2458 (78.9%) whom we were able to contact, 1149 (46.7%) completed the phone screen; 1282 (52.2%) were not interested, 9 (0.4%) were too young, 8 (0.3%) were not patients at the participating hospitals, 6 (0.2%) were deceased, 2 (.08%) were cognitively impaired, and 2 (.08%) were severely hearing impaired. Of the 1149 who completed the phone screen, 150 (13.1%) screened negative. Of the 999 (86.9%) who screened positive at the phone screen, 562 (56.3%) signed consent. Of the 562 consented patients, 3 (0.5%) were too young, and 10 (1.8%) screened negative at the in-person visit, leaving 549 (97.7%) eligible for diagnosis, 493 (90.0%) of whom participated in a diagnostic interview.
Of the 493 who completed a diagnostic interview, 239 (48.5%) met inclusion criteria, 8 (1.6%) served as nonstudy clinical-training cases, and 246 (49.9%) met exclusion criteria for the following reasons: no GAD (n = 185; 37.5%), GAD not principal or co-principal (n = 20; 4.1%), psychosis or bipolar disorder (n = 8; 1.6%), alcohol or other substance abuse (n = 11; 2.2%), current suicidal intent (n = 7; 1.4%), or cognitive impairment (n = 15; 3.0%). Of the 239 patients who met inclusion criteria, 223 (93.3%) completed the baseline assessment and are, therefore, included in the current analyses.
Mean age of the 223 included patients with GAD was 66.9 years (SD = 6.64 years). There were 119 (53.4%) women and 104 (46.6%) men. Racial distribution was as follows: 176 (78.9%) Caucasian, 40 (17.9%) African American, 3 (1.3%) Asian, 1 (0.5%) American Indian/Alaskan Native, and 3 (1.3%) multiracial. Twenty-four (10.8%) patients were Hispanic. Mean education level was 15.5 years (SD =2.92 years).
Alcohol use
Most patients (n = 123, 55.2%) endorsed alcohol use (yes/no) in the past month, but the overall average weekly frequency for the entire sample was low, with a mean number of 2.97 drinks a week (SD = 5.69). Among those who endorsed alcohol use in the past month, average weekly frequency of drinks was 5.38 (SD = 6.78). With regard to frequency of use over an average week in the past month, 41.7% of participants (n = 93) drank moderately (i.e., an average of ≤ 7 drinks per week), 8.5% participants (n = 19) reported at-risk drinking (i.e., an average of 8 to 14 drinks per week), and 5.0% (n = 11) indicated heavy drinking (i.e., an average of more than 14 drinks per week). These frequencies differed significantly from those reported in a general study of older adults in primary care (10), Χ23) = 73.98, p < 0.0001. Relative to this previously published report, fewer older adults in the current study reported not drinking (44.8% versus 70.0%); and more reported moderate (41.7% versus 21.5%) and at-risk (8.5% versus 4.1%) weekly drinking.
Average weekly frequency of alcohol use was positively skewed, with 5 participants who were outliers (i.e., scoring at least 3 standard deviations above the mean). Results of all analyses after removing the outliers were the same that those that included them. Alternately, the variable was transformed by taking its square root to achieve normality. We reran analyses on the entire sample with the transformed variable, and results remained the same as they were prior to transformation. Based on these findings, we report results only for the non-transformed weekly frequency of alcohol-use variable that included all participants.
Patients who reported using alcohol in the past month (yes/no) were more educated (M = 16.22 years, SD = 2.68 years) than those who denied use of alcohol during that time period [(M = 14.65 years, SD = 2.98 years; t(220) = −4.13, p < 0.0001]. Alcohol users also were more likely to be women (N = 73, 59.4%) than men (N = 50, 40.6%), Χ21) = 3.95, p= .0.047]. The majority of both women and men who used alcohol reported moderate drinking (86% of women drinkers [n = 63]; 60% of men drinkers [n = 30]), but more men reported either at risk or heavy drinking (33% of women drinkers [n = 10]; 40% of men drinkers [n = 20]). Higher levels of education were associated with a greater number of drinks per week [r(222) = 0.20, p =.003], and Caucasians reported more drinks per week (M = 3.46, SD = 6.19) than African Americans [(M = 1.11, SD = 2.62), t(147.6) = −3.77, p = 0.0002. All other associations with presence and weekly frequency were nonsignificant, including use of sedative-hypnotic medications (yes/no) (all ps > 0.05).
