Abstract
CD4+ T cells stimulate immune responses through distinct patterns of cytokine produced by Th1, Th2 or Th17 cells, or inhibit immune responses through Foxp3-expressing regulatory T cells (Tregs). Paradoxically, effector T cells were recently shown to activate Tregs, however, it remains unclear which Th subset is responsible for this effect. In this study, we found that Th17 cells expressed the highest levels of TNF among in vitro generated Th subsets, and most potently promoted expansion and stabilized Foxp3 expression by Tregs when co-transferred into Rag1−/− mice. Both TNF and IL-2 produced by Th17 cells contributed to this effect. The stimulatory effect of Th17 cells on Tregs was largely abolished when co-transferred with TNFR2-deficient Tregs. Furthermore, Tregs deficient in TNFR2 also supported a much lower production of IL-17A and TNF expression by co-transferred Th17 cells. Thus, our data indicate that the TNF-TNFR2 pathway plays a crucial role in the reciprocal stimulatory effect of Th17 cells and Tregs. This bidirectional interaction should be taken into account when designing therapy targeting Th17 cells, Tregs, TNF and TNFR2.
Keywords: Th17, regulatory T cell, IL-2, TNF, TNFR2
1. INTRODUCTION
CD4+ T helper (Th) cells play central roles in orchestrating innate and adaptive immune responses to offending pathogens, and to self- or tumor-antigens [1]. The immune stimulatory effects of Th cells are mediated by distinct patterns of cytokine produced by diverse subsets of effector Th cell (Teff) including Th1, Th2 and Th17 cells, while the immune suppressive effects of Th cells are exerted by CD4+FoxP3+ regulatory T cells (Tregs) [1]. Up- or down-regulation of Th subset activity has become an important therapeutic strategy in the treatment of a wide spectrum of diseases including autoimmunity and cancer. For example, it has been well established that an increase in Treg activity dampens autoimmune inflammatory responses [2], while depletion of Tregs is able to boost anti-tumor immune responses [3]. More recently, targeting Th17 cells has been shown to be a promising means of treating autoimmune disorders. This was achieved by 1) blocking the differentiation and amplification of Th17 cells; or 2) inhibiting or neutralizing of Th17 cytokines and their receptors, or 3) suppressing the transcription factors specific for Th17 cells [4, 5]. Although Th17-cytokines may be associated with tumor initiation [6], Th17 cells have also been reported to contribute to protective anti-tumor immunity [6] and adoptive transfer of Th17 cells elicited remarkable anti-tumor immune responses which was superior to the efficacy of Th1 cells, a classical anti-tumor Th subset [7]. Since immunity and tolerance are dynamically determined by the interplay and balance of Tregs and Teffs [8], further understanding of the complex interactions between these two functional distinct CD4 cells is crucial for devising effective and safe treatment of autoimmunity and immunotherapy of cancer.
Tregs constitutively express high levels of functional cytokine receptors such as CD25 and TNFR2, but do not have the capacity to produce their ligands [9, 10]. Consequently, as has been reported, IL-2 produced by activated Teff cells is crucial for the expansion and suppressive function of Tregs [9]. TNF, another product of activated Teff cells, by interacting with TNFR2, is crucial for the phenotypic stability, expansion and in vivo function of Tregs [10, 11]. The stimulatory signals from activated pathogenic effector T cells were a prerequisite for Tregs to acquire the capacity to suppress murine autoimmune diabetes [12]. Thus, paradoxically the activation of Teffs may attenuate immune responses by boosting Treg activity. The identity of the subset of Th1, or Th2, or Th17 cells most responsible for the activation of Tregs remains to be determined.
In this study, we found that Th17 cells expressed the highest levels of TNF among Th subsets generated in vitro from naïve mouse CD4 cells by using a standard differentiation protocol [13, 14]. Accordingly, Th17 cells were the most potent Th subset in supporting the expansion and in stabilizing Foxp3 expression by Tregs when co-transferred into Rag1−/− mice. The stimulatory effect of Th17 cells on Tregs deficient in TNFR2 was attenuated by up to 80%. Furthermore, this was accompanied by a marked reduction in characteristic Th17 cytokine expression. This observation accentuates the contribution of TNF-TNFR2 signaling to the reciprocal stimulatory effects of these two Th subsets. This effect should be taken into account when designing future treatments for autoimmune diseases and immunotherapy for cancer by targeting Th17 cells, Treg cells or the TNF-TNFR2 pathway.
2. MATERIALS and METHODS
2.1. Mice and reagents
Normal C57BL/6 mice, Ly5.2 C57BL/6 mice, Rag 1–/– mice, TNFR2–/– mice and FoxP3/gfp KI (knock in) mice were provided by the Animal Production Area of the NCI (Frederick, MD). Frederick National Laboratory for Cancer Research is accredited by AAALAC International and follows the Public Health Service Policy for the Care and Use of Laboratory Animals. Animal care was provided in accordance with the procedures outlined in the "Guide for Care and Use of Laboratory Animals" (National Research Council; 1996; National Academy Press; Washington, D.C.). Anti-mouse antibodies (Abs) were purchased from BD Biosciences (San Diego, CA) consisted of anti-mouse CD3 (145-2C11), CD4 (GK1.5), CD25 (PC61), CD45.2 (104), CD45RB (16A), CD45 (30-F11), Ki-67 (B56), TNF (MP6-XT22), IFNγ (XMG1.2), IL-13 (JES10-5A2) and IL-17A (TC11-18H10). Functional grade purified anti-mouse CD3e (eBio500A2), CD28 (37.51) and IL-4 (11B11) Abs, Foxp3 Staining Set (FJK-16s) and anti-mouse TCRβ Ab (H57-597) were purchased from eBioscience (San Diego, CA). Murine IL-2, IL-4, IL-6, IL-12 and TNF were purchased from PeproTech (Rocky Hill, NJ). Human rTGFβ1 was from R&D Systems (Minneapolis, MN). Anti-IL-2 Ab (JES6-5H4) was purchased from Bio X Cell (West Lebanon, NH). TNFR2-fusion protein (Enbrel) was from Amgen, Inc. (Thousand Oaks, CA). Purified anti-mouse IL-12p40 Ab (C17.8) and IFNγ Ab (XMG-6) were kindly gifts from Dr. Giorgio Trinchieri (NCI).
2.2. Purification of T cells
To prepare a single-cell suspension, spleens and lymph nodes (inguinal, axillary and mesenteric regions) were mashed and passed through a 70-µm mesh (BD Labware, San Jose, CA). CD4+ T cells were purified using magnetic beads coated with anti-CD4 Ab (clone L3T4) according to the manufacturer’s instructions (Miltenyi Biotec Inc., Anburn, CA). Subsequently, the CD4+ cells were stained with anti-CD4, anti-CD25, and anti-CD45RB Abs and sorted into naïve CD4+CD25− CD45RBhi T cells. CD4+FoxP3/gfp+ Tregs were purified using Cytomation MoFlo cytometer (Fort Collins, CO), yielding a purity of >96% Foxp3+ cells, and CD4+CD25+ Tregs were flow sorted from WT or TNFR2−/− mice with >92% of Foxp3+ cells.
