Abstract
A reversed-phase high performance liquid chromatographic (LC), tandem mass spectrometry (MS/MS) assay for the determination of tenofovir (TFV) and emtricitabine (FTC) in dried blood spots (DBS) from human whole blood was developed and validated. Whole blood samples were spotted, dried, and a 3mm punch was extracted with methanol for analysis by LC-MS/MS utilizing stable isotope labeled internal standards. The assay was validated over the range of 2.5ng/mL to 1,000ng/mL for TFV and 2.5ng/mL to 5,000ng/mL for FTC. The method was accurate (within ± 15% of control) and precise (coefficient of variation ≤ 15%) for hematocrit concentrations ranging from 25% to 76%; using edge punches versus center punches; and spot volumes of 10µL to 50µL. Analytes were stable for five freeze/thaw cycles and up to 6 days at room temperature, whereas long-term storage required −20°C or −80°C. Comparison of TFV and FTC in DBS versus plasma yielded r2 ≥ 0.96, indicating that DBS can be used as a plasma alternative for pharmacokinetic analyses in vivo.
Keywords: Analytical method, dried blood spot, nucleoside analog, antiretroviral therapy, LC-MS/MS
1. Introduction
Tenofovir (TFV) and emtricitabine (FTC) are widely-prescribed antiretroviral drugs used for the treatment and prophylaxis of HIV infection [1]. These agents are co-formulated as Truvada, among other co-formulation products. Both agents are also active against Hepatitis B virus (HBV) infection, although only tenofovir has an indication for HBV treatment [2, 3]. Measurement of TFV and FTC in plasma has been used to monitor adherence to therapy, and to evaluate pharmacokinetics in special populations such as infants, children, pregnancy, and for drug-drug interaction studies [1, 4–6]. Thus far, most assays have utilized plasma or serum matrices for these studies.
Plasma processing is time consuming, and it requires technical personnel and onsite or nearby centrifugation. Blood collection for harvesting plasma typically requires ≥ 4mL of blood, which can add up to large volumes when conducting pharmacokinetic studies among infants or pediatric patients. The dried blood spot (DBS) blood sampling strategy offers advantages to plasma drawbacks: blood for DBS can be collected and processed at any clinic or research facility quickly and easily, and DBS requires only ~25 µL of whole blood, making it suitable for pharmacokinetic studies in special patient populations [7]. Because of these advantages, many DBS methods have been developed for a variety of drugs, including antiretrovirals [7], but a DBS method for tenofovir and emtricitabine has not been developed, to our knowledge. An important consideration for tenofovir and emtricitabine in DBS will be the high accumulation of phosphorylated tenofovir in red blood cells [8]. We previously described preliminary analyses and the potential clinical application of DBS for adherence monitoring in subjects receiving tenofovir and emtricitabine, but we did not describe the method validation [8]. This communication provides the methodology and supporting analytical validation results for the measurement of TFV and FTC in DBS.
2. Methods
2.1. Chemicals and materials
TFV and FTC were acquired from the NIH AIDS Research & Reference Reagent Program (Germantown, MD, USA). TFV isotopic internal standard (13C5 TFV-iso, MW=292.2) and FTC-isotopic internal standard (15 N2,13C1 FTC-iso, MW=250.2) were purchased from Moravek Biochemicals, Inc (Brea, CA, USA). Methanol (HPLC Grade) and formic acid were purchased from Fisher Scientific, (Fairlawn, NJ, USA), and acetonitrile was purchased from J.T. Baker (Phillipsburg, NJ, USA). Whatman 903 Protein Saver Cards, desiccants, and humidity indicators were obtained from Fisher Scientific. Human blank whole blood with EDTA anticoagulant was obtained from Biological Specialty Corporation and from consented participants in human research protocols that were approved by the local institutional review board (IRB).
2.2. Preparation of stocks and standard calibrators, quality control and internal standard samples
Individual standard prep stocks (1mg/mL) of TFV and FTC were prepared in ultra-pure water (UPH2O). These were used to make the combined standard working stocks of TFV/FTC in UPH2O. Twenty microliters of working stocks were combined with 480 microliters whole blood to arrive at final concentrations of TFV/FTC for standard calibrators (2.5/2.5, 5.0/5.0, 10/10, 25/25, 50/50, 100/100, 250/250, 500/500, 750/1000, 1000/5000 ng/mL ). Separate quality control (QC) prep stocks (1 mg/mL) of TFV and FTC were prepared in UPH2O. These were combined to prepare QC working stocks and the validation QC samples in the same way as standard calibrators. The concentrations of validation QC samples were prepared at 6 different levels: Level 1: 2.5/2.5, Level 2: 5.0/5.0, Level 3: 7.5/7.5, Level 4: 15/15, Level 5: 200/400, and Level 6: 800/4000 ng/mL. The combined working internal standard (IS) solution of TFV-iso and FTC-iso were made in UPH2O yielding a final concentration of approximately 16.7 ng/mL. Purity and salt content were taken into consideration when preparing prep stocks. All solutions were stored at 4°C in glass vials.
2.3. Preparation of dried blood spot sample
DBS were prepared by spotting 25 µL of whole blood that contained either standard or QC onto 903 Protein Saver Cards (except for testing the effect of spot volumes which were spotted using serial volumes of 5 µL to 50 µL). The pipette tip was not allowed to touch the paper. After spotting, the cards were allowed to dry for at least for 2 hours and up to overnight. Once dried, cards were placed in plastic bags and stored in a sample box with desiccant and humidity indicators at room temperature (RT), 4°C, −20°C, and/or −80°C for the storage condition tests.
2.4. Sample extraction from DBS
One 3mm diameter disc was punched with a Harris micro-punch (Sigma Aldrich, St. Louis, MO, USA) from the blood spots for extraction. An additional punch from a clean card was performed between each DBS sample punch to minimize the potential for carry-over. The punched discs were placed in a micro-centrifuge tube and TFV and FTC were extracted with 200 µL of 100% methanol, and 20 µL of working IS. Samples were sonicated for 10 minutes and centrifuged for 1 minute. Supernatants were then dried and reconstituted in 100 µL UPH2O for LC-MS/MS analysis.
