Abstract
Iron is essential for many metabolic pathways, but is toxic in excess. Recent identification of the ferric iron reductase LFR1, the ferrous iron transporter LIT1, and the heme transporter LHR1 greatly advanced our understanding of how Leishmania parasites acquire iron and regulate its uptake. LFR1 and LIT1 have close orthologs in plants, and are required for Leishmania virulence. Consistent with the lack of heme biosynthesis in trypanosomatids, LHR1 and LABCG5, a protein involved in heme salvage from hemoglobin, seem essential for Leishmania survival. LFR1, LIT1 and LHR1 are upregulated under low iron availability, in agreement with the need to prevent excessive iron uptake. Future studies should clarify how Leishmania interacts with the iron homeostasis machinery of its host cell, the macrophage.
Introduction
Leishmaniasis, which is caused by the intracellular protozoan parasite Leishmania, is reported to be the ninth largest infectious disease burden worldwide. The World Health Organization currently estimates that as many as 1.6 million cases of leishmaniasis occur annually, of which approximately 40,000 result in death [1]. The disease affects humans, livestock and pets, and the latter two can act as reservoirs for the parasites. The disease's manifestation, which varies depending upon the infecting species and host, ranges from self-healing cutaneous lesions to a more severe visceralizing form that can be fatal if left untreated (Table 1) [2]. Infection is initiated by the bite of an infected sand fly, which inoculates the host with the infective, metacyclic form of the parasite [3]. Once the infection is established, the parasites replicate as amastigotes within parasitophorous vacuoles (PV) of macrophages, which resemble acidic phagolysosomes [4]. Whereas most microorganisms are destroyed in this harsh environment, the intracellular stages of Leishmania have specialized adaptive mechanisms that allow them to survive and acquire essential nutrients and minerals from the host cell. One of such essential nutrients, iron, can be obtained within the PV as inorganic iron or in the form of iron-containing porphyrins such as heme. Recent studies revealed that Leishmania expresses several membrane proteins that are specialized in the acquisition of inorganic iron or heme. It is important to note that, due to its redox potential, iron can be toxic in high amounts; therefore acquisition systems must be tightly regulated. Although significant strides have been made in determining how Leishmania acquires and utilizes iron, many questions remain. Here we discuss recent developments in this area and their implications for our understanding of the delicate balance that Leishmania parasites must achieve in their quest for iron.
Table 1. Leishmania species reported to cause clinical symptoms in humans.
| Major Disease Manifestation | ||||
|---|---|---|---|---|
| Species | Cutaneous | Diffuse Cutaneous | Mucocutaneous | Visceral |
| L. tropica | □ | |||
| L. major | □ | |||
| L. aethiopica | □ | □ | ||
| L. donovani | □ | |||
| L. infantum (L. chagasi) | □ | □ | ||
| L. mexicana | □ | □ | ||
| L. amazonensis | □ | □ | □ | |
| L. pifanoi | □ | |||
| L. braziliensis | □ | □ | □ | |
| L. panamensis | □ | □ | ||
| L. peruviana | □ | |||
| L. guyanensis | □ | □ | ||
| L. siamensis | □ | |||
Leishmania possess a plant-like system for the acquisition of inorganic iron
Inorganic iron is largely available as the ferric (Fe3+) form, which is insoluble at physiological pH. Within the mammalian host, ferric iron (Fe3+) is delivered to cells bound to the carrier protein transferrin. Upon binding to transferrin receptors (TfR), the iron-containing holotransferrin is internalized via endocytosis, and Fe3+ is released when it reaches an acidified intracellular compartment [5]. To cross membranes Fe3+ must be reduced to Fe2+, a highly reactive form of iron that must be tightly controlled. Most of the Fe3+ entering cells complexed to tranferrin is reduced to Fe2+ by a host ferric reductase and translocated to the cytosol by Nramp2/DMT1, an endosomal membrane transporter [6]. However, it is believed that a small amount of holotransferrin can keep moving deeper into the endocytic pathway and reach Leishmania PVs [7], where it becomes available for acquisition by the parasites.
