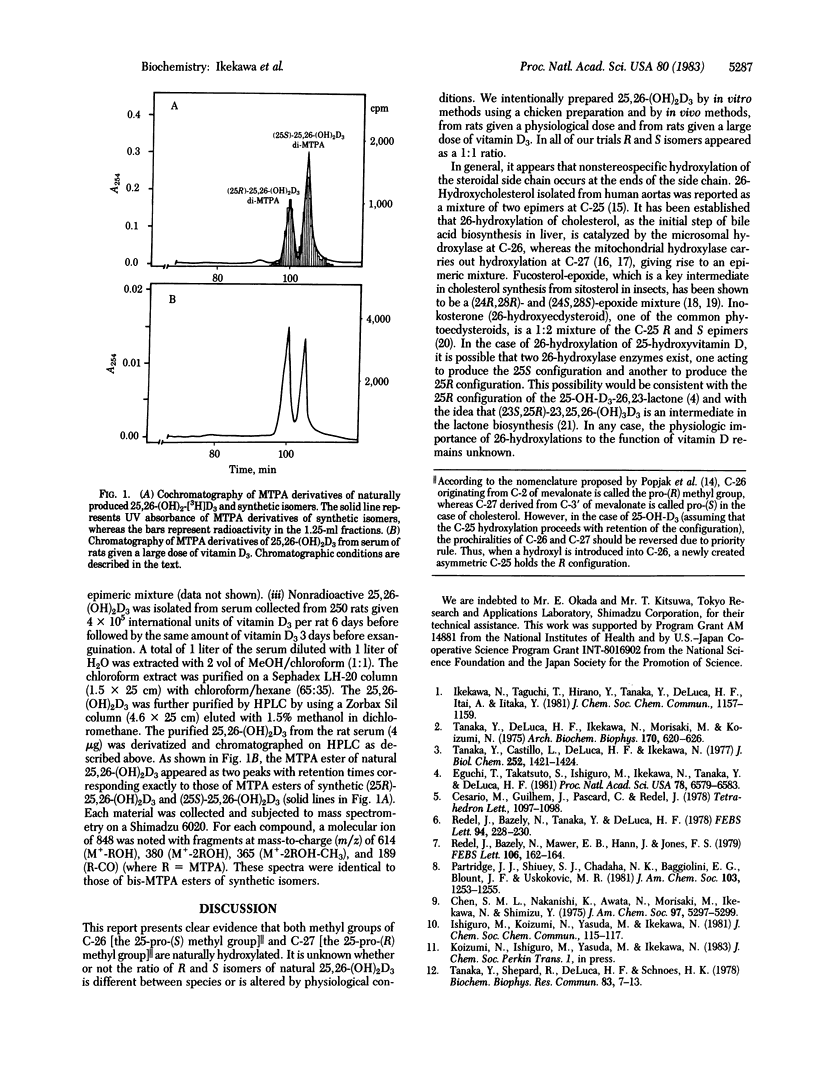
Abstract
Radiolabeled 25,26-dihydroxyvitamin D3 was prepared in vitro by using chicken kidney homogenates and in vivo in rats from [23,24-3H]-25-hydroxyvitamin D3. These compounds were mixed with synthetic (25S)- and (25R)-25,26-dihydroxyvitamin D3, converted to the corresponding (+)-alpha-methoxy-alpha-trifluoromethylphenylacetyl esters, and subjected to high-performance liquid chromatography that separates the derivatized epimers. The radiolabeled 25,26-dihydroxyvitamin D3 derivatives were a 1:1 mixture of the 25S and 25R isomers. Similarly unlabeled 25,26-dihydroxyvitamin D3 isolated from the plasma of rats given large amounts of vitamin D3 was shown to be a 1:1 mixture of the S and R isomers. Therefore, naturally occurring 25,26-dihydroxyvitamin D3 is a mixture of the 25R and 25S isomers and not just the S isomer reported previously.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atsuta Y., Okuda K. On the stereospecificity of cholestanetriol 26-monooxygenase. J Biol Chem. 1981 Sep 10;256(17):9144–9146. [PubMed] [Google Scholar]
- Chen S. M., Nakanishi K., Awata N., Morisaki M., Ikekawa N. Letter: Stereospecificity in the conversion of fucosterol 24,28-epoxide to desmosterol in the silkworm, Bombyx mori. J Am Chem Soc. 1975 Sep 3;97(18):5297–5299. doi: 10.1021/ja00851a056. [DOI] [PubMed] [Google Scholar]
- Eguchi T., Takatsuto S., Ishiguro M., Ikekawa N., Tanaka Y., Deluca H. F. Synthesis and determination of configuration of natural 25-hydroxyvitamin D(3) 26,23-lactone. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6579–6583. doi: 10.1073/pnas.78.11.6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto Y., Morisaki M., Ikekawa N. Stereochemical importance of fucosterol epoxide in the conversion of sitosterol into cholesterol in the silkworm Bombyx mori. Biochemistry. 1980 Mar 18;19(6):1065–1069. doi: 10.1021/bi00547a003. [DOI] [PubMed] [Google Scholar]