2.5. Clinical study samples
DBS samples were collected from consented study subjects participating in IRB approved protocols with a DBS component [8]. Blood was collected in EDTA Vacutainers and 25 µL were spotted as described above. Samples were collected from subjects at variable times post dose and variable durations of TFV/FTC therapy. Samples from human subjects were used to complement QC samples to evaluate punch location, spot volume, and stability testing. To evaluate TFV/FTC DBS concentrations versus plasma concentrations, a total of 30 selected DBS samples with paired plasma samples were analyzed, as described previously [8].
2.6. LC-MS/MS instrumentation
Quantification of TFV and FTC and their respective internal standards was performed with high performance liquid chromatography tandem mass spectrometry (LC-MS/MS) with an approach similar to previously published methods [9, 10]. The instrumentation was a Thermo Scientific TSQ Vantage® triple quadrupole mass spectrometer coupled with a Thermo Scientific Accela® UHP pump (Thermo Scientific, San Jose, USA) and CTC Analytics HTC PAL® auto sampler. The Vantage® system utilized the Ion Max HESI II® electrospray ionization (ESI) probe, which was operated in ESI + mode. The selective (SRM) and highly selective reaction monitoring (HSRM) [precursor/product]+ transitions (m/z) were: TFV (288.04/176.11), TFV-IS (293.04/181.11), FTC (248.10/130.00), FTC-IS (251.10/133.00). 20 µL of extracted DBS solution was injected onto the system and analytes were separated with an isocratic mobile phase (0.1% Formic Acid in 0.5% Acetonitrile: 99.5% UPH2O) with a flow rate of 250 µL/min. The analytical column was Synergi Polar RP 2.5 µM, 100A, 2.0X100mm, purchased from Phenomenex (Torrance, CA, USA). The retention times were approximately 2.3 minutes for TFV and 4.6 minutes for FTC. Data were captured and analyzed with Xcalibur™ 2.0.7 SP1 software (Thermo Scientific San Jose, USA).
2.7. Validation strategy
DBS is regarded as an alternative matrix bio-analytical methodology with no standard validation guidelines. Therefore, accepted bio-analytical methodology validation practices were used as a guide to evaluate accuracy and precision [11, 15]. Validation was demonstrated if inter-assay and intra-assay accuracy (% deviation from nominal) and precision (% coefficient of variation (CV)) were within 15% at all QC levels, except the lower limit of quantitation (LLOQ) where within 20% was allowed. Additionally, literature references for DBS methodologies were utilized to determine other important factors that should be considered when validating a method for a DBS matrix [7, 12–14]. These included effects of punch location, spot volume, hematocrit, and dilution/punch-stacking. Acceptance criteria for tested conditions were within ± 15% deviation from the control and/or nominal and ≤ 15% CV for replicate analysis.
The validation also included the use of human subject samples to assess validation factors that cannot be imitated by spiking TFV/FTC into whole blood and spotting. This was especially important for TFV/FTC because these drugs are phosphorylated in red blood cells. For TFV, this phosphorylation leads to substantial accumulation of phosphorylated anabolites with repeated dosing. Because these phosphorylated a nabolites are susceptible to hydrolysis when released from the intracellular compartment, it was important to include samples from various durations of therapy in the validation. In order to evaluate samples at different levels of drug accumulation, samples were utilized from initial dosing (Day 1) and accumulated dosing (Day 20 and/or Day 30) from human subjects receiving a daily TDF/FTC regimen.
3. Validation results and discussion
3.1. Accuracy and precision
3.1.1. Standard performance
A single calibration curve was run with each of 5 analytical runs that assessed accuracy and precision. The calibration curve included 10 concentration levels ranging from 2.5 to 1000 ng/mL for TFV and 2.5 to 5000 ng/mL for FTC, as described above. Back-calculated concentration values and resulting calibration curve parameters were recorded to assess inter-day accuracy and precision of the standards used for calibration of the validation analytical runs. Each analyte utilized a stable isotope labeled internal standard and the resulting peak area ratios were fitted linearly with 1/x2 weighting. Acceptance criteria for back-calculated standards were ±20% accuracy (within runs) and precision (between runs) at the LLOQ and ±15% at all other levels. If a standard was outside the acceptable range the standard point was dropped from the calibration curve and the calibration curve was recalculated. The calibration curve performance is summarized in Table 1a. For the TFV calibration curve, the mean slope was 0.00679 with a %CV of 11.7%; the mean r2 value was 0.9952. For FTC, the mean slope was 0.00611 with a %CV of 7.9%; the mean r2 value was 0.9959. The accuracy (% deviation) was ≤ 7.9% and 7.2% for TFV and FTC, and precision was ≤ 7.1% and 6.5%, respectively.
Table 1.
Assay accuracy and precision validation results: 1a Standard performance from 5 validation runs. Linear regression curves were fit with 1/x2 weighting. 1b Quality control performance at 5 quality control concentrations.