Zero-order correlations of alcohol use and frequency with insomnia, worry, and anxiety
Table 1 presents zero-order correlations between both alcohol use (yes/no) and weekly frequency and 1) insomnia, 2) worry, and 3) anxiety. Alcohol use in the past month was associated significantly with less severe insomnia, lower self-reported anxiety, and less clinician-rated worry and anxiety (all ps <0.05). The number of drinks per week was significantly associated with clinician-rated anxiety such that more drinks per week were associated with lower anxiety.
Table 1.
Zero-order correlations between drinking and anxiety and worry measures.
| Alcohol Use† (0= no, 1 = yes) |
Number of drinks/week‡ |
|
|---|---|---|
| Sleep | ||
| ISI | −0.15* | −0.10 |
| Worry | ||
| PSWQ-A | −0.12 | −0.11 |
| GADSS | −0.17* | −0.11 |
| Anxiety | ||
| STAI-T | −0.19** | −0.11 |
| SIGH-A | −0.18** | −0.16* |
Point-biserial correlation
Pearson’s zero-order correlations, all degrees of freedom = 223
p < 0.05,
p < 0.01
ISI = Insomnia Severity Index; PSWQ-A = Penn State Worry Questionaire − Abbreviated; GADSS = Generalized Anxiety Disorder Severity Scale; STAI-T = Trait subscale of the State-Trait Anxiety Inventory; SIGH-A − Structured Interview Guidelines for the Hamilton Anxiety Rating Scale
Moderation analyses
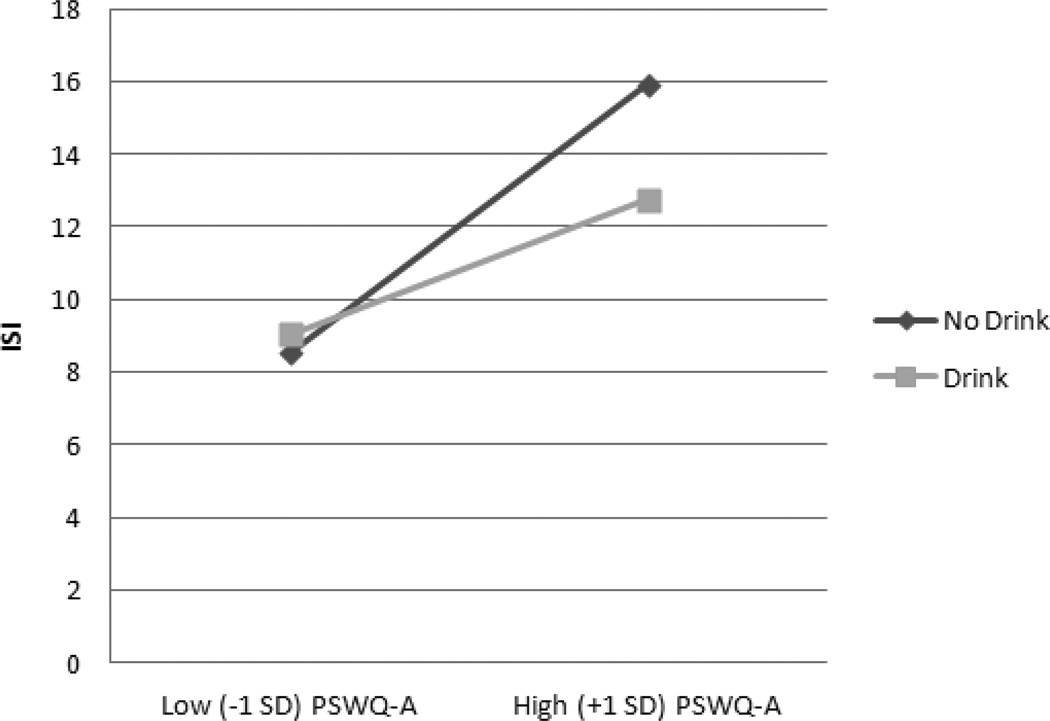
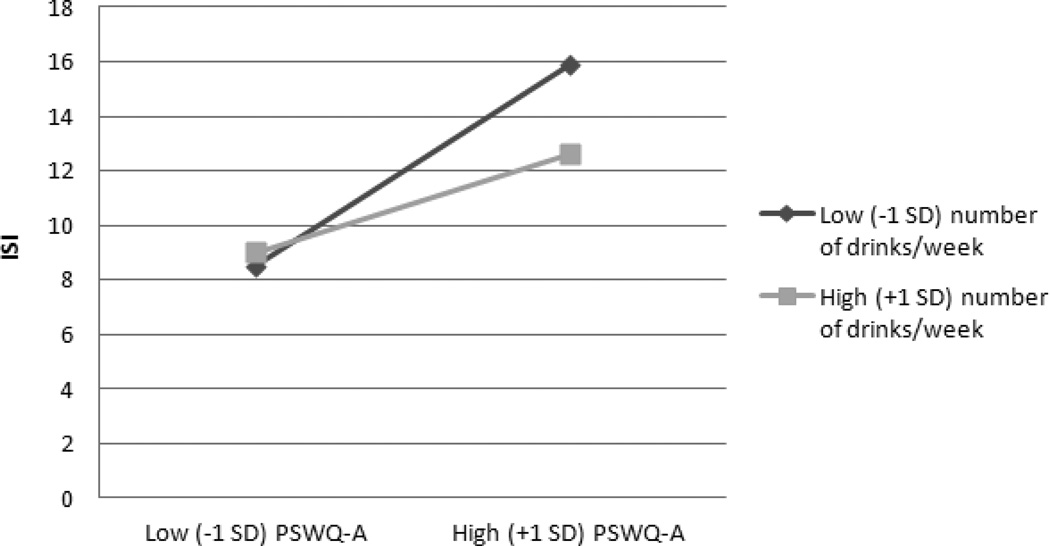
Tables 2 and 3 present regression coefficients for moderator analyses. We controlled demographic variables that were significantly related to presence of drinking (i.e., education and gender) in all presence (yes/no) analyses, and we controlled demographic variables significantly related to frequency of drinks per week (i.e., education and race) in all drinking frequency analyses. For all 8 main-effects models, higher levels of anxiety and worry, whether self or clinician-rated, were related to higher levels of insomnia symptoms. However, neither presence