- Gustafsson J., Sjöstedt S. On the stereospecificity of microsomal "26"-hydroxylation in bile acid biosynthesis. J Biol Chem. 1978 Jan 10;253(1):199–201. [PubMed] [Google Scholar]
- Ishizuka S., Ishimoto S., Norman A. W. Metabolic pathway to 25-hydroxyvitamin D3-26,23-lactone from 25-hydroxyvitamin D3. FEBS Lett. 1982 Feb 8;138(1):83–87. doi: 10.1016/0014-5793(82)80400-6. [DOI] [PubMed] [Google Scholar]
- Popják G., Edmond J., Anet F. A., Easton N. R., Jr Carbon-13 NMR studies on cholesterol biosynthesized from [13C]mevalonates. J Am Chem Soc. 1977 Feb 2;99(3):931–935. doi: 10.1021/ja00445a041. [DOI] [PubMed] [Google Scholar]
- Redel J., Bazely N., Mawer E. B., Hann J., Jones F. S. The configuration at C-25 of human 25,26-dihydroxycholecalciferol. FEBS Lett. 1979 Oct 1;106(1):162–164. doi: 10.1016/0014-5793(79)80718-8. [DOI] [PubMed] [Google Scholar]
- Redel J., Bazely N., Tanaka Y., DeLuca H. F. The absolute configuration of the natural 25,26-dihydroxycholecalciferol. FEBS Lett. 1978 Oct 15;94(2):228–230. doi: 10.1016/0014-5793(78)80943-0. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Castillo L., DeLuca H. F. The 24-hydroxylation of 1,25-dihydroxyvitamin D3. J Biol Chem. 1977 Feb 25;252(4):1421–1424. [PubMed] [Google Scholar]
- Tanaka Y., DeLuca H. F., Ikekawa N., Morisaki M., Koizumi N. Determination of stereochemical configuration of the 24-hydroxyl group of 24,25-dihydroxyvitamin D3 and its biological importance. Arch Biochem Biophys. 1975 Oct;170(2):620–626. doi: 10.1016/0003-9861(75)90157-5. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., DeLuca H. F. Measurement of mammalian 25-hydroxyvitamin D3 24R-and 1 alpha-hydroxylase. Proc Natl Acad Sci U S A. 1981 Jan;78(1):196–199. doi: 10.1073/pnas.78.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Shepard R. A., DeLuca H. F., Schnoes H. K. The 26-hydroxylation of 25-hydroxyvitamin D3 in vitro by chick renal homogenates. Biochem Biophys Res Commun. 1978 Jul 14;83(1):7–13. doi: 10.1016/0006-291x(78)90390-x. [DOI] [PubMed] [Google Scholar]