| a. Back Calculated Calibration Standards | TFV | FTC |
|---|---|---|
| Calibration Standard Range | 2.5 to 1000 ng/mL | 2.5 to 5000 ng/mL |
| Interassay Accuracy (% Dev ) (n=5) | −7.9% to 5.4% | −7.2% to 4.8% |
| Interassay Precision (%CV ) (n=5) | 2.7% to 7.1% | 2.0% to 6.5% |
| Slope Mean (n=5) | 6.79E-03 | 6.11E-03 |
| Slope Precision (n=5) | 11.7% | 7.90% |
| Coefficient of Determination (r^2) Mean (n=5) | 0.9952 | 0.9959 |
| b. Quality Control Accuracy and Precision | TFV | FTC |
|---|---|---|
| Quality Control Level 1 (LLOQ) | 2.5 ng/mL | 2.5 ng/mL |
| Interassay Accuracy (%Dev) (n=25) | −1.4 | 3.1 |
| Interassay Precision (%CV) (n=25) | 13.3 | 9.1 |
| Intraassay Accuracy (%Dev)(n=5) | −14.6 to 10.0 | −1.4 to 8.0 |
| Intraassay Precision (%CV) (n=5) | 4.1 to 18.8 | 5.4 to 12.0 |
| Quality Control Level 2 | 5.0 ng/mL | 5.0 ng/mL |
| Interassay Accuracy (%Dev) (n=25) | 5.2 | 0.1 |
| Interassay Precision (%CV) (n=25) | 9.1 | 6.6 |
| Intraassay Accuracy (%Dev)(n=5) | −1.1 to12.0 | −3.8 to 6.0 |
| Intraassay Precision (%CV) (n=5) | 5.0 to12.4 | 4.0 to 7.2 |
| Quality Control Level 4 | 15 ng/mL | 15 ng/mL |
| Interassay Accuracy (%Dev) (n=25) | 3.3 | 0.9 |
| Interassay Precision (%CV) (n=25) | 6.0 | 5.6 |
| n I traassay Accuracy (%Dev)(n=5) | −1.7 to 7.2 | −1.8 to 6.4 |
| Intraassay Precision (%CV) (n=5) | 3.8 to 8.1 | 2.8 to 7.0 |
| Quality Control Level 5 | 200 ng/mL | 400 ng/mL |
| Interassay Accuracy (%Dev) (n=25) | 3.1 | −0.4 |
| Interassay Precision (%CV) (n=25) | 5.4 | 4.5 |
| Intraassay Accuracy (%Dev)(n=5) | −2.5 to 7.7 | −3.0 to 5.5 |
| Intraassay Precision (%CV) (n=5) | 1.5 to 6.9 | 1.4 to 4.4 |
| Quality Control Level 6 | 800 ng/mL | 4000 ng/mL |
| Interassay Accuracy (%Dev) (n=25) | 7.1 | 3.9 |
| Interassay Precision (%CV) (n=25) | 8.0 | 5.5 |
| Intraassay Accuracy (%Dev)(n=5) | −1.7 to 14.5 | 0.8 to 7.4 |
| Intraassay Precision (%CV) (n=5) | 4.1 to 6.5 | 3.5 to 7.3 |
3.1.2. Quality control validation samples
Five quality control validation levels (Levels 1, 2, 4, 5, and 6; previously described) were analyzed in five replicates in each of the 5 validation runs to assess inter- and intra-run accuracy and precision. QC validation levels 1 and 2 were utilized to assess the LLOQ for the assay. Level 6 was within 80% of the highest calibration standard, as described above. A summary of the results for accuracy and precision for both within run (intra-assay) and between run (inter-assay) are shown in Table 1b. The accuracy and precision were within ±14.6% and ±18.8% at 2.5ng/mL for both TFV and FTC, establishing 2.5ng/mL as the LLOQ for the assay. The accuracy and precision for all other TFV and FTC QC levels were within ±14.5% and ±12.4%, respectively. Thus, the lower limit of TFV/FTC quantification for a 3mm DBS punch (approximately 3µL of whole blood) was 2.5 ng/mL, which is comparable with previous plasma methodologies that extract ≥100µL of plasma [10, 16, 17]. This high sensitivity with low blood volumes could be utilized for intensive pharmacokinetic studies in infants and pediatrics where blood volume limitations are important considerations [4, 7].
3.1.3 Precision using clinical research samples
Intra-assay precision was further assessed by analyzing clinical study subject samples (n=9) from six subjects. Each sample was extracted in 5 replicates. The mean concentration ranges of the measured TFV and FTC were 16.9 to 104 ng/mL and 36.5 to 1437 ng/mL, respectively. The precision (%CV) of the determinations were all within 8.8%. The subject sample results were consistent with the QC validation sample results indicating a DBS method precise in measurement of TFV/FTC, consistent with accepted validation guidelines [11].
3.2. Specificity and selectivity
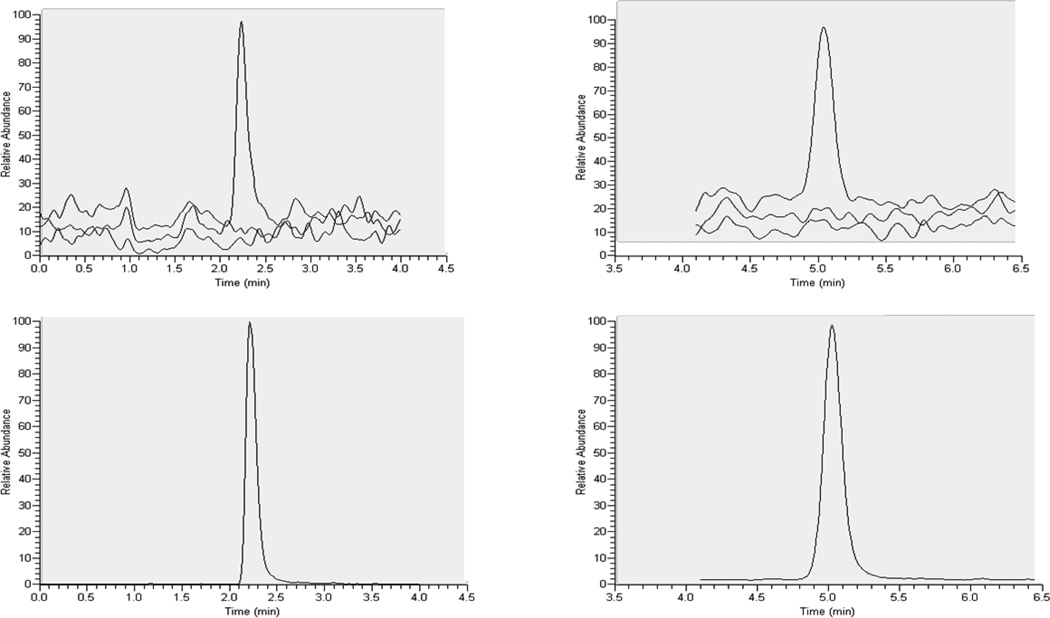
DBS specificity was assessed by preparing DBS from 6 separate lots of blank whole blood and one pooled lot of blank whole blood (n=6 lots). Cross talk was evaluated by injection of extracted DBS high standard (STD A; TFV/FTC of 1000/5000 ng/mL) without IS and an extracted Blank containing IS. The carryover was evaluated by injecting extracted STD A followed by an extracted DBS blank sample. There was no significant TFV/FTC or respective IS response observed in any of the lots of blank DBS tested. There was no significant cross-talk or carryover observed, as well. Representative chromatographs for extracted DBS blank and DBS blank with IS and the lower limit of quantitation are shown in Figure 1. System suitability test mix was injected on both unit resolution (SRM) and HSRM and peak area ratio responses compared. No significant difference in peak area ratios was observed between HSRM and unit resolution.