nor weekly frequency of alcohol use was related to insomnia symptoms. Importantly, moderation analyses revealed significant interactions between the presence of drinking and all anxiety or worry measures predicting insomnia (all ps < 0.05). Simple slopes analyses revealed that the relationships between the PSWQ-A, GADSS, STAI-T, and SIGH-A and insomnia were lower for those who reported drinking (βs between 0.29 and 0.40), relative to those who reported not drinking (βs between 0.49 and 0.63) (see Figure 1 for an illustration of the pattern for all 4 worry/anxiety variables). However, the frequency of drinks per week significantly moderated only the association between PSWQ-A and insomnia (see Figure 2), such that the positive association between self-reported worry and insomnia was lessened the more weekly drinks reported.
Table 2.
Presence of Drinking and Anxiety Measures Predicting ISI.
| Anxiety/Worry Measures |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PSWQ-A | GADSS | STAI-T | SIGH-A | |||||||||
| F† | β‡ | p | F† | β‡ | p | F† | β‡ | p | F† | β‡ | p | |
| Step 1: Covariates and Main Effects | ||||||||||||
| Years of Education | 0.32 | −0.04 | 0.57 | 0.08 | −0.02 | 0.78 | 0.01 | 0.00 | 0.94 | 0.01 | 0.01 | 0.91 |
| Gender (0 = male, 1 = female) | 0.18 | −0.03 | 0.67 | 0.14 | 0.02 | 0.71 | 0.00 | 0.00 | 0.97 | 0.08 | 0.02 | 0.78 |
| Drink (yes/no) | 1.78 | −0.09 | 0.18 | 1.07 | −0.06 | 0.30 | 0.86 | −0.06 | 0.35 | 0.97 | −0.06 | 0.33 |
| Anxiety/Worry measure | 39.64 | 0.39 | <0.0001 | 73.72 | 0.51 | <0.0001 | 66.76 | 0.49 | <0.0001 | 81.17 | 0.53 | <0.0001 |
| Step 2: Interaction | ||||||||||||
| Drink* Anxiety/Worry measure | 3.98 | −0.42 | 0.04 | 5.96 | −0.41 | 0.015 | 7.00 | −0.75 | 0.009 | 7.85 | −0.38 | 0.006 |
| Full Model R2 | 0.19 | 0.29 | 0.28 | 0.32 | ||||||||
Step 1 covariates and main effects: dfnumerator= 1, dfdenominator= 217; Step 2 interaction: dfnumerator= 1, dfdenominator= 216.