Initial reports postulated that Leishmania expressed proteins capable of acting as receptors for transferrin [8], but subsequent studies suggested that these were non-specific interactions [9]. Experiments with L. chagasi revealed the presence a NADPH-dependent ferric reductase activity associated with the cell surface of live parasites [9], indicating for the first time the existence of a potential pathway for direct membrane translocation of Fe2+. Genome searches based on homology to plant reductases [10] led to identification of LFR1, the L. amazonensis plasma membrane-associated ferric reductase [11]. LFR1 is a119 kDa membrane protein that contains FAD- and NADPH-binding sites and putative heme-binding sites within its transmembrane regions. Similar to its close homologue FRO2 in Arabidopsis thaliana, LFR1 uses cytosolic NADPH as an electron source and contains heme molecules that facilitate the transport of electrons across the lipid bilayer, reducing Fe3+ to Fe2+ [10, 11]. Fe2+ is then transported directly to the parasite's cytosol by LIT1, the transmembrane ferrous iron transporter from the ZIP family first identified in L. amazonensis [10, 12], and already reviewed elsewhere [13]. Together, LFR1 and LIT1 provide Leishmania with an inorganic iron acquisition pathway that has several similarities with the system found in plants (Figure 1).
Figure 1. Schematic representation of iron acquisition pathways in Leishmania and in plants.
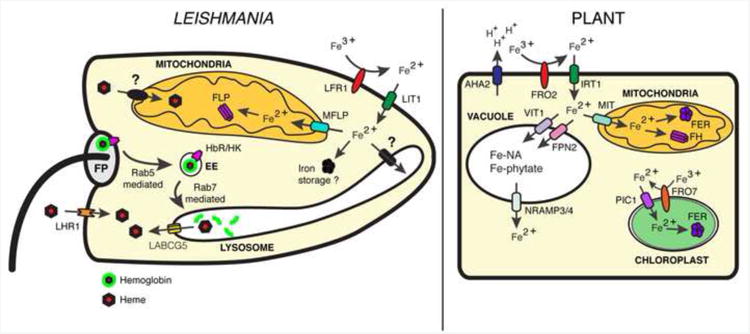
Leishmania and plants utilize a ferric reductase (LFR1/FRO2) to reduce iron and a ferrous iron transporter (LIT1/IRT1) to transport it into the cytosol. In plants, specific membrane proteins transport iron into the vacuole (VIT1/FPN2), mitochondria (MIT), or chloroplast (PIC1) and store it bound to nicotiamine (Fe-NA) or phytate (Fe-phytate) in the vacuole, bound to ferritin (FER) or frataxin (FH) in the mitochondria, or to ferritin in the chloroplast. In Leishmania, there are no identified iron transporters in the lysosome, but a mitoferrin-like protein (MFLP) may transport iron into the mitochondria for storage in association with a frataxin-like protein (FLP). There are no known ferritin orthologues in Leishmania. Iron-containing heme is also be acquired by Leishmania in two ways. Hemoglobin may bind to a hexokinase (HbR/HK) and traffic to the parasite's lysosome, where it is degraded releasing heme which is translocated to the cytosol by the ABC transporter, LABCG5. Heme is also transported directly into the cytosol by the heme transporter, LHR1.
Importantly, as in plants, LFR1 and LIT1 are regulated by iron levels, with the expression of both genes being upregulated under low iron conditions [11, 14, 15]. The LIT1 protein is only detected on the plasma membrane of iron-deprived promastigotes [10] or intracellular amastigotes [9]. The ferric reductase activity associated with promastigotes is also elevated in a low iron environment, presumably reflecting an increase in LFR1 protein levels [11]. LFR1 and LIT1 null mutant promastigotes can grow axenically in iron rich media but are unable to grow as amastigotes within macrophage PVs, unless supplemented with cationic ferritin as a source of iron [11].
Once iron has been transferred into the cytosol, its subsequent processing and storage is unknown. In higher eukaryotes, cytoplasmic iron is stored in association with the protein ferritin [16]. Extensive searches of the published genomes of various Leishmania species have so far failed to yield a putative ferritin orthologue candidate. This suggests several possible scenarios. One is that Leishmania may not have a system in place to store cytosolic iron. This possibility may explain the observation that moderate amounts of Fe-NTA (ferric nitriloacetate) are toxic to Leishmania [15]. Another possible scenario is that, much like yeast and brown algae which also lack ferritin, Leishmania may store iron in a mineralized form within lysosomal compartments [17]. Alternatively, it may utilize frataxin, which contains >16 atoms of iron per molecule, to store iron in the mitochondria [18]. This latter possibility is particularly appealing, since the Leishmania genome includes genes encoding a frataxin-like protein, and also a mitoferrin-like protein possibly responsible for iron transport into the mitochondria. Additional studies are needed to clarify the alternative mechanisms used by Leishmania for iron storage in the apparent absence of ferritin.