2.3. Differentiation of Th subsets
Flow-sorted naïve CD4+ T cells were cultured at a concentration of 1 × 106 cells/ml. The polarized culture condition of Th subsets was adapted from previous reports [13, 14]. The cells were stimulated with plate-bound anti-CD3e Ab (5 µg/ml) and soluble anti-CD28 Ab (2 µg/ml) as Th0 cells. Th1 cells were generated by addition of IL-12 (10 ng/ml) and anti-IL-4 Ab (2 µg/ml) into the culture. Th2 cells were generated via addition of IL-4 (50 ng/ml), anti-IFNγ (2 µg/ml) and anti-IL-12 (2 µg/ml) Abs. For the generation of Th17 cells, naïve T cells were cultured with IL-6 (100 ng/ml), TGFβ (2 ng/ml), anti-IFNγ (2 µg/ml), anti-IL2 Ab (2 µg/ml) and anti-IL4 Ab (2 µg/ml). Iscove’s modified Dulbecco’s medium (IMDM, Sigma-Aldrich) was used in Th17 polarizing culture, and RPMI-1640 (Lonza Bio Whittaker, Walkersville, MD) was used in all other cultures. The medium was supplemented with 10% fetal bovine serum (FBS, Hyclone, Logan, UT) containing 2 mM glutamine, 100 IU/ml penicillin, and 100 µg/ml streptomycin, 10 mM HEPES, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids and 50 mM 2-ME.
2.4. T cell co-transfer model
Flow-sorted CD45.2+ Tregs were transferred alone or co-transferred with polarized CD45.1+ (CD45.2−) Th0, Th1, Th2 or Th17 cells at 1:1 ratio into Rag1−/− mice (i.p., 0.8 × 105 /mouse, each). Mouse body weight was monitored weekly. In some experiments, mice were injected i.p. with neutralizing anti-IL-2 mAb or/and TNFR2-Fc fusion protein, or Rat IgG as control, once a week for 5 weeks, starting from one day after cell transfer. Spleen, and LNs in axillary, inguinal and mesenteric regions were harvested 5 weeks after transfer.
2.5. Flow Cytometry and intracellular cytokine staining
After blocking FcR, cells were incubated with appropriately diluted antibodies. Appropriate species matched Abs served as isotype control. For intracellular cytokines staining, cells were re-stimulated with phorbol myristate acetate (PMA, 50 ng/ml; Sigma-Aldrich St. Louis, MO) and ionomycin (1 µM; Sigma-Aldrich) in the presence of GolgiPlug (BD Biosciences) for 5 h, and then stained with anti IFNγ, or IL-13, or IL-17, or IL-2 or TNF Ab. For detection of Foxp3, cells were fixed and permeabilized using the anti-mouse Foxp3 staining kit (FJk-16S, eBioscience). Acquisition was performed using a LSRII (BD Biosciences, Mountain View, CA) and data analysis was conducted using FlowJo software (Tree Star Inc., Ashland, OR). FACS analysis was gated on the live cells only by using LIVE/DEAD Fixable Dead Cell Stain Kit.
2.6. Statistical analysis
Comparison of data was analyzed by two-tailed Student’s t test using Graphpad Prism 6.0. Differences were considered statistically significant when the p value was < 0.05.
3. RESULTS
3.1. In vitro differentiated Th17 cells express high levels of TNF
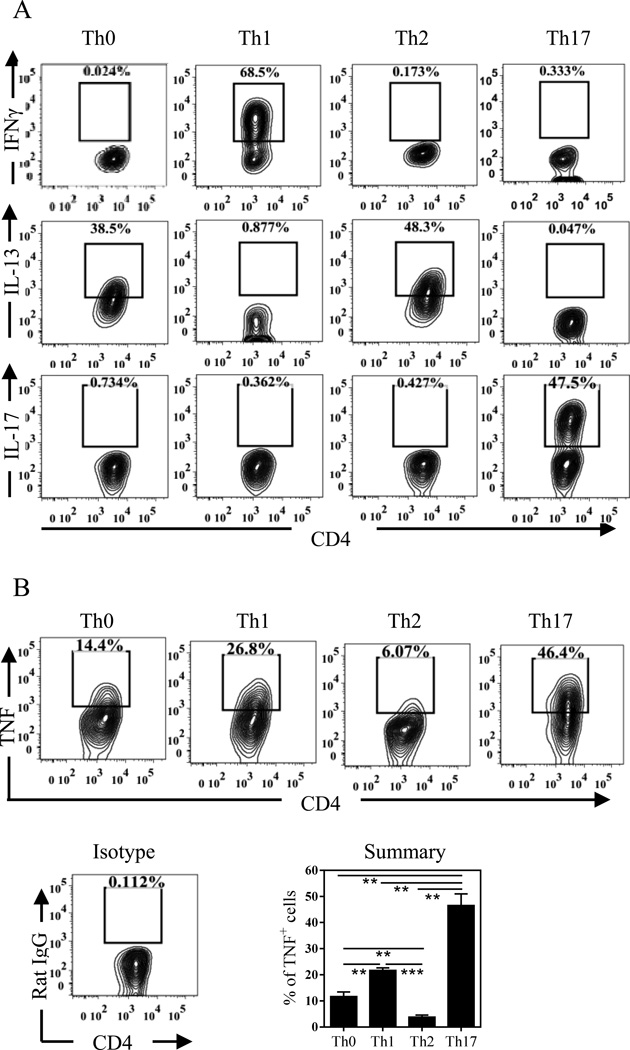
To determine the effect of various Th subsets on Tregs, we generated Th0, Th1, Th2 and Th17 subsets in vitro by stimulating naïve CD4 cells under the respective polarized culture conditions as described in Methods. After culture for 5 days, the phenotype of Th subsets was verified by intracellular cytokine staining (Fig 1A). Since TNF was reported to stimulate the activation of Tregs through TNFR2 [9, 10], we also determined the expression of this cytokine by Th subsets. Th17 cells expressed a high level of TNF (46%, Fig 1B), in addition to their expression of IL-17A (47%, Fig 1A). Th1 cells also expressed TNF at a markedly lower level of 26% than Th17 cells (p < 0.01, Fig 1B), but significantly higher than Th0 (14%) and Th2 cells (6%) (p < 0.01~0.001). Thus, in vitro differentiated Th17 cells expressed the highest level TNF and thus had the potential to promote phenotype stability and proliferative expansion of Tregs.
Figure 1.
Phenotype and expression of TNF by in vitro-generated CD4+ Th subsets. Flow-sorted naïve CD4 T cells from Ly5.2 C57BL/6 mice were cultured under Th0-, Th1-, Th2- and Th17-polarizing conditions with TCR stimulation for 5 days. The phenotype of Th subsets was analyzed by FACS (A). Intracellular TNF expression by Th subsets was determined by FACS (B). Representative FACS data from at least three separate experiments with similar results are shown on (A) and the upper panel of (B). The lower panel of (B) show isotype staining control, and a summary pooled from 2 to 3 experiments (N=6~12). Comparison of indicated groups: *p < 0.05; **p < 0.01. *** p < 0.001.