Standardized beta weight
ISI = Insomnia Severity Index; PSWQ-A = Penn State Worry Questionnaire-Abbreviated; GADSS = Generalized Anxiety Disorder Severity Scale; STAI-T = Trait subscale of the State-Trait Anxiety Inventory; SIGH-A = Structured Interview Guidelines for the Hamilton Anxiety Rating Scale
Table 3.
Weekly Frequency of Drinking and Anxiety Measures Predicting ISI.
| Anxiety/Worry Measures | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PSWQ-A | GADSS | STAI-T | SIGH-A | |||||||||
| F† | β‡ | p | F† | β‡ | p | F† | β‡ | p | F† | β‡ | p | |
| Step 1: Covariates and Main Effects | ||||||||||||
| Years of Education | 1.22 | −0.07 | 0.27 | 0.21 | −0.03 | 0.64 | 0.08 | −0.02 | 0.78 | 0.07 | −0.02 | 0.79 |
| Race (0 = African American, 1 = Caucasian) | 9.16 | −0.19 | 0.003 | 2.75 | −0.10 | 0.10 | 6.04 | −0.15 | 0.01 | 2.35 | −0.09 | 0.13 |
| Number of Drinks/week | 0.06 | −0.02 | 0.81 | 0.20 | −0.03 | 0.66 | 0.14 | −0.02 | 0.71 | 0.01 | 0.00 | 0.94 |
| Anxiety/Worry measure | 42.19 | 0.40 | <0.0001 | 69.47 | 0.50 | <0.0001 | 64.33 | 0.48 | <0.0001 | 77.00 | 0.52 | <0.0001 |
| Step 2: Interaction | ||||||||||||
| Drinks/week* Anxiety/Worry | 4.26 | −0.40 | 0.04 | 2.90 | −0.27 | 0.09 | 2.71 | −0.43 | 0.10 | 1.67 | −0.15 | 0.20 |
| Full Model R2 | 0.22 | 0.29 | 0.28 | 0.31 | ||||||||
Step 1 covariates and main effects: dfnumerator= 1, dfdenominator= 210; Step 2 interaction: dfnumerator= 1, dfdenominator= 209
Standardized beta weights
ISI = Insomnia Severity Index; PSWQ-A = Penn State Worry Questionnaire-Abbreviated; GADSS = Generalized Anxiety Disorder Severity Scale; STAI-T = Trait subscale of the State-Trait Anxiety Inventory; SIGH-A = Structured Interview Guidelines for the Hamilton Anxiety Rating Scale
Figure 1.
Interaction between Presence of Drinking and PSWQ-A Predicting ISI. PSWQ-A = Penn State Worry Questionnaire, ISI = Insomnia Severity Index.
Figure 2.
Interaction between Weekly Frequency of Drinking and PSWQ-A Predicting ISI. PSWQ-A Penn State Worry Questionnaire-Abbreviated; ISI = Insomnia Severity Index.
Conclusions
The majority of older primary care patients with GAD in this study reported some alcohol use (55%), and frequency was higher overall than in a prior report of geriatric patients in primary care (11). This finding is particularly notable given that patients with alcohol abuse were excluded from the current study. Although most alcohol use among the older GAD sample was moderate, at-risk and heavy drinking also were more common than in the Kirchner et al. sample. Differences in patterns of alcohol use between these 2 samples may reflect age differences (67 years vs. 72 years), as alcohol consumption decreases with age (46). However, differences also may reflect the prominent role of anxiety/worry for patients in the current study. Moderate alcohol use in the late-life GAD sample may serve a self-medicating function, a notion that is supported by data here suggesting that self-reported alcohol use was associated with lower levels of anxiety, worry, and insomnia. Alcohol use also seemed to have a “protective” effect on the relation between anxiety/worry and insomnia, such that patients who reported some alcohol use showed a weaker relation between anxiety and sleep disturbance. In a similar vein, Kirchner et al. (11) found that nondrinkers reported more depressive/anxiety symptoms than moderate drinkers, and Jaussent et al. (12) also found some alcohol use to be protective against insomnia in a subgroup of elderly individuals. The data here, however, need to be considered in light of our exclusion of patients with alcohol abuse. Hypotheses about differential effects of alcohol use for heavy drinkers and bingers could not be tested adequately here because of the small number of heavy drinkers. As such, the current study findings are unlikely to generalize to populations of older adults with higher average amounts of alcohol use. Number of drinks per week also was associated only with clinician-rated anxiety and did not consistently moderate relations between anxiety/worry and insomnia.