Leishmania express molecules that allow heme acquisition from the host
Heme is an iron-coordinated porphyrin with iron at the center of the molecule, which can adopt either an oxidized ferric state or a reduced ferrous state [19]. Heme is a critically important prostetic group that allows proteins to participate in various functions such as oxidative [20] and lipid metabolism [20, 21], redox homeostasis [22], and iron acquisition [11]. In metazoans, heme is synthesized in an eight-step pathway with the first and three last steps occurring within the mitochondria [23]. Genome sequencing revealed that all but the last three enzymes in the heme biosynthetic pathway are missing in trypanosomatid parasites [24, 25]. These findings explain the requirement for addition of either hemin or protoporphyrin IX (PPIX) to growth media used for axenically cultivating Leishmania [26]. In vivo, this auxotrophy explicitly requires Leishmania to acquire heme from the host. The evidence available to date indicates that Leishmania can acquire heme by two mechanisms, hemoglobin receptor-mediated endocytosis followed by heme salvage [27-29] and direct transmembrane transport (Figure 1) [29, 30]. These two pathways are outlined below.
Sengupta et. al. showed that hemoglobin binds to the N-terminal region of a 46 kDa protein in the flagellar pocket of L. donovani, followed by endocytosis [31]. The hemoglobin-binding protein was later identified as a hexokinase (LdBPK_210300.1) [27], but it remains unclear if this has a functional significance as a link between the iron acquisition pathway and other metabolic processes. Additional work demonstrated that hemoglobin is internalized by a clathrin-mediated process [28] and traffics along the endocytic pathway to the parasite's lysosome, in a process mediated by the small GTPase Rab proteins Rab 5 [32] and Rab 7 [33]. Once in the lysosome hemoglobin is degraded releasing heme, which was proposed to be salvaged by translocation into the cytosol by the ATP-binding cassette protein, LABCG5 [29].
A second, more rapid mechanism for heme acquisition in Leishmania occurs by direct transport of extracellular heme into the parasites' cytosol [30]. The existence of a specific heme receptor and/or transporter in these parasites was suggested by studies showing specific binding of heme to L. amazonensis [34] and L. infantum [35], but no specific protein mediating this process was identified. The molecular machinery responsible for heme transport was also unknown in higher eukaryotes, until recently when the first bona fide eukaryotic heme permeases were identified. Genetic screens in C. elegans identified HRG-1, which exports heme from the lysosome into the cytosol, and HRG-4, which functions as a heme transporter at the plasma membrane [36, 37]. A genome-wide search of proteins with similarity to C. elegans HRG proteins led to identification of L. amazonensis LHR1, a small 20 kDa protein predicted to have four transmembrane domains and ∼45% similarity to HRG-4 [30]. LHR1 localizes to both the plasma membrane and lysosomes in L. amazonensis, controls the size of the parasite intracellular heme pool, and directly promotes uptake of radioactive heme when expressed in yeast [30]. LHR1 null mutants could not be obtained and thus appear not to be viable, suggesting an essential role for this transporter in the promastigote stages of L. amazonensis. LHR1 orthologs are present in several trypanosomatid genomes, and are likely to correspond to the long sought-after heme transporter previously proposed to exist in several parasite species, including Trypanosoma cruzi [38].
How does Leishmania regulate their iron-responsive genes?