3.2. Th17 cells most potently promote the stabilization and expansion of Tregs in vivo
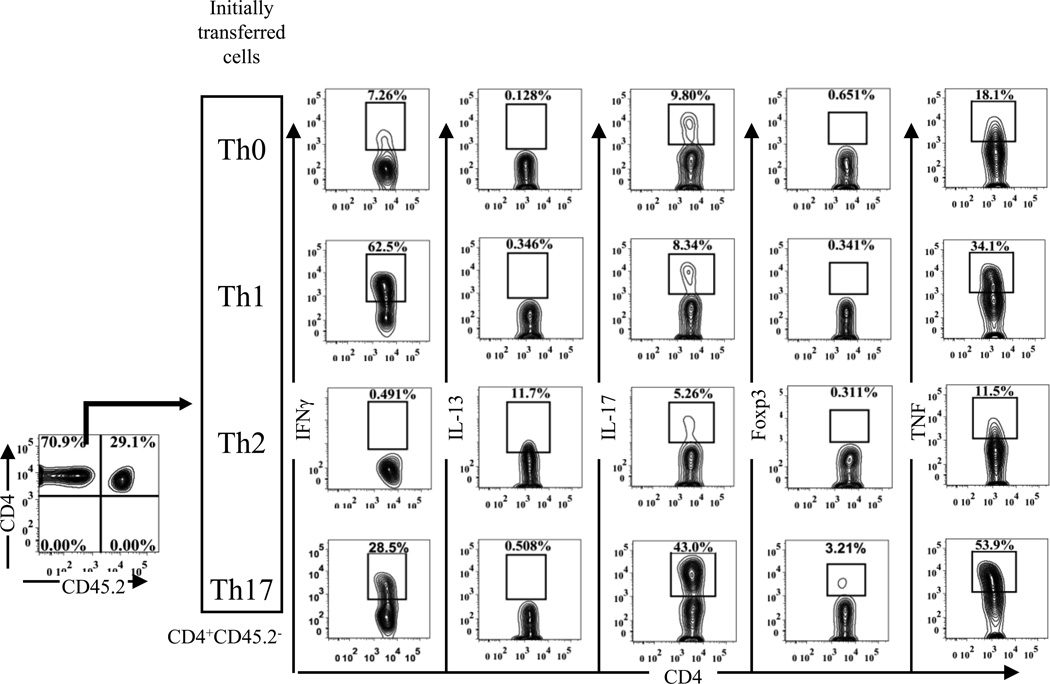
To test this idea, highly purified CD45.2+CD4+Foxp3/gfp+ Tregs were transferred alone or co-transferred with congenic CD45.1+ Th0, Th1, Th2 or Th17 cells into Rag1−/− mice at a 1:1 ratio. During the experimental period (5 weeks), the body weight of mice did not decreased (data not shown) and no signs of colitis were observed in any mice. Presumably, the proinflammatory effects of Th cells were suppressed by the co-transferred Tregs. The phenotype of transferred Th cells remained largely unchanged, except that a substantial fraction of Th17 cells (28%) expressed IFNγ (Fig 2). Furthermore, Th17 cells maintained the highest levels of TNF expression as compared with other Th subsets (Fig 2).
Figure 2.
Phenotype and expression of TNF by Th subsets after transfer into Rag1−/− mice. In vitro differentiated Th subsets were co-transferred with freshly flow-sorted Tregs at 1:1 ratio into Rag1−/− mice. After 5 weeks, Expression of IFNγ, IL-13, IL-17, Foxp3, and TNF by Th subsets recovered from spleen of recipient mice was analyzed by FACS, gating on CD4+CD45.2− cells. Representative FACS data from 3 separate experiments are shown.
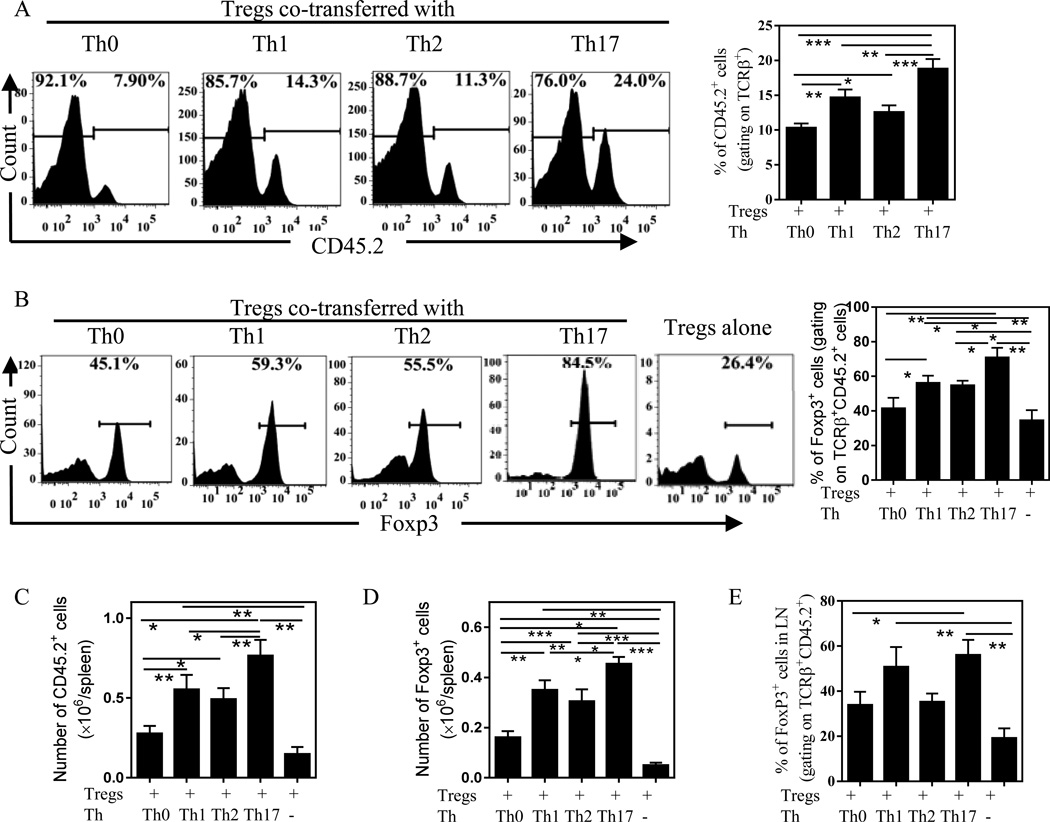
It has been shown that the transferred Foxp3+ Treg cells, similar to Foxp3− Teff cells, replicate in the lymphopenic mouse [15, 16]. Therefore, we first examined the effect of Th subsets on the expansion of Tregs in vivo. Although a 1:1 ratio of Th cells to Tregs was transferred into Rag1−/− mice, only 7.9% of Tregs co-transferred with Th0 cells were found in the total CD4 T cells recovered from recipient mouse spleens (Fig 3A). This was presumably caused by the greater proliferative nature of pre-activated Th cells or caused by a homeostatic mechanism to restore the natural ratio of Teffs:Tregs in the lymphopeneic mice in the course of re-population. Nevertheless, when co-transferred with Th17 cells, 24% of splenic CD4 T cells were Tregs, which was markedly higher than those co-transferred with other subsets of Th cells (Fig 3A, p < 0.01~0.001). Consequently, the absolute number of transferred Tregs in the spleen of recipient mice was highest when co-transferred with Th17 cells (Fig 3C, p < 0.05~0.01).