Figure 1.
Representative overlay of extracted DBS blank, DBS blank with internal standard and the lower limit of quantitation for TFV and FTC are in the upper left and right panels, respectively. The TFV and FTC retention times were approximately 2.2 and 5.0 minutes respectively. The y-axis was scaled to the lower limit of quantification response. The lower left and right panels represent the TFV-IS and FTC-IS signals, respectively.
3.3. Matrix Effect (ME), Recovery (RE), and Process Efficiency (PE)
Since stable isotope labeled internal standards were utilized, an abbreviated experiment was conducted to assess the assay matrix effect (ME), extraction recovery (RE), and process efficiency (PE) of parent TFV/FTC from DBS matrix [15]. The experiment was determined at two TFV/FTC QC validation sample concentrations: Level 4 (15/15 ng/mL) and Level 6 (800/4000 ng/mL). Three sets of samples were prepared to assess ME, RE and PE. Set 1 was neat samples (in UPH20), set 2 was spiked into blank extracted DBS, and set 3 was extracted DBS samples. The matrix utilized for this experiment was the pooled whole blood described above. The ME was determined by comparing the analyte peak response from Set 2 to Set 1. RE was determined by comparing peak response from Set 3 to Set 2. The PE was determined comparing peak response from Set 3 to Set 1. The ME, RE, and PE were consistent over the tested concentration ranges for analyte and analyte-IS (all individual determinations within ± 3% of the mean determined value). The mean ME, RE, and PE for TFV was 90.5%, 75.1%, and 68.0%, respectively. The mean ME, RE, and PE for FTC was 88.5%, 87.9%, and 77.8%. The TFV-IS and FTC-IS ME, RE, and PE were 82.6/85.1 104/104%, and 85.6/88.3%, respectively.
3.4. Stability testing
DBS quality control samples were considered stable under tested conditions if the mean accuracy was within ±15% the nominal value and/or ±15% to control, and precision was within 15% for the replicates. At least 50% of the tested samples should meet the acceptance limits for the mean to be acceptable for comparison to nominal or control.
It should be noted that long term stability of parent drug preparation stocks and working stocks stored at 4°C was not specifically determined, as they have previously been assessed and shown to be stable at least 2.2 years [9].
3.4.1. Stability in whole blood prior to spotting (QC samples)
The allowable time a TFV/F TC spiked whole blood sample can remain at room temperature prior to spotting on a DBS card was determined. A set of TFV/FTC QC validation samples (Level 2: 5.0/5.0 ng/mL and Level 6: 800/4000 ng/mL) were prepared in blank human whole blood. 25µL of each QC was immediately spotted and used as control (T=0). The remaining whole blood QC samples remained at room temperature and 25µL of blood was removed and spotted at various time points up to 24 hrs. (T=5, 15, 30, 60 min, 2, 4, 6 and 24 hrs.). The tested DBS were allowed to dry and three 3mm discs from each timed spot were punched and extracted for TFV/FTC analysis. Mean responses from the treated samples were compared to the mean response from T=0 (untreated control). All deviations from control were within ±11.8% except one time point (2 hour) tested for TFV Level 6 (800 ng/mL) which was +18.3%. These data provide evidence that the preparation of TFV/FTC spiked in whole blood may remain at room temperature for up to 24 hours prior to spotting on DBS cards.
3.4.1.1. Stability in whole blood prior to spotting (clinical samples)
TFV/FTC stability in whole blood drawn into EDTA Vacutainer tubes from study subjects was assessed to determine the period of time allowable prior to spotting DBS cards. Six different subjects were tested providing 6 different sources of whole blood/DBS matrix for the measurements. Subjects had been taking TDF/FTC for 2 to 7 years. Three blood tubes were drawn for each subject and reserved for testing at T=0 hours (control), T=24 hours and 48 hours, respectively. The TFV 24 hour samples compared to control (T=0) were all within ±9%. FTC had 5/6 samples within ±8.9%; one value was at 16.4%. The mean %Diff at T=24hr for TFV was 5.3% and for FTC was 3.0%. All 48 hour samples for TFV were outside the acceptable range of ±15% with a mean of 42.1%. FTC had 3/6 subjects outside the acceptable range with a mean of 19.9%. The increased TFV and FTC at 48 hours likely represents release of phosphorylated TFV and FTC anabolites from red blood cells, and degradation of these anabolites to TFV and FTC [8]. Together, the data demonstrated that whole blood can remain at room temperature for up to 24 hr. prior to spotting.
3.4.2. Long term storage stability (QC samples)
Long term storage stability of QC validation samples at Levels 1, 2, 4, 5, and 6 (2.5/2.5, 5.0/5.0, 15/15, 200/400, 800/4000ng/mL) stored at RT was assessed at 5 months and approximately 18 months. The mean (n=5) results were compared with nominal concentrations and the original value. All deviations from control were ≤ 13.9%, except the Level 1 (2.5/2.5 ng/mL) for TFV (LLOQ), which was ≤ 15.7% control.
Long term storage stability at −20°C and −80°C was assessed at 4 QC validation sample concentrations (Level 2, 3, 5, and 6; 5.0/5.0, 7.5/7.5, 200/400, 800/4000 ng/mL), in triplicate. Samples were re-assayed after approximately 11 months. All were within 13.3% of the original value except the TFV Level 2 result at −80°C for 11 months, which was −15.6%. Overall results demonstrated consistent stability at multiple concentrations and storage conditions for quality controls.