High iron concentrations can generate highly toxic hydroxyl radicals, creating the need for mechanisms for tightly regulating iron acquisition pathways. In L. amazonensis, a genome-wide analysis revealed a set of genes that are differentially regulated by iron levels in the culture medium [15]. Importantly, as expected from their role in iron and heme acquisition, transcripts from LFR1, LIT1, and LHR1 were upregulated in the response to iron deprivation [11, 14, 15, 30]. Trypanosomatid parasites do not control gene expression through classic transcriptional promoters, utilizing instead post-transcriptional strategies such as regulation of mRNA stability or of protein translation initiation. This form of regulation is thought to be mediated by control elements found within the 5′ and 3′ untranslated regions (UTRs) of mature mRNAs [33-35]. In metazoans, expression of iron-responsive proteins is controlled by iron response proteins (IRPs) that bind to iron response elements (IREs), hairpin structures present in the 3′UTR of gene transcripts [39]. A protein that binds mammalian IREs was identified in L. tarentolae [40], but studies in T. brucei showed that a protein highly related to a mammalian IRP was not required for regulation of the iron-responsive transferrin receptor [41]. The latter study led to the suggestion that trypanosomatids may regulate iron-responsive genes by a mechanism distinct from the IRE/IRP paradigm. Although potential regulatory motifs have not yet been identified in Leishmania iron-regulated genes, this goal is likely to be facilitated by the increasing availability of data on global transcriptional responses to iron [15].
A promising future line of investigation will be to determine whether iron-responsive genes are differentially regulated in species of Leishmania that cause different forms of clinical disease. Because of the extensive phagocytosis of senescent red blood cells performed by macrophages from deeper organs such as the spleen [42], markedly different levels of iron are expected to be available within skin macrophages where cutaneous species of Leishmania replicate, when compared to liver Kupffer cells and spleen macrophages that harbor visceralizing species [43]. Suggesting the existence of species-specific adaptations in heme uptake regulation, differences are observed in iron-dependent expression of the heme transporter LHR1 and in uptake of a fluorescent heme analog when the cutaneous species L. amazonensis and the visceral species L. infantum are compared (Figure 2).
Figure 2. Species-specific gene regulation of the Leishmania heme transporter LHR1.
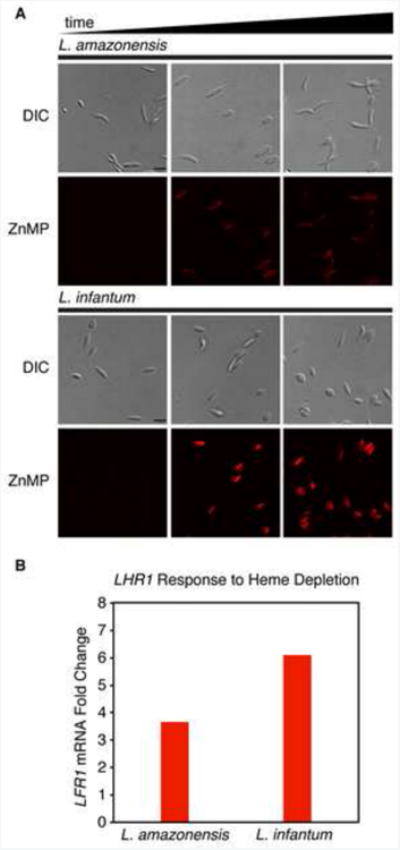
(A) L. amazonensis and L. infantum were grown in heme depleted media containing the fluorescent heme analog, zinc mesoporphyrin (ZnMP) and imaged over a period of 16 hours. L. infantum showed greater accumulation of ZnMP than L. amazonensis. (B) Transcript levels of LHR1 after heme depletion in the culture medium, showing higher upregulation in L. infantum when compared to L. amazonensis.
Conclusions
Several molecular players responsible for iron acquisition in Leishmania have been identified recently. Earlier studies postulated that the parasites might express receptors for transferrin, but this has not been confirmed. The discovery of reductase activity associated with the cell surface of these parasites [9] opened the way to the subsequent identification of the ferric iron reductase LFR1, which generates the substrate for the ferrous iron transporter LIT1 [6,9]. More recently, identification of the heme transporter LHR1 [30], a hemoglobin receptor [33] and the ATP-binding cassette protein LABCG5 involved in heme salvage from endocytosed hemoglobin [29] solved the long-standing puzzle of how trypanosomatids acquire this essential iron-containing molecule in the absence of a functional biosynthetic pathway.