Figure 3.
Effect of CD4+ Th subsets on co-transferred Tregs in Rag1−/− mice. Flow-sorted CD4+FoxP3/gfp+ cells (CD45.2+8.5 × 104/mose) were transferred alone or co-transferred with Th0, or Th1, or Th2 or Th17 cells at 1:1 ratio into Rag1–/– mice. After 5 weeks, the proportion of Tregs in total transferred CD4+ T cells in the spleen of recipient mice was determined, based on CD45.2 expression (A). Foxp3 expression by transferred Tregs present in the spleen (B) and LNs (E) was analyzed by FACS, gating on TCRβ+CD4+CD45.2+ cells. Representative FACS data from three separate experiments with similar results are shown on the left and summary from 2 to 3 experiments (N=6~12) is shown on the right. The absolute number of transferred Tregs (TCRβ+CD45.2+) in the spleen is shown in (C) and the absolute number of Foxp3+ cells in the spleen is shown in (D), summarized from 3 separate experiments (N=10~12). Comparison of indicated groups: *p < 0.05; **p < 0.01. *** p < 0.001.
Next we asked if Th17 cells were the most effective Th subsets to maintain Treg phenotype by stabilizing Foxp3 expression. In Rag1−/− mice injected with Tregs alone, only 26% of Tregs present in the spleen of recipient mice expressed Foxp3 (Fig 3B), which was consistent with our previous report [11]. In contrast, Rag1−/− mice given Tregs and Th17 cells, the majority of Tregs (~85%) present in the spleen maintained their Foxp3 expression, which was markedly higher than that of Tregs co-transferred with Th0 (45%), Th2 (55%) and Th1 cells (59%, p < 0.05~0.01, Fig 3B). Furthermore, the absolute number of Foxp3-expressing cells in the spleen was markedly higher in mice when co-transferred with Th17 cells, as compared with mice co-transferred with other Th subsets (p<0.001~0.05, Fig 3D). Notably, the number of Foxp3+ cells in the spleen of mice co-transferred with Th0 cells, although 2.5~3.7-fold lower than those co-transferred with other Th subsets (p<0.01~0.001), was still 2.4-fold higher as compared with the number recovered when Treg alone (p<0.05, Fig 3D). Examination of LNs also showed the similar results (Fig 3E). These data clearly show that Th17 cells are the most potent population of Th cells in stabilizing the phenotype and in supporting the expansion of Tregs in the lymphopenic mice.
3.3. Both TNF and IL-2 contribute to the Treg-stimulatory effect of Th17 cells
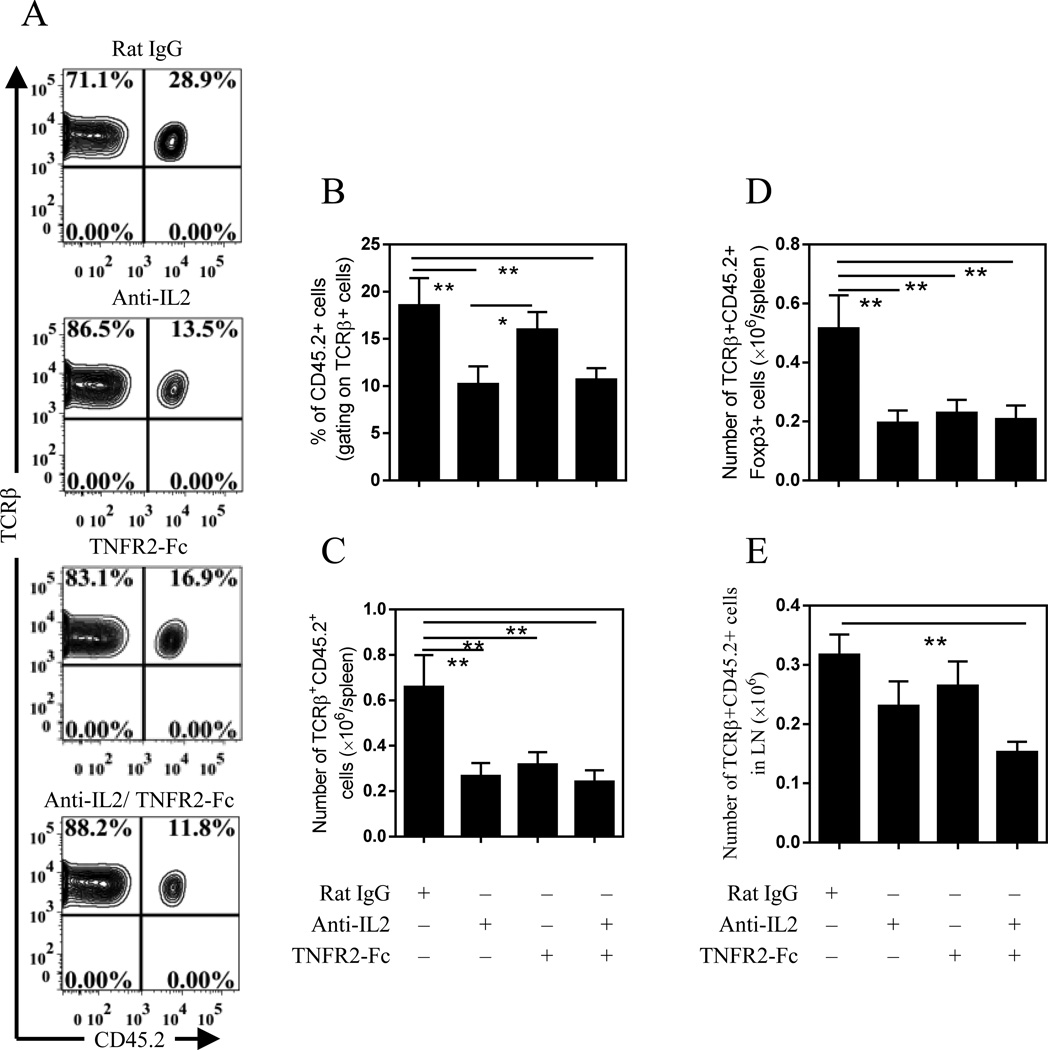
Since Th17 cells produced high levels of TNF, we investigated the role of this cytokine in the stimulatory effect of Th17 cells on Tregs in vivo. Further, it has been reported that Th17 cells persistently express IL-2 although at lower levels as compared with other Th subsets [17, 18]. We therefore also examined IL-2 derived from Th17 cells on Tregs. To this end, Rag1−/− mice co-administered with Th17 cells and Tregs were treated with neutralizing anti IL-2 Ab and/or TNFR2 Fc fusion protein (TNFR2-Fc). As shown in Fig 4 treatment with anti IL-2 Ab alone, or in combination with TNFR2-Fc, markedly decreased the proportion of Tregs in the spleen (Fig 4 A-B, p < 0.01). These inhibitors individually or in combination markedly reduced the absolute number of transferred Tregs (Fig 4C, p < 0.01) or Foxp3+ cells (Fig 4D, p < 0.01) recovered from the spleen, indicating that the expansion of Tregs in the recipient mice was at least partially dependent on IL-2 and TNF. A similar effect was also observed in the mesenteric LNs (mLNs), with a more profound reduction of Tregs when these two inhibitors were used together (p<0.01, Fig 4E). The combination of anti-IL-2 Ab and TNFR2-Fc did not further inhibit the expansion of Tregs in the spleen, presumably due to the maximal effect being achieved by each inhibitor alone. Consequently, IL-2 and TNF are each required to support Treg expansion. Treatment with anti IL-2 Ab and TNFR2-Fc did not inhibit the expansion of Th17 cells in Rag1−/− mice, and did not reduce their expression of IL-17A, IL-2 and TNF, as compared with Rat IgG treatment (Data not shown). Thus, the inhibitors acted on Treg cells.