3.4.2.1. Long term storage stability (Clinical Samples)
The use of clinical DBS samples for assessment of long term stability was imperative since spiked QC samples cannot entirely imitate the DBS sample contents from clinical subject specimens. In vivo, the parent TFV and FTC are anabolized to their intracellular nucleotide forms inside cells, including in red blood cells, which comprise a large component of the DBS sample. TFV-diphosphate has a 17 day half-life in red blood cells, which translates to high accumulation of TFV-diphosphate with repeated daily doses in humans, estimated at ~25-fold accumulation between the first dose and steady-state [8, 19]. Therefore, the clinical DBS specimens utilized for the stability testing were derived from samples (n=3 subjects) obtained at initial dose (day 1) and days 3, 7, 20, and 30 of daily TDF/FTC dosing. Samples were also obtained at study days 35, 45, and 60 after subjects had discontinued dosing at Day 30 representing samples that were 5, 15, and 30 days off drug, respectively.
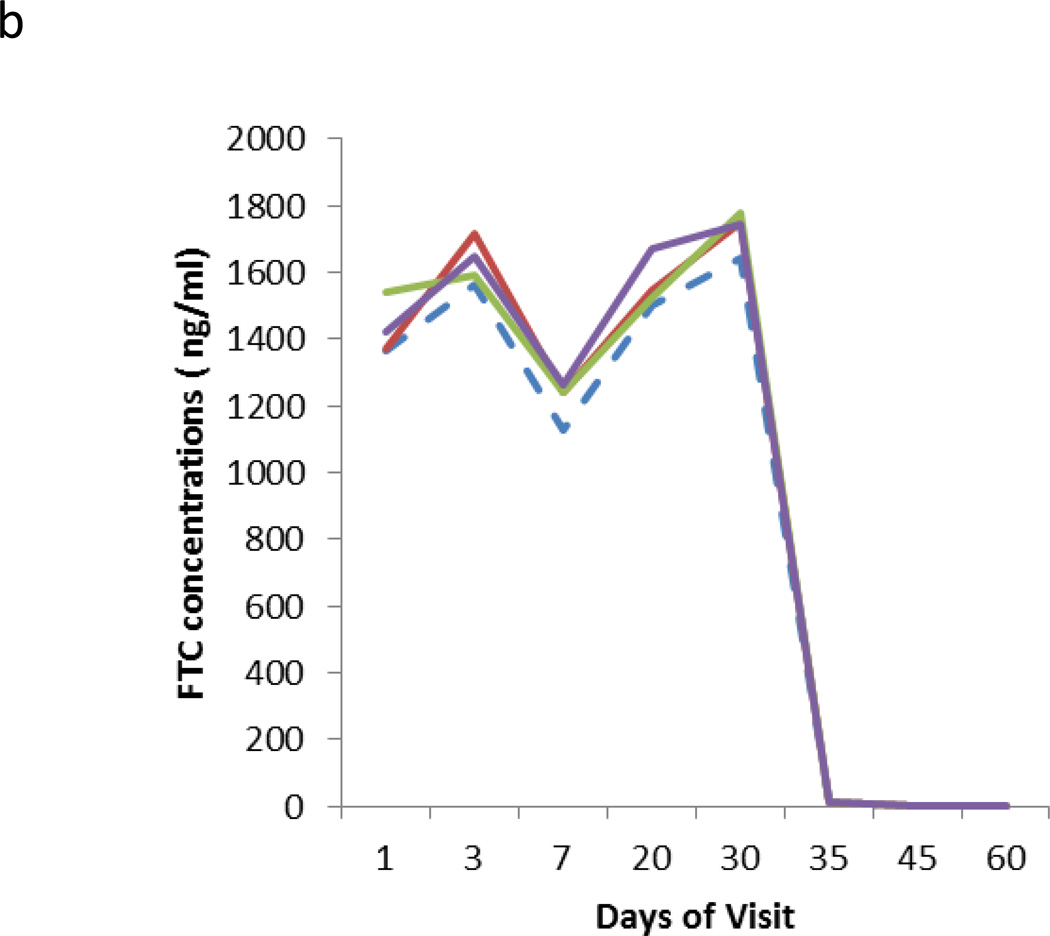
Three individual subjects were utilized to assess the effects of DBS card storage temperature and long term stability. The 2 hour post dose sample from study days 1, 3, 7, 20, and 30 and the sample obtained at off drug visit days 35, 45, and 60 were analyzed for this analysis. Samples were stored at various temperatures from 12.4 to 17.9 months. The DBS card storage condition TFV/FTC data are shown in Figure 2. The data show that RT storage caused an overall increase in TFV concentrations while storage at conditions below RT (sub-RT) showed virtually no effects to the resulting data. The data also reflect that RT storage begins to deviate significantly from sub-RT storage with approximately 1 week of TDF/FTC dosing. The greatest effects were seen as subjects discontinued dosing after 30 days of daily dosing. The day 45 and 60 sub-RT storage were largely below the lower limit of quantitation (2.5 ng/mL) while the RT stored samples showed TFV concentrations well above the lower limit of quantitation. For FTC, which does not accumulate as significantly in RBC, storage conditions did not appear to have as great of an effect on resulting concentrations.
Figure 2.
Results from the different temperature storage stability of clinical samples. Four storage conditions were tested represented by a dashed line (RT) and solid lines (4°C, −20°C, −80°C). The Y axis represents the mean of measured concentrations at the different storage conditions of TFV (2a) and FTC (2b) from three subjects receiving 30 days of daily dosing followed by off-drug 30 days.