Female gender and higher education level among older patients with GAD were associated with a higher incidence of alcohol use in the previous month. Caucasians and more educated patients reported more drinks per week than African Americans or patients with lower education levels. Previous data have suggested similar racial and ethnic differences, with Caucasians reporting more moderate or at-risk drinking than African Americans, Hispanic/Latino Americans, and other ethnic minority older patients (11). However, in prior reports, older men typically report more alcohol use than older women (11,46,47). Gender differences across reports may be explained in part by differential gender distributions in study samples (e.g., Kirchner et al. sample was 81% men). However, the increased use of alcohol among women in the current study may reflect the reinforcing properties of moderate drinking for sleep difficulties and anxiety that are more generally common in women.(12) Other factors that warrant further attention in future research include differential roles of chronic illness and social interactions that impact alcohol use. Future research also might include objective measures of sleep, including actigraphy or polysomnography, to examine whether findings reflect perceptions or actual patterns of sleep.
Older adults with GAD appear to use alcohol at an increased rate, but mild-to-moderate drinkers do not appear to experience negative effects of alcohol with regard to sleep difficulties. In fact, the use of a modest amount of alcohol may be beneficial for minimizing the association between anxiety/worry and insomnia among older adults with GAD. Nevertheless, findings may be limited, given the exclusion of patients with alcohol-use disorders and the overall low frequency of alcohol use in the study sample.
Acknowledgments
This material is based upon work supported by a grant from the National Institute of Mental Health (NIMH) (R01-MH53932) and supported in part with resources and the use of facilities at the Houston VA Health Services Research and Development Center of Excellence (HFP90-020) at the Michael E. DeBakey VA Medical Center. The views expressed are those of the authors and not necessarily those of the NIMH, National Institutes of Health, Department of Veterans Affairs, the US government or Baylor College of Medicine. The NIMH had no role in the design and conduct of the study; the collection, management, analysis and interpretation of the data; or the preparation, review or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No disclosures to report
References
- 1.Thase ME. Correlates and consequences of chronic insomnia. Gen Hosp Psychiatry. 2005;27(2):100–112. doi: 10.1016/j.genhosppsych.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Brenes GA, Miller ME, Stanley MA, et al. Insomnia in older adults with generalized anxiety disorder. Am J Geriatr Psychiatry. 2009;17:465–472. doi: 10.1097/jgp.0b013e3181987747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Magee J, Carmin C. The relationship between sleep and anxiety in older adults. Curr Psychiatry Rep. 2010;12:13–19. doi: 10.1007/s11920-009-0087-9. [DOI] [PubMed] [Google Scholar]
- 4.Spira AP, Stone K, Beaudreau SA, et al. Anxiety symptoms and objectively measured sleep quality in older women. Am J Geriatr Psychiatry. 2009;17:136–143. doi: 10.1097/JGP.0b013e3181871345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolitzky-Taylor K, Castriotta N, Lenze E, et al. Anxiety disorders in older adults: A comprehensive review. Depress Anxiety. 2010;27:190–211. doi: 10.1002/da.20653. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson J, Ostling S, Waern M, et al. The 1-month prevalence of generalized anxiety disorder according to DSM-IV, DSM-V, and ICD-10 among nondemented 75-year-olds in Gothenburg, Sweden. Am J Geriatr Psychiatry. 2012;20:963–972. doi: 10.1097/JGP.0b013e318252e749. [DOI] [PubMed] [Google Scholar]
- 7.Kunik ME, Roundy K, Veazey C, et al. Surprisingly high prevalence of anxiety and depression in chronic breathing disorders. Chest. 2005;127:1205–1211. doi: 10.1378/chest.127.4.1205. [DOI] [PubMed] [Google Scholar]