Future studies should be focused on answering two questions: (i) Which are the additional components of the Leishmania machinery for iron acquisition, storage and utilization? (ii) How does Leishmania effectively gain access to iron within their host cell, the macrophage? The later question can be addressed by taking advantage of the extensive information available on the iron homeostasis system of mammalian macrophages. Macrophages play an important role in vivo as iron reservoirs, releasing iron into the circulation through the export pump ferroportin, which can be rapidly removed from the cell surface in response to the peptide hepcidin generated during inflammation. Therefore, it is conceivable that intracellular amastigotes of Leishmania have strategies to modulate the capacity of macrophages to store iron. It is already known that L. donovani and L. major increase iron uptake in macrophages by upregulating expression of transferrin receptors (TfR) [44], and that L. amazonensis enhances fusion of TfR-containing vesicles with the PV [7]. With the numerous additional molecular tools now available, our knowledge of how Leishmania manipulates iron homeostasis pathways in macrophages is poised to expand rapidly in the near future.
Highlights.
Iron and heme acquisition are essential for Leishmania virulence
Leishmania express LFR1, a transmembrane ferric reductase that converts Fe3+ in Fe2+
The Leishmania ferrous iron transporter express LIT1 translocates Fe2+ to the cytosol
The Leishmania heme transporter LHR1 translocates heme into the parasite's cytosol
The closest orthologs of LFR1 and LIT1 are found in plants
LFR1, LIT1 and LHR1 expression is upregulated under low iron availability
Acknowledgments
Work discussed in this review was supported by NIH grant R01 AI067979 to Norma W. Andrews.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
*of special interest (9, 10, 23, 36, 38, 42, 44)
**of outstanding interest (11, 14, 15, 24, 29, 30)
- 1.Alvar J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5):e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCall LI, Zhang WW, Matlashewski G. Determinants for the development of visceral leishmaniasis disease. PLoS Pathogens. 2013;9(1):e1003053. doi: 10.1371/journal.ppat.1003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaye P, Scott P. Leishmaniasis: complexity at the host-pathogen interface. Nat Rev Microbiol. 2011;9(8):604–15. doi: 10.1038/nrmicro2608. [DOI] [PubMed] [Google Scholar]
- 4.Antoine JC, et al. The biogenesis and properties of the parasitophorous vacuoles that harbour Leishmania in murine macrophages. Trends Microbiol. 1998;6(10):392–401. doi: 10.1016/s0966-842x(98)01324-9. [DOI] [PubMed] [Google Scholar]
- 5.Andrews NC. Iron homeostasis: insights from genetics and animal models. Nature reviews. Genetics. 2000;1(3):208–217. doi: 10.1038/35042073. [DOI] [PubMed] [Google Scholar]
- 6.Andrews NC. The iron transporter DMT1. Int J Biochem Cell Biol. 1999;31(10):991–4. doi: 10.1016/s1357-2725(99)00065-5. [DOI] [PubMed] [Google Scholar]
- 7.Borges VM, Vannier-Santos MA, de Souza W. Subverted transferrin trafficking in Leishmania-infected macrophages. Parasitol Res. 1998;84(10):811–22. doi: 10.1007/s004360050493. [DOI] [PubMed] [Google Scholar]
- 8.Voyiatzaki CS, Soteriadou KP. Identification and isolation of the Leishmania transferrin receptor. J Biol Chem. 1992;267(13):9112–9117. [PubMed] [Google Scholar]
- 9*.Wilson ME, et al. Leishmania chagasi: uptake of iron bound to lactoferrin or transferrin requires an iron reductase. Exp Parasitol. 2002;100(3):196–207. doi: 10.1016/s0014-4894(02)00018-8. By detecting a ferric iron reductase activity on the surface of Leishmania chagasi, this study was the first important step towards clarification of the iron uptake pathway in these parasites. [DOI] [PubMed] [Google Scholar]