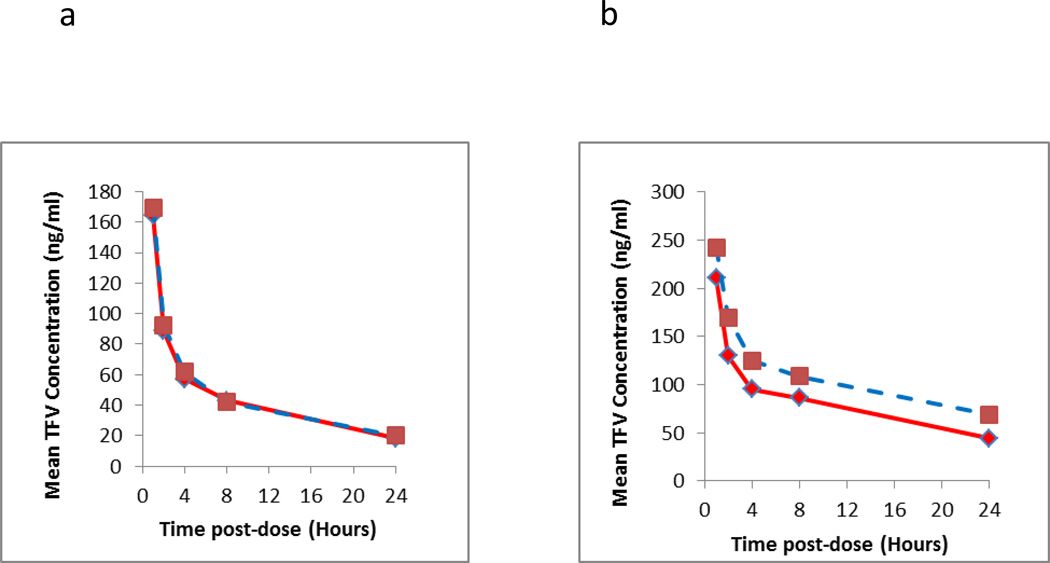
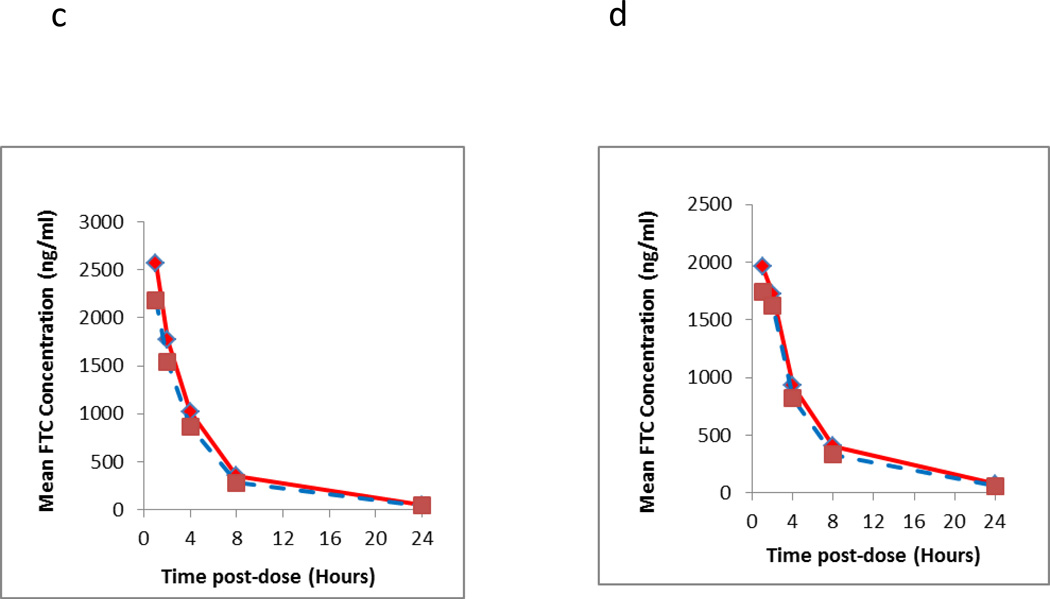
Further testing was performed with clinical DBS samples (n=3 subjects) stored at RT (n=30 samples) to determine if the nucleotide forms of the TFV/FTC could potentially effect the parent TFV/FTC concentration determinations from the DBS matrix at different times post dose within a dosing profile. Clinical DBS specimens were chosen to reflect first dose (Day 1) and accumulated doses (Day 30) where sampling was performed over the post dose time interval of 1, 2, 4, 8 and 24 hours. Each DBS sample was extracted and analyzed for TFV/FTC concentration measurement initially and analyzed again 404 days (13.5 months) after RT storage. The data are shown in Figure 3. The TFV day 1 data show no significant difference for long term RT storage, confirming results shown above, where the deviation becomes significant around day 7 of therapy. The day 30 (accumulated doses) data showed a significant deviation with RT storage. The average difference over the entire profile was +31.1%, while at the individual PK post dose time points (1, 2, 4, 8 and 24 hours) was 14.5%, 30.0%, 30.4%, 25.3%, and 55.5%, respectively. These data again were consistent with data presented above. The FTC data did not exhibit the same instability as TFV and was stable at RT storage.
Figure 3.
Intensive pharmacokinetic results evaluating the long term storage stability of clinical samples at room temperature from two different dosing days (first dose, i.e. Day 1 and Day 30). The Y axis represents the mean of measured TFV (3a Day 1;3b Day 30) and FTC (3c Day 1;3d Day 30) of three subjects at the respective time point and dosing days from the initial run (solid line) and re-run (dashed line). The elapsed time between two runs was 404 days.
Together, RT storage of clinical DBS samples caused increases of TFV as doses accumulated, which was explained by the degradation of TFV-DP in DBS stored at RT. To preserve the integrity of TFV in DBS samples, study samples should be stored long term at −20°C or below. The inclusion of clinical research samples was critical to the stability testing, as demonstrated above. These findings underscore the importance of evaluating clinical samples with the validation when drug metabolites or anabolites might degrade to parent drug.