Figure 4.
Effect of neutralizing anti IL-2 Ab and inhibitor of TNF on Tregs co-transferred with Th17 cells. Flow-sorted Tregs (CD45.2+) were co-transferred with Th17 cells (CD45.2−) into Rag1–/– mice as described in Fig 2. The mice were i.p. treated with anti-IL2 Ab or/and TNFR-Fc fusion protein (100 µg) once a week. After 5 wks, the proportion of Tregs in transferred T cells present in the spleen was analyzed by FACS. Typical FACS plots are shown in (A) and summary (N=12) is shown in (B). The number of transferred Treg cells in spleen is shown in (C, N=12), in LNs (mesenteric region) is shown in (E, N=6) and number of Foxp3+ Tregs in the spleen is shown in (D, N=12). Data in summary are pooled from 3 separate experiments. Comparison between indicated groups: *p < 0.05; **p < 0.01.
3.4. TNF-TNFR2 interaction is crucial for the reciprocal stimulatory effect of Th17 cells and Tregs
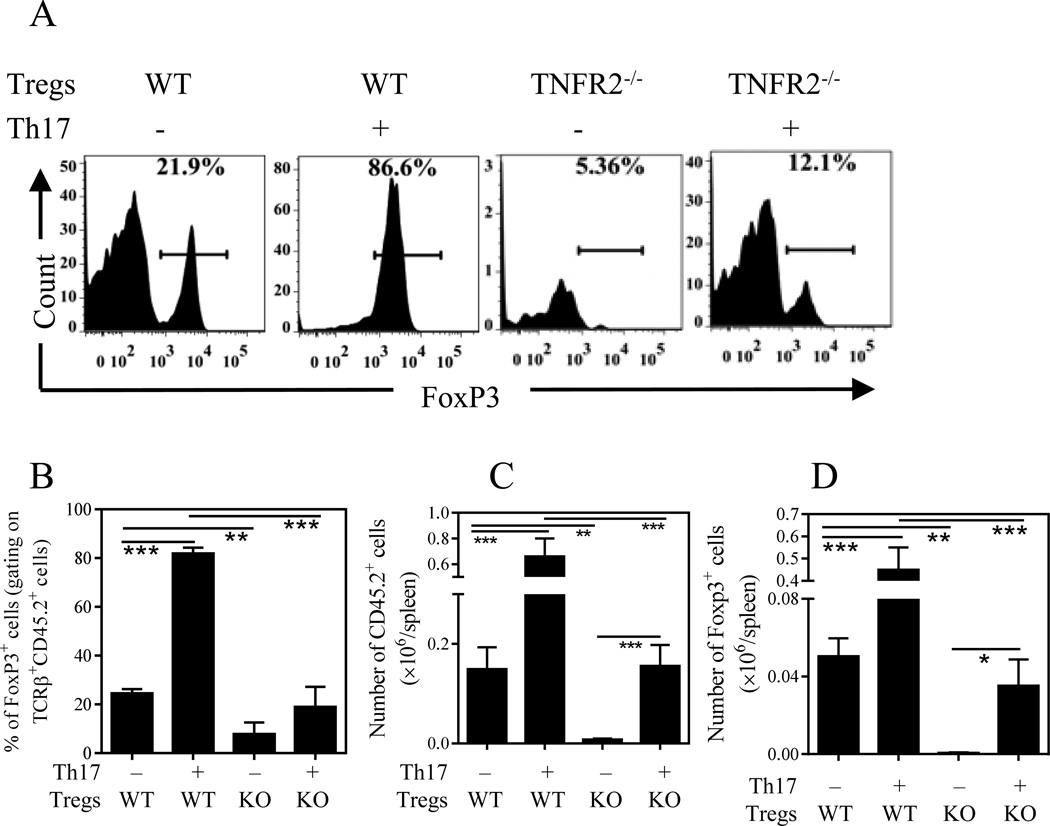
TNFR2-Fc was known to block the soluble form but not membrane-bound form of TNF [13], while the membrane-bound TNF preferentially activates TNFR2 [19]. Thus, TNFR2-Fc may not be able to completely block the stimulatory effect of membrane-bound TNF on TNFR2 expressed by Tregs. To further clarify the role of TNF-TNFR2 pathway in the boosting effect of Th17 cells on Tregs, we experimentally examined the effect of Th17 cells on Tregs obtained from TNFR2 KO mice. Consistent with our previous report [11], only 5% of TNFR2-deficient Tregs retained Foxp3 expression when they were transferred alone, which was markedly lower than when WT Tregs were transferred alone into Rag1−/− mice (22%, p < 0.01, Fig 5A). Although co-transferred Th17 cells markedly increased Foxp3 expression by WT Tregs (85%, p < 0.001), Th17 cells did not result in an increase in Foxp3 expression by TNFR2-deficient Tregs (p>0.05, in comparison with TNFR2-deficient Tregs transferred alone, Fig 5B). Furthermore, co-transfer of Th17 cells resulted in >3-fold increase in the number of transferred WT Tregs than TNFR2-deficient Tregs, and >10-fold increase in number of Foxp3+ cells in the spleen of mice treated with WT Tregs than with TNFR2-deficient Tregs (p<0.001, Fig 5C-D). Therefore, TNF-TNFR2 interaction was crucial to enable Th17 cells to stimulate Tregs. Nevertheless, the number of transferred TNFR2-deficient Tregs and the number of Foxp3+ cells were markedly increased by the co-transfer of Th17 cells, as compared with TNFR2-deficient Tregs transfer alone (p<0.05~0.0001, Fig 5C~D). This residual stimulatory effect of Th17 cells to Tregs in the absence of TNF-TNFR2 signaling is likely mediated by other pathways including the interaction of IL-2 with CD25.