- 8.Tolin DF, Robinson JT, Gaztambide S, et al. Anxiety disorders in older Puerto Rican primary care patients. Am J Geriatr Psychiatry. 2005;1392:150–156. doi: 10.1176/appi.ajgp.13.2.150. [DOI] [PubMed] [Google Scholar]
- 9.Ohayon MM, Roth T. What are the contributing factors for the insomnia in the general population? J Psychosom Res. 2001;51:745–755. doi: 10.1016/s0022-3999(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 10.Gillin JC, Drummond SPA. Medication and substance abuse. In: Kryger MH, Roth T, Dement WE, editors. In Principles of Practice of Sleep Medicine. Philadelphia: W.B. Saunders Co.; 2000. pp. 1176–1195. [Google Scholar]
- 11.Kirchner JE, Zubritsky C, Docy M. Alcohol consumption among older adults in primary care. J Gen Intern Med. 2007;22:92–97. doi: 10.1007/s11606-006-0017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaussent I, Dauvilliers Y, Ancelin M-L, et al. Insomnia symptoms in older adults: Associated factors and gender differences. Am J Geriatr Psychiatry. 2011;19:88–97. doi: 10.1097/JGP.0b013e3181e049b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stein MD, Friedman PD. Disturbed sleep and its relationship to alcohol use. Subst Abu. 2005;26(1):1–13. doi: 10.1300/j465v26n01_01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vitiello MV. Sleep in normal aging. Sleep Med Clin. 2006;1(2):171–176. [Google Scholar]
- 15.Fuller KH, Waters WF, Binks PG, et al. Generalized anxiety and sleep architecture: a polysomnographic investigation. Sleep. 1997;20(5):370–376. doi: 10.1093/sleep/20.5.370. [DOI] [PubMed] [Google Scholar]
- 16.Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45:347–360. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- 17.Horne J. Human slow wave sleep: a review and appraisal of recent findings, with implications for sleep functions, and psychiatric illness. Experientia. 1992;48:941–954. doi: 10.1007/BF01919141. [DOI] [PubMed] [Google Scholar]
- 18.Williams D, MacLean A, Cairns J. Dose-response effects of ethanol on the sleep of young women. J Stud Alcohol. 1983;44:515–523. doi: 10.15288/jsa.1983.44.515. [DOI] [PubMed] [Google Scholar]
- 19.Roehrs T, Roth T. Sleep, sleepiness, sleep disorders and alcohol use and abuse. Sleep Med Rev. 2001;5:287–297. doi: 10.1053/smrv.2001.0162. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association: Diagnostic & Statistical Manual of Mental Disorders. Arlington VA: American Psychiatric Press; 2000. [Google Scholar]
- 21.Spitzer RL, Williams JB, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994;272(22):1749–1756. [PubMed] [Google Scholar]
- 22.Callahan CM, Frederick UW, Hui SL, et al. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40:771–781. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 23.First MB, Spitzer RL, Miriam G, et al. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Patient Edition with Psychotic. New York: Biometrics Research, New York State Psychiatric Institute; 1997. [Google Scholar]
- 24.Morin CM. Insomnia: Psychological Assessment and Management. New York: Guilford; 1993. [Google Scholar]
- 25.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 236.Brenes GA, Miller ME, Williamson JD, et al. A randomized controlled trial of telephone-delivered cognitive-behavioral therapy for late-life anxiety disorders. Am J Geriatr Psychiatry. 2012;20:707–716. doi: 10.1097/JGP.0b013e31822ccd3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer T, Metzger R, Borkovec TD. Development and validity of the Penn State Worry Scale. Behav Res Ther. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- 28.Crittendon J, Hopko DR. Assessing worry in older and younger adults: Psychometric properties of an abbreviated Penn State Worry Questionnaire (PSWQ-A) J Anxiety Disord. 2006;20:1036–1054. doi: 10.1016/j.janxdis.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Hopko DR, Stanley MA, Reas DL, et al. Assessing worry in older adults: Confirmatory factor analysis of the Penn State Worry Questionnaire and psychometric properties of an abbreviated model. Psychol Assess. 2003;15:173–183. doi: 10.1037/1040-3590.15.2.173. [DOI] [PubMed] [Google Scholar]