3.4.3. Freeze/Thaw stability and room temperature stability (QC samples)
Freeze/thaw assessments and room temperature stability were conducted in triplicate at two QC validation sample concentrations, Level 3 (7.5/7.5 ng/mL) and Level 6 (800/4000 ng/mL). Freeze/Thaw samples underwent 5 freeze/thaw cycles starting from either −20°C or −80°C. At each cycle, sample cards were thawed at RT for 2 to 3 hrs. Room temperature stability after thaw was conducted. Thawed DBS card was allowed to remain at room temperature for 7 days. The conditional stability tests were compared with control samples (n=3) that were not subjected to the test condition. All of the determinations were within ± 6.0% that of control for Freeze/Thaw and 10.4% that of the controls for RT up to 7 days, indicating stability at tested conditions for quality control samples.
3.4.3.1. Freeze/Thaw stability and room temperature stability (clinical samples)
Stability of freeze/thaw (n=5 cycles) and room temperature (n=6 days) post initial thaw were assessed with DBS subject samples stored at −20°C and −80°C. DBS samples (n=4 ) were selected at 1hr, 4 hr, and 24hr after an observed dose following 30 days of daily TDF/FTC therapy and approximately 30 days after dosing was stopped to cover a wide range of drug levels and accumulation of phosphorylated TFV in RBC. Samples underwent 5 freeze/thaw cycles starting from either −20°C or −80°C. At each cycle, cards were thawed at RT for 2 to 3 hrs.
For additional RT stability testing, samples were thawed and allowed to remain at RT for 6 days. The sample from 30 days off drug was BLQ for control and the conditions tested. The precision determinations for all detectable results were within 6.5% and the %Diff to control (not subjected to treatment condition) were within ±7.2%, demonstrating that 5 freeze/thaw cycles and 6 days at RT (after thaw) have no impact on TFV/FTC measured from DBS subject samples when stored at −20°C and/or −80°C. Furthermore, comparison of the data from the −20°C stored DBS to that of −80°C stored DBS were within ±13.3%, providing evidence that storage at either −20°C and/or −80°C is acceptable for TFV/FTC in DBS.
Additional stability data will be generated for room temperature storage to more precisely identify the time clinical samples can remain at room temperature prior to −20°C or −80°C storage (currently 6 days); and long-term storage data will continue to be accrued.
3.4.4. Extracted sample stability (Injection Matrix Stability)
Extracted sample stability was assessed by retaining five replicate QC validation samples (utilized from one accuracy/precision run) in the auto sampler, which was maintained at 20°C for 3 days followed by 4 days at room temperature. The samples were re-injected against the calibration curve and quality controls of a later run. Mean response was compared to both nominal and mean response from freshly prepared QC that was not subjected to the test condition. The greatest % deviation compared to control was −6.0%, and the accuracy and precision were within ±15% indicating stability for extracted samples for up to 7 days at conditions tested.
3.5. Spot volume assessment (QC samples)
The effect of spot volume was assessed at two QC validation sample levels, Level 4 (15.0/15.0 ng/mL) and Level 6 (800/4000 ng/mL) in triplicate by spotting different volumes (5, 10, 50 µL) of blood on DBS cards. The analyte concentrations of the DBS spotted with each spot volume were compared to that of a DBS QC with a standard 25µL spot volume. Acceptance criteria were a mean difference to control value ±15% for each analyte. All the samples were punched from center because the area generated with 5 µL and 10 µL of blood spotted only allowed for 1 punch from the center of the spot. When compared to nominal, the 5 µL spot had both TFV/FTC outside acceptance criteria for both tested concentrations. The precision (%CV) for all other spot volume measurements (10 µL to 50 µL) were <8% for both TFV/FTC DBS QC samples tested. When compared to control (25 µL spot volume), all spot volumes compared well to control (≤ 14.6%) except for the 50 µL spot volume at 800/4000 ng/mL samples for TFV/FTC (19.8% and 15.5%).
3.5.1. Spot volume (clinical samples)
Additional testing for effect of spot volume was performed utilizing a subject sample from day 20 of daily TDF/FTC dosing and the 2 hour post dose sample. This sample was chosen so TFV/FTC concentrations would be clinically at their peak concentrations. The mean observed concentration of TFV and FTC were 134 ng/mL and 1976 ng/mL, respectively for the control (25 µL) spot volume. The results showed good precision (<3.6% CV) for all determinations. TFV DBS results when compared to control were all within ±12.2%. FTC DBS results were within ±6.7%, except with the 10µL spot volume (−15.1%).
Taken together, the spot volume data generated from QC validation samples and the subject samples helped establish that a spot volume range of 10–50µL is valid for the method. This provides a relatively wide margin of spot size acceptance for clinical practice.