Figure 5.
TNF-TNFR2 pathway is crucial for the stimulation of Th17 on Tregs. Flow-sorted Tregs (CD45.2+) from WT mice or from TNFR2–/– mice were transferred into Rag1–/– mice, with or without Th17 cells (CD45.2−). After 35 days, the expression of Foxp3 by transferred Tregs was analyzed by FACS, gating on live TCRβ+CD45.2+ cells. (A) Shows typical flow plots and (B) shows summary (N=3). (C) The absolute number of transferred Treg cells (C, N=3) and (D) absolute number of Foxp3+ Tregs in the spleen (N=3). Data shown are representatives of 2 separate experiments with same results. Comparison between indicated groups: *p < 0.05; **p < 0.01; *** p < 0.001.
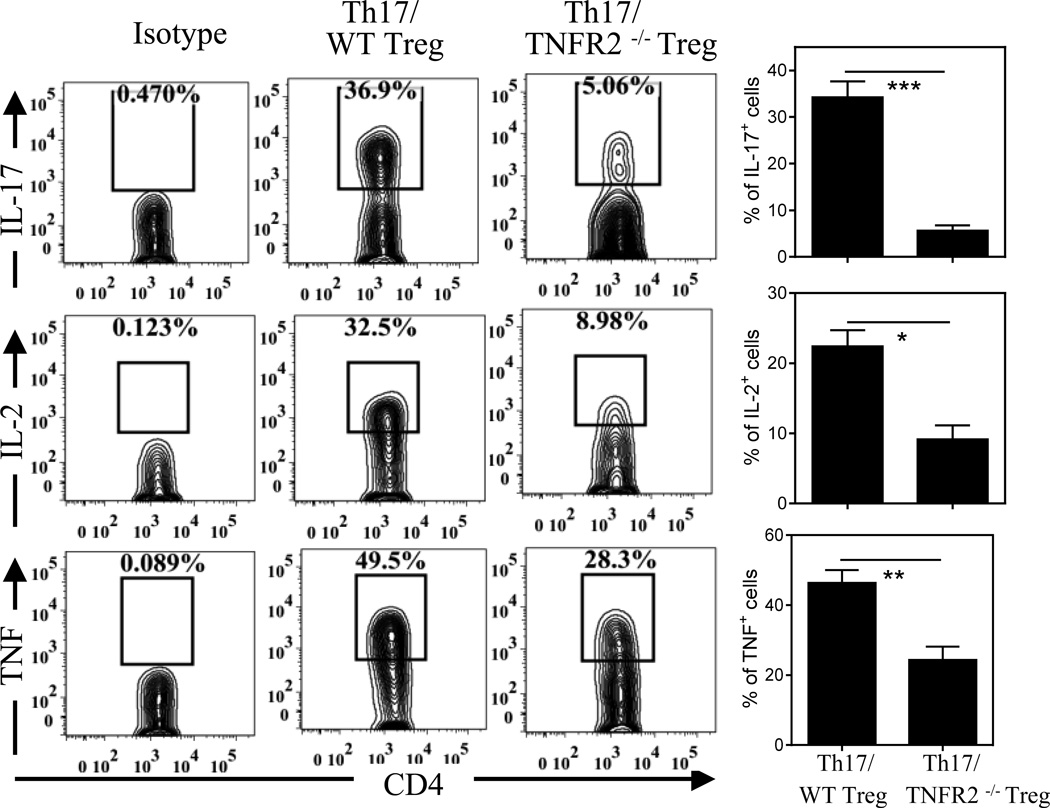
It has been shown in previous studies [20, 21] including our own [11] that co-transfer of WT Tregs resulted in a marked increase in the proportion of IL-17A-producing cells developed from transferred naïve CD4 cells in Rag1−/− mice. We thus hypothesized that Tregs might also help maintain the phenotype of Th17 cells. Indeed, when co-transferred with WT Tregs, Th17 cells recovered from spleen of recipient Rag1−/− mice were able to maintain the expression of Th17 signature cytokines, e.g., expressed high levels of IL-17A (36%), IL-2 (32%) and TNF (49%, Fig 6). To our surprise, when co-transferred with TNFR2−/− Tregs, the expression of IL-17A, IL-2 and TNF by Th17 cells was reduced by 86%, 72% and 42%, respectively (Fig 6p < 0.05~0.001), suggesting that TNFR2 expression by Tregs was also important for the maintenance of the Th17 phenotype. Presumably, the decrease in IL-2 production by Th17 cells may contribute to the down-regulation of Foxp3 expression and reduced expansion of co-transferred TNFR2−/− Tregs, in addition to the absence of TNF-TNFR2 stimulatory signaling.
Figure 6.
TNFR2 expression on Tregs promotes phenotypic stability of co-transferred Th17 cells. Th17 cells (CD45.2−) were co-transferred with flow-sorted Tregs (CD45.2+) from WT mice or from TNFR2–/– mice into Rag1–/– mice. After 35 days, lymphoid tissues were harvested. Expression of IL-17A, IL-2 and TNF by transferred Th17 cells recovered from spleen of recipient mice were analyzed by FACS, gating on TCRβ+CD45.2− cells in spleen. Typical FACS plots are shown on the left and summary (N = 3~8) are shown on the right. Data shown are representatives of 2 separate experiments with same results. Comparison between indicated groups: *p < 0.05; **p < 0.01; *** p < 0.001.
4. DISCUSSION
Development of distinct Th1, Th2 and Th17 effector function by Teff cells are crucial for host protection against diverse offending pathogens, while appropriately expanded and activated Tregs are indispensable for restraining excessive and prolonged inflammation and preventing collateral damage to normal tissues. The capacity of activated Teffs to stimulate Tregs, as revealed by recent studies [11, 12], is likely to represent a major mechanism by which a dynamic equilibrium is established between Teffs and Tregs in an ongoing immune or inflammatory response. In this study, we confirmed that all types of activated Teff cells, including Th0 cells, were able to support expansion and phenotypic stability of Tregs in vivo. However, our study indicates that the Treg-stimulating effect of Th subsets was distinct: Th17 cells appeared to be the most potent subset, which was followed in order by Th1, Th2 and Th0 cells.
Th17 cells have the capacity to produce members of the IL-17 family such as IL-17A and IL-17F, that target innate immune cells and epithelial cells [22]. This subset also produces the highest levels of TNF. Interestingly, although named for the production of IL-17, Th17 cells also expressed similar or even higher levels of TNF than IL-17A in vitro (Fig 1) and in vivo (Fig 2 and Fig 6). Since having been identified as a novel CD4 Th subset, Th17 cells were shown to be responsible for the pathogenetic effects of an increasing number of chronic inflammatory disorders, which was previously attributed to Th1 cells [22]. Unlike Th1 cells, Th17 cells have stem cell-like features [23, 24], and are able to mediate sustained autoimmune inflammation [25]. It is reasonable to hypothesize that the potent Treg-stimulatory activity of Th17 cells co-evolved with their robust and prolonged proinflammatory effector function. Indeed, most Th17 cells in the body reside in the barrier tissues including intestinal tracts and skin [22], while Tregs are also abundant in these interfaces of host and microbiota [26, 27]. This co-existence may enable the immune system to mount effective responses against pathogens, while simultaneously maintaining self-tolerance.