- 10*.Morrissey J, Guerinot ML. Iron uptake and transport in plants: the good, the bad, and the ionome. Chem Rev. 2009;109(10):4553–4567. doi: 10.1021/cr900112r. A comprehensive review of iron acquisition and trafficking in plants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11**.Flannery AR, et al. LFR1 ferric iron reductase of Leishmania amazonensis is essential for the generation of infective parasite forms. J Biol Chem. 2011;286(26):23266–23279. doi: 10.1074/jbc.M111.229674. This study identified and functionally characterized LFR1, the Leishmania amazonensis ferric iron reductase. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacques I, Andrews NW, Huynh C. Functional characterization of LIT1, the Leishmania amazonensis ferrous iron transporter. Molecular and Biochem Parasitol. 2010;170(1):28–36. doi: 10.1016/j.molbiopara.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huynh C, Andrews NW. Iron acquisition within host cells and the pathogenicity of Leishmania. Cellular Microbiology. 2008;10(2):293–300. doi: 10.1111/j.1462-5822.2007.01095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14**.Huynh C, Sacks DL, Andrews NW. A Leishmania amazonensis ZIP family iron transporter is essential for parasite replication within macrophage phagolysosomes. J Exp Med. 2006;203(10):2363–2375. doi: 10.1084/jem.20060559. This study identified and functionally characterized LIT1, the Leishmania amazonensis ferrous iron transporter. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15**.Mittra B, et al. Iron uptake controls the generation of Leishmania infective forms through regulation of ROS levels. J Exp Med. 2013;210(2):401–416. doi: 10.1084/jem.20121368. This study demonstrates that iron uptake regulates differentiation of infective Leishmania amazonensis amastigotes, and includes the first RNAseq analysis of parasite global transcriptional responses to iron limiting conditions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theil EC. Iron, ferritin, and nutrition. Annu Rev Nutr. 2004;24:327–43. doi: 10.1146/annurev.nutr.24.012003.132212. [DOI] [PubMed] [Google Scholar]
- 17.Böttger LH, et al. Atypical iron storage in marine brown algae: a multidisciplinary study of iron transport and storage in Ectocarpus siliculosus. J Exp Bot. 2012;63(16):5763–5772. doi: 10.1093/jxb/ers225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adamec J, et al. Iron-dependent self-assembly of recombinant yeast frataxin: implications for Friedreich ataxia. Am J Hum Gen. 2000;67(3):549–562. doi: 10.1086/303056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Severance S, Hamza I. Trafficking of heme and porphyrins in metazoa. Chem Rev. 2009;109(10):4596–4616. doi: 10.1021/cr9001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tripodi KEJ, Menendez Bravo SM, Cricco JA. Role of heme and heme-proteins in trypanosomatid essential metabolic pathways. Enzyme Res. 2011;2011:873230. doi: 10.4061/2011/873230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verma S, Mehta A, Shaha C. CYP5122A1, a novel cytochrome P450 is essential for survival of Leishmania donovani. PLoS ONE. 2011;6(9):e25273. doi: 10.1371/journal.pone.0025273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adak S, Pal S. Ascorbate Peroxidase Acts As a Novel Determiner of Redox Homeostasis in Leishmania. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2012.4745. [DOI] [PubMed] [Google Scholar]
- 23*.Hamza I, Dailey HA. One ring to rule them all: trafficking of heme and heme synthesis intermediates in the metazoans. Biochem Biophys Acta. 2012;1823(9):1617–1632. doi: 10.1016/j.bbamcr.2012.04.009. An outstanding review discussing heme biosynthesis and trafficking. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24**.Alves JMP, et al. Identification and phylogenetic analysis of heme synthesis genes in trypanosomatids and their bacterial endosymbionts. PLoS ONE. 2011;6(8):e23518. doi: 10.1371/journal.pone.0023518. This paper includes an in-depth bioinformatic analysis of genes involved in heme biosynthesis in trypanosomatids in parallel with their bacterial endosymbionts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kořený L, Lukeš J, Oborník M. Evolution of the haem synthetic pathway in kinetoplastid flagellates: an essential pathway that is not essential after all? Int J Parasitol. 2010;40(2):149–156. doi: 10.1016/j.ijpara.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Chang CS, Chang KP. Heme requirement and acquisition by extracellular and intracellular stages of Leishmania mexicana amazonensis. Mol Biochem Parasitol. 1985;16(3):267–276. doi: 10.1016/0166-6851(85)90069-6. [DOI] [PubMed] [Google Scholar]
- 27.Krishnamurthy G, et al. Hemoglobin receptor in Leishmania is a hexokinase located in the flagellar pocket. J Biol Chem. 2005;280(7):5884–5891. doi: 10.1074/jbc.M411845200. [DOI] [PubMed] [Google Scholar]