3.6. Hematocrit (HCT) assessment
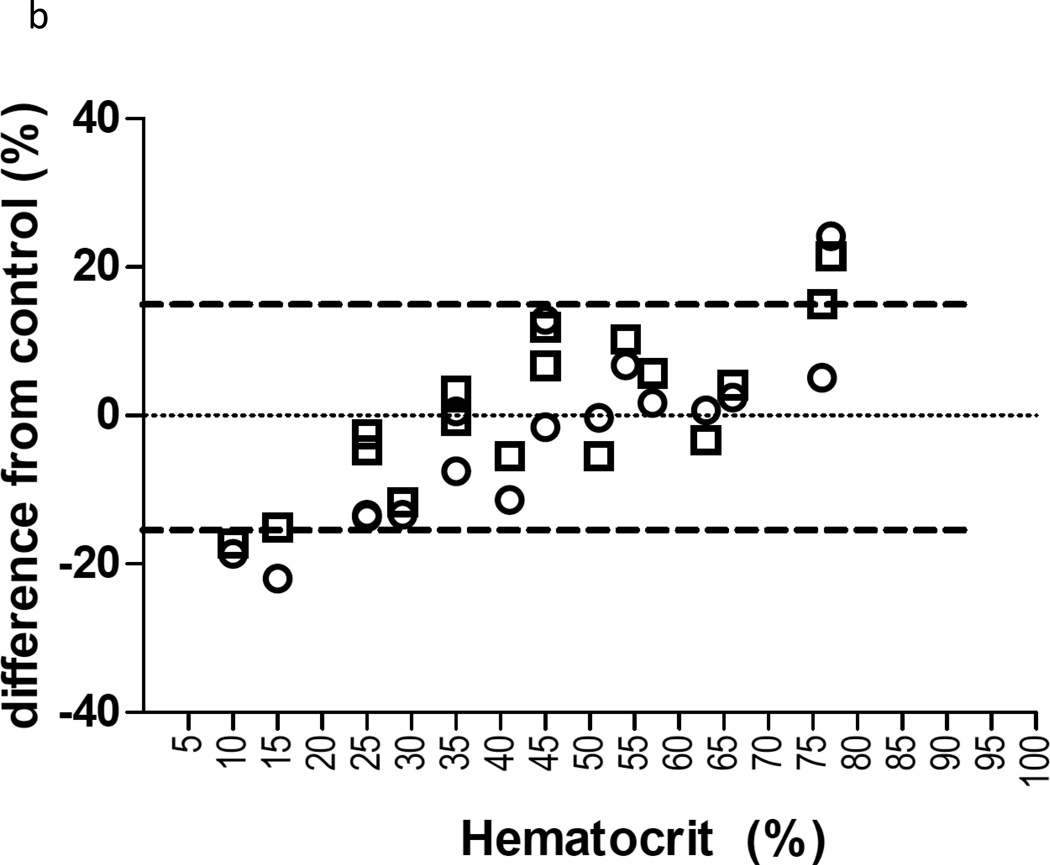
Three lots of whole blood with known HCT% were purchased for HCT testing. Samples were spun and plasma was added or removed to mimic a range of HCT percentages. The final HCT ranges of the samples were 29%, 41%, 51%, 63%, 76% from Lot 1; 25%, 35%, 45%, 57% and 77% from Lot 2; and 10%, 15%, 25%, 35%, 45% 54% and 66% from Lot 3. Each HCT sample was then used to spike with the respective QC validation sample level 4 (15.0/15.0 ng/mL) and Level 6 (800/4000 ng/mL) concentrations and extracted in triplicate. The mean analyte values from the various HCT levels had to be within ±15% of the mean analyte value from the original HCT for acceptance. The results of the HCT effects are shown in Figure 4. Comparing tested HCT to original HCT, the determinations were generally acceptable within the HCT range of 25% to 76%. The low HCT in the range of 25% to 29% showed half of the TFV values within ±15%, whereas all FTC values were within ±15%. All determinations for both drugs were within ±15% between HCT of 35% to 76%. Collectively, the results show that: 1) The TFV/FTC response of extracted samples tended to increase with increasing HCT. 2) The acceptable HCT range was 25% to 76%. 3) The acceptable performance surrounding the normal HCT range is important for implementation in clinical practice, where patients may have diseases or circumstances leading to HCT abnormalities. However, for HCT out of this range (e.g. severe anemia), further studies will be needed to determine how to account for severe HCT abnormalities. Additionally, further HCT testing is needed with clinical samples from subjects exhibiting a wider range of HCT values, as this method is implemented.
Figure 4.
Results from hematocrit experiments for TFV (4a) and FTC (4b). The Y axis represents the deviation from the control condition (original hematocrit) for the QC Level 4 (15/15 ng/mL) represented by open circles and the Level 6 (800/4000 ng/mL) represented by open squares.
3.7. Effect of punch location
Punches were taken from the edge or the center of the spot to test the effect of punch location. Two quality control levels were tested in triplicate, Level 4 (15.0/15.0 ng/mL) and Level 6 (800/4000 ng/mL). Additionally, the hematocrit studies described above were used to evaluate the effect of different HCT values on edge versus center punch. The edge punch (including the edge punch for the original HCT) was used as a control and the mean analyte value from the center punch had to be within ±15% of the mean analyte value from the edge punch for acceptance. The % difference was ≤ 14.8% for TFV and ≤ 6.3% for FTC. For the HCT experiment, center punch was ≤ 9.4% for TFV/FTC between the HCT levels of 25% to 57%. Together, these results indicate that center versus edge punches did not impact the determination of TFV/FTC including HCT that extends past the normal range (35% to 50%). Of note, the use of all edge punching allows for multiple punches to be taken from the same blood spot; three 3mm punches can be extracted from a 25µL spot. Additionally, using all edge punches (or all center punches) within an analytical run minimizes potential variability.
3.8. Overcoming the limits of quantitation: “punch stacking” and dilution
The effect of “punch stacking” (extraction of multiple DBS punches from the same spot) was investigated for those samples that might be initially below the limit of quantification. Three, 3mm punches from DBS with two DBS QC validation sample levels at (7.50/7.50 vs. 200/400 of TFV/FTC) were “stacked” for extraction in triplicate. Mean responses were divided by 3 and compared to nominal concentration. Acceptance criteria were precision and mean %difference of ±15%. Both the %CV (≤ 3.4%) and mean % difference (within ±8.1%) were acceptable when compared to nominal value for both TFV a nd FTC. Multiple punches may be stacked for extraction if increased sensitivity is needed.
An approach called internal standard-tracked (IS-tracked) dilution was used to dilute DBS samples that might be initially above the upper limit of quantification [13]. If an incurred DBS sample required dilution by “X” times to fall within the assay reportable range, an “X” times concentration of regular IS solution was added to the sample to be re-assayed with dilution. Prior to drying the methanol extraction solution, a volume is removed corresponding to the “X” times dilution and replaced with extracted blank DBS extraction solution to complete the dilution factor. This makes the IS response in the final reconstitution of the diluted specimen equivalent in response to the other IS samples in the analysis. Two-fold and five-fold dilution factors were investigated using the QC validation sample Level 6 (800/4000 ng/mL) in triplicate. Mean responses were multiplied by 2x and 5x, respectively and were compared to nominal concentration. Acceptance criteria were precision and mean %difference of ±15%. The %CV and %Dev were both within 9% for both dilution factors tested, demonstrating that IS-tracked dilution may be used for TFV/FTC DBS samples.
3.9. Relationship of TFV/FTC concentrations in DBS vs. plasma