In fact, both Th17 cells and Treg cells are frequently most abundant in the same anatomical compartment, such as lamina propria of intestine [27], inflamed synovial fluids [28] and in aggressively growing breast cancer [29], which may suggest that their relationship is far more complicated than just counteracting each other’s functions. Indeed, differentiation of Th17 cells and Tregs from naïve CD4 cells are reciprocally induced in the presence of TGFβ, contingent upon the presence of either IL-6 or IL-2, respectively [30–32]. Tregs have clearly been shown to inhibit the activation of Th1 and Th2 cells [33, 34], however, the susceptibility of Th17 cells to Treg-mediated inhibition is still controversial. Although a certain subset of Tregs was reported to inhibit Th17 cells [35], nevertheless, Tregs were also able to promote the differentiation of Th17 cells [11, 20, 21], by providing TGFβ [11, 20, 21] and inhibiting Th1 and Th2 responses [36, 37]. Furthermore, it was reported that Tregs increased the production of IL-17 cytokine by Teffs and therefore promoted the capacity of Th17 responses to suppress infections of mucosal fungus [38]. Treg cells were also shown to play a critical role in promoting Th17-mediated rejection of skin allograft [39] and in promoting pulmonary fibrosis [40]. These studies further corroborate the role of Tregs in the differentiation of Th17 cells and consequently in promoting Th17 function in vivo. Our study provides novel evidence that Tregs also contribute to the stability of Th17 phenotype of previously differentiated Th17 cells. Increasing Treg activity, by either stimulating in vivo expansion of Tregs or adoptive transfer of in vitro expanded Tregs, has become a strategy in the treatment of autoimmunity, allograft rejection and GVHD [41]. Our observation, together with the aforementioned studies, clearly suggests the potential risk of developing Th17-mediated inflammation by such Treg-based treatment. On the other hand, caution should also be used when targeting Th17 cells in the treatment of autoimmunity, since it may be undesirable to reduce immunosuppressive Treg activity as a side effect.
It has also been proposed that IL-2 signals are crucial for the reciprocal balance between Th17 cells and Tregs [27]. By consuming IL-2, which inhibits the differentiation of Th17 cells [42], Tregs cells could also promote Th17 cell survival and function [20, 38]. Our data accentuate the crucial role of TNF-TNFR2 signal in the reciprocal stimulatory effect of proinflammatory Th17 cells and immunosuppressive Tregs. Intriguingly, Tregs deficient in TNFR2 resulted in the reduction of IL-2 expression by co-transferred Th17 cells. This suggests a primary role of TNF produced by Th17 cells in the stimulation of Tregs. Supportive to this notion, the potency of Treg-stimulatory effect of Th subsets (Th17>Th1>Th0) is correlated with their capacity to express TNF (Th17>Th1>Th0), but not with their capacity to express IL-2 [18]. Nevertheless, in addition to TNF and IL-2, other factor(s) may also contribute to this effect, since Th2 cells expressed lower levels of TNF and IL-2 while having more potent Treg-stimulatory activity than Th0 cells.
Taken together, our data provide novel insights into the complex interplay between Th subsets and revealed that Th17 cells potently stimulate the expansion and promote the phenotypic stability of Tregs. This effect is mainly through a mechanism dependent on TNF-TNFR2 signaling, which also plays a key role in the maintenance of Th17 phenotype by Tregs. This effect should be taken into account when designing future therapy of autoimmunity and cancer by targeting Th17 cells, Tregs and TNF or TNFR2.
Highlights.
Th17 cells express the highest levels of TNF than other Th subsets
Th17 cells most potently stimulate co-transferred Tregs in Rag1 KO mice
This effect of Th17 cells is TNF- and IL-2-dependent
TNF-TNFR2 plays crucial role in reciprocal stimulation of Th17 cells and Tregs
Acknowledgments
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E. This Research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Abbreviations
- FoxP3
forkhead box P3
- KI
knock in
- KO
knock out
- nCD4
naïve CD4 cells
- Teffs
effector T cells
- TNFR2
tumor necrosis factor receptor type II
- TNFR2-Fc
TNFR2 Fc fusion protein
- Tregs
regulatory T cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. Government
References
- 1.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annual review of immunology. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X, Oppenheim JJ, Winkler-Pickett RT, Ortaldo JR, Howard OM. Glucocorticoid amplifies IL-2-dependent expansion of functional FoxP3(+)CD4(+)CD25(+) T regulatory cells in vivo and enhances their capacity to suppress EAE. European journal of immunology. 2006;36:2139–2149. doi: 10.1002/eji.200635873. [DOI] [PubMed] [Google Scholar]
- 3.Ruter J, Barnett BG, Kryczek I, Brumlik MJ, Daniel BJ, Coukos G, et al. Altering regulatory T cell function in cancer immunotherapy: a novel means to boost the efficacy of cancer vaccines. Frontiers in bioscience : a journal and virtual library. 2009;14:1761–1770. doi: 10.2741/3338. [DOI] [PubMed] [Google Scholar]
- 4.Maddur MS, Miossec P, Kaveri SV, Bayry J. Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. The American journal of pathology. 2012;181:8–18. doi: 10.1016/j.ajpath.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 5.Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nature reviews Drug discovery. 2012;11:763–776. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- 6.Wilke CM, Bishop K, Fox D, Zou W. Deciphering the role of Th17 cells in human disease. Trends in immunology. 2011;32:603–611. doi: 10.1016/j.it.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korn T, Anderson AC, Bettelli E, Oukka M. The dynamics of effector T cells and Foxp3+ regulatory T cells in the promotion and regulation of autoimmune encephalomyelitis. Journal of neuroimmunology. 2007;191:51–60. doi: 10.1016/j.jneuroim.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. Journal of immunology. 2004;172:6519–6523. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Baumel M, Mannel DN, Howard OM, Oppenheim JJ. Interaction of TNF with TNF receptor type 2 promotes expansion and function of mouse CD4+CD25+ T regulatory cells. Journal of immunology. 2007;179:154–161. doi: 10.4049/jimmunol.179.1.154. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Wu X, Zhou Q, Howard OM, Netea MG, Oppenheim JJ. TNFR2 is critical for the stabilization of the CD4+Foxp3+ regulatory T. cell phenotype in the inflammatory environment. Journal of immunology. 2013;190:1076–1084. doi: 10.4049/jimmunol.1202659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grinberg-Bleyer Y, Saadoun D, Baeyens A, Billiard F, Goldstein JD, Gregoire S, et al. Pathogenic T cells have a paradoxical protective effect in murine autoimmune diabetes by boosting Tregs. J Clin Invest. 2010;120:4558–4568. doi: 10.1172/JCI42945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stummvoll GH, DiPaolo RJ, Huter EN, Davidson TS, Glass D, Ward JM, et al. Th1, Th2, and Th17 effector T cell-induced autoimmune gastritis differs in pathological pattern and in susceptibility to suppression by regulatory T cells. Journal of immunology. 2008;181:1908–1916. doi: 10.4049/jimmunol.181.3.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Howard OM, Oppenheim JJ. Pertussis toxin by inducing IL-6 promotes the generation of IL-17-producing CD4 cells. Journal of immunology. 2007;178:6123–6129. doi: 10.4049/jimmunol.178.10.6123. [DOI] [PubMed] [Google Scholar]