- 28.Agarwal S, et al. Clathrin-mediated hemoglobin endocytosis is essential for survival of Leishmania. Biochem Biophys Acta. 2013;1833(5):1065–1077. doi: 10.1016/j.bbamcr.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 29**.Campos-Salinas J, et al. A new ATP-binding cassette protein is involved in intracellular haem trafficking in Leishmania. Molecular Microbiology. 2011;79(6):1430–1444. doi: 10.1111/j.1365-2958.2010.07531.x. This paper demonstrates that there are two independent pathways of heme uptake in Leishmania donovani, one involving rapid specific transport and one involving hemoglobin degradation followed by lysosomal degradation and heme salvage by the ATP-binding cassette protein LABCG5. [DOI] [PubMed] [Google Scholar]
- 30**.Huynh C, et al. Heme Uptake by Leishmania amazonensis Is Mediated by the Transmembrane Protein LHR1. PLoS Pathogens. 2012;8(7):e1002795. doi: 10.1371/journal.ppat.1002795. This study identified and functionally characterized LHR1, the Leishmania amazonensis heme transporter. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sengupta S, et al. Hemoglobin endocytosis in Leishmania is mediated through a 46-kDa protein located in the flagellar pocket. J Biol Chem. 1999;274(5):2758–2765. doi: 10.1074/jbc.274.5.2758. [DOI] [PubMed] [Google Scholar]
- 32.Singh SB, et al. Rab5-mediated endosome-endosome fusion regulates hemoglobin endocytosis in Leishmania donovani. EMBO J. 2003;22(21):5712–5722. doi: 10.1093/emboj/cdg557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel N, et al. Leishmania requires Rab7-mediated degradation of endocytosed hemoglobin for their growth. Proc Natl Acad SciUSA. 2008;105(10):3980–3985. doi: 10.1073/pnas.0800404105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galbraith RA, McElrath MJ. Heme binding to Leishmania mexicana amazonensis. Mol Biochem Parasitol. 1988;29(1):47–53. doi: 10.1016/0166-6851(88)90118-1. [DOI] [PubMed] [Google Scholar]
- 35.Carvalho S, et al. Heme as a source of iron to Leishmania infantum amastigotes. Acta Tropica. 2009;109(2):131–135. doi: 10.1016/j.actatropica.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 36*.Rajagopal A, et al. Haem homeostasis is regulated by the conserved and concerted functions of HRG-1 proteins. Nature. 2008;453(7198):1127–1131. doi: 10.1038/nature06934. An important study describing how a genetic screen in C. elegans led to identification of the first eukaryotic proteins capable of mediating heme transport. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White C, et al. HRG1 is essential for heme transport from the phagolysosome of macrophages during erythrophagocytosis. Cell Metabolism. 2013;17(2):261–270. doi: 10.1016/j.cmet.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Cupello MP, et al. The heme uptake process in Trypanosoma cruzi epimastigotes is inhibited by heme analogues and by inhibitors of ABC transporters. Acta Tropica. 2011;120(3):211–218. doi: 10.1016/j.actatropica.2011.08.011. A study showing that Trypanosoma cruzi parasites are able to take up heme directly from the environment. [DOI] [PubMed] [Google Scholar]
- 39.Anderson CP, et al. Mammalian iron metabolism and its control by iron regulatory proteins. BBA - Mol Cell Res. 2012;1823(9):1468–1483. doi: 10.1016/j.bbamcr.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meehan HA, Lundberg RA, Connell GJ. A trypanosomatid protein specifically interacts with a mammalian iron-responsive element. Parasitol Res. 2000;86(2):109–114. doi: 10.1007/s004360050019. [DOI] [PubMed] [Google Scholar]
- 41.Fast B, et al. Iron-dependent regulation of transferrin receptor expression in Trypanosoma brucei. Biochem J. 1999;342(3):691. [PMC free article] [PubMed] [Google Scholar]
- 42*.Ganz T. Macrophages and systemic iron homeostasis. J Innate Immun. 2012;4(5-6):446–453. doi: 10.1159/000336423. An excellent review outlining the macrophage iron response to infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCall LI, Zhang WW, Matlashewski G. Determinants for the development of visceral leishmaniasis disease. PLoS Pathogens. 2013;9(1):e1003053. doi: 10.1371/journal.ppat.1003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44*.Das NK, et al. Leishmania donovani depletes labile iron pool to exploit iron uptake capacity of macrophage for its intracellular growth. Cellular Microbiology. 2009;11(1):83–94. doi: 10.1111/j.1462-5822.2008.01241.x. A study providing evidence that the intracellular growth of Leishmania causes depletion of the labile iron pool in its host cell, the macrophage. [DOI] [PMC free article] [PubMed] [Google Scholar]