- 30.Stanley MA, Beck JG, Novy DM, et al. Cognitive-behavioral treatment of late-life generalized anxiety disorder. J Consult Clin Psychol. 2003;71:309–319. doi: 10.1037/0022-006x.71.2.309. [DOI] [PubMed] [Google Scholar]
- 31.Stanley MA, Wilson N, Novy DM. Cognitive behavior therapy for older adults with generalized anxiety disorder in primary care: A randomized clinical trial. JAMA. 2009;301:1460–1467. doi: 10.1001/jama.2009.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wetherell JL, Gatz M, Craske MG. Treatment of generalized anxiety disorder in older adults. J Consult Clin Psychol. 2003;71:31–40. doi: 10.1037//0022-006x.71.1.31. [DOI] [PubMed] [Google Scholar]
- 33.Webb SA, Diefenbach G, Wagener P, et al. Comparison of self-report measures for identifying late-life generalized anxiety disorder. J Geriatr Psychiatry Neurol. 2008;21:223–231. doi: 10.1177/0891988708324936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shrestha S, Armento MEA, Bush AL. Pilot findings from a community-based treatment program for late-life anxiety. Int J Pers Cent Med. 2012;293:400–409. [Google Scholar]
- 35.Shear K, Belnap BH, Mazumdar S, et al. Generalized anxiety disorder severity scale (GADSS): A preliminary validation study. Depress Anxiety. 2006;23:77–82. doi: 10.1002/da.20149. [DOI] [PubMed] [Google Scholar]
- 36.Andreescu C, Belnap BH, Rollman BL, et al. Generalized Anxiety Disorder Severity Scale validation in older adults. Am J Geriatr Psychiatry. 2008;16:813–818. doi: 10.1097/JGP.0b013e31817c6aab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss BJ, Calleo J, Rhoades HM, et al. The utility of the Generalized Anxiety Disorder Severity Scale (GADSS) with older adults in primary care. Depress Anxiety. 2009;26:E10–E15. doi: 10.1002/da.20520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spielberger CD, Gorsuch RI, Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto CA: Consulting Psychologist Press; 1970. [Google Scholar]
- 39.Kabacoff RI, Segal DL, Hersen M, et al. Psychometric properties and diagnostic utility of the Beck Anxiety Inventory and the State-Trait Anxiety Inventory with older adult psychiatric outpatients. J Anxiety Disord. 1997;11:33–47. doi: 10.1016/s0887-6185(96)00033-3. [DOI] [PubMed] [Google Scholar]
- 40.Stanley MA, Roberts RE, Bourland SL, et al. Anxiety disorders among older primary care patients. Journal of Clinical Geropsychology. 2001;7:105–116. [Google Scholar]
- 41.Hamilton M. Diagnosis and rating of anxiety. Br J Psychiatry Special Publication. 1969;3:76–79. [Google Scholar]
- 42.Lenze EJ, Mulsant BH, Shear MK, et al. Efficacy and tolerability of citalopram in the treatment of late-life anxiety disorders: Results from an 8-week randomized, placebo-controlled trial. Am J Psychiatry. 2005;162:146–150. doi: 10.1176/appi.ajp.162.1.146. [DOI] [PubMed] [Google Scholar]
- 43.Shear MK, Vander BJ, Rucci P, et al. Reliability and validity of a structured interview guide for the Hamilton Anxiety Rating Scale (SIGH-A) Depress Anxiety. 2001;13:166–178. [PubMed] [Google Scholar]
- 44.Skopp NA, Novy D, Kunik M, et al. Investigation of cognitive behavior therapy. Am J Geriatr Psychiatry. 2006;14:282. doi: 10.1097/01.JGP.0000192505.56434.7e. [DOI] [PubMed] [Google Scholar]
- 45.Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. London Sage Publications, Inc.; 1991. [Google Scholar]
- 46.Moos RH, Schutte KK, Brennan PL, Moos BS. Older adults’ alcohol consumption and late-life drinking problems: a 20-year perspective. Addiction. 2009;104:1293–1302. doi: 10.1111/j.1360-0443.2009.02604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin JC, Karno MP, Grella CE, et al. Alcohol, tobacco, and nonmedical drug use disorders in U.S. adults aged 65 years and older: data from the 2001−2002 National Epidemiologic Survey of Alcohol and Related Conditions. Am J Geriatr Psychiatry. 2011;19:29209. doi: 10.1097/JGP.0b013e3181e898b4. [DOI] [PMC free article] [PubMed] [Google Scholar]