- 15.Yurchenko E, Shio MT, Huang TC, Da Silva Martins M, Szyf M, Levings MK, et al. Inflammation-Driven Reprogramming of CD4(+)Foxp3(+) Regulatory T Cells into Pathogenic Th1/Th17 T Effectors Is Abrogated by mTOR Inhibition in vivo. PLoS One. 2012;7:e35572. doi: 10.1371/journal.pone.0035572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duarte JH, Zelenay S, Bergman ML, Martins AC, Demengeot J. Natural Treg cells spontaneously differentiate into pathogenic helper cells in lymphopenic conditions. Eur J Immunol. 2009;39:948–955. doi: 10.1002/eji.200839196. [DOI] [PubMed] [Google Scholar]
- 17.Yu CR, Oh HM, Golestaneh N, Amadi-Obi A, Lee YS, Eseonu A, et al. Persistence of IL-2 expressing Th17 cells in healthy humans and experimental autoimmune uveitis. European journal of immunology. 2011;41:3495–3505. doi: 10.1002/eji.201141654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quintana FJ, Jin H, Burns EJ, Nadeau M, Yeste A, Kumar D, et al. Aiolos promotes TH17 differentiation by directly silencing Il2 expression. Nature immunology. 2012;13:770–777. doi: 10.1038/ni.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grell M, Douni E, Wajant H, Lohden M, Clauss M, Maxeiner B, et al. The transmembrane form of tumor necrosis factor is the prime activating ligand of the 80 kDa tumor necrosis factor receptor. Cell. 1995;83:793–802. doi: 10.1016/0092-8674(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Haines CJ, Gutcher I, Hochweller K, Blumenschein WM, McClanahan T, et al. Foxp3(+) regulatory T cells promote T helper 17 cell development in vivo through regulation of interleukin-2. Immunity. 2011;34:409–421. doi: 10.1016/j.immuni.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Sujino T, Kanai T, Ono Y, Mikami Y, Hayashi A, Doi T, et al. Regulatory T cells suppress development of colitis, blocking differentiation of T-helper 17 into alternative T-helper 1 cells. Gastroenterology. 2011;141:1014–1023. doi: 10.1053/j.gastro.2011.05.052. [DOI] [PubMed] [Google Scholar]
- 22.Weaver CT, Elson CO, Fouser LA, Kolls JK. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annual review of pathology. 2013;8:477–512. doi: 10.1146/annurev-pathol-011110-130318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kryczek I, Zhao E, Liu Y, Wang Y, Vatan L, Szeliga W, et al. Human TH17 cells are long-lived effector memory cells. Science translational medicine. 2011;3:104ra0. doi: 10.1126/scitranslmed.3002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, Sanchez-Perez L, et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity. 2011;35:972–985. doi: 10.1016/j.immuni.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi G, Ramaswamy M, Vistica BP, Cox CA, Tan C, Wawrousek EF, et al. Unlike Th1, Th17 cells mediate sustained autoimmune inflammation and are highly resistant to restimulation-induced cell death. Journal of immunology. 2009;183:7547–7556. doi: 10.4049/jimmunol.0900519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirahara K, Liu L, Clark RA, Yamanaka K, Fuhlbrigge RC, Kupper TS. The majority of human peripheral blood CD4+CD25highFoxp3+ regulatory T cells bear functional skin-homing receptors. Journal of immunology. 2006;177:4488–4494. doi: 10.4049/jimmunol.177.7.4488. [DOI] [PubMed] [Google Scholar]
- 27.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 28.Grose RH, Millard DJ, Mavrangelos C, Barry SC, Zola H, Nicholson IC, et al. Comparison of blood and synovial fluid th17 and novel peptidase inhibitor 16 Treg cell subsets in juvenile idiopathic arthritis. The Journal of rheumatology. 2012;39:2021–2031. doi: 10.3899/jrheum.111421. [DOI] [PubMed] [Google Scholar]
- 29.Benevides L, Cardoso CR, Tiezzi DG, Marana HR, Andrade JM, Silva JS. Enrichment of regulatory T cells in invasive breast tumor correlates with the upregulation of IL-17A expression and invasiveness of the tumor. European journal of immunology. 2013 doi: 10.1002/eji.201242951. [DOI] [PubMed] [Google Scholar]
- 30.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 31.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 32.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Venuprasad K, Kong YC, Farrar MA. Control of Th2-mediated inflammation by regulatory T cells. The American journal of pathology. 2010;177:525–531. doi: 10.2353/ajpath.2010.090936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koch MA, Thomas KR, Perdue NR, Smigiel KS, Srivastava S, Campbell DJ. T-bet(+) Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor beta2. Immunity. 2012;37:501–510. doi: 10.1016/j.immuni.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fletcher JM, Lonergan R, Costelloe L, Kinsella K, Moran B, O'Farrelly C, et al. CD39+Foxp3+ regulatory T Cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. Journal of immunology. 2009;183:7602–7610. doi: 10.4049/jimmunol.0901881. [DOI] [PubMed] [Google Scholar]
- 36.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nature immunology. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 37.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature immunology. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pandiyan P, Conti HR, Zheng L, Peterson AC, Mathern DR, Hernandez-Santos N, et al. CD4(+)CD25(+)Foxp3(+) regulatory T cells promote Th17 cells in vitro and enhance host resistance in mouse Candida albicans Th17 cell infection model. Immunity. 2011;34:422–434. doi: 10.1016/j.immuni.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vokaer B, Van Rompaey N, Lemaitre PH, Lhomme F, Kubjak C, Benghiat FS, et al. Critical role of regulatory T cells in Th17-mediated minor antigen-disparate rejection. Journal of immunology. 2010;185:3417–3425. doi: 10.4049/jimmunol.0903961. [DOI] [PubMed] [Google Scholar]
- 40.Song L, Weng D, Liu F, Chen Y, Li C, Dong L, et al. Tregs promote the differentiation of Th17 cells in silica-induced lung fibrosis in mice. PloS one. 2012;7:e37286. doi: 10.1371/journal.pone.0037286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tran DQ, Shevach EM. Therapeutic potential of FOXP3(+) regulatory T cells and their interactions with dendritic cells. Human immunology. 2009;70:294–299. doi: 10.1016/j.humimm.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liao W, Lin JX, Wang L, Li P, Leonard WJ. Modulation of cytokine receptors by IL-2 broadly regulates differentiation into helper T cell lineages. Nature immunology. 2011;12:551–559. doi: 10.1038/ni.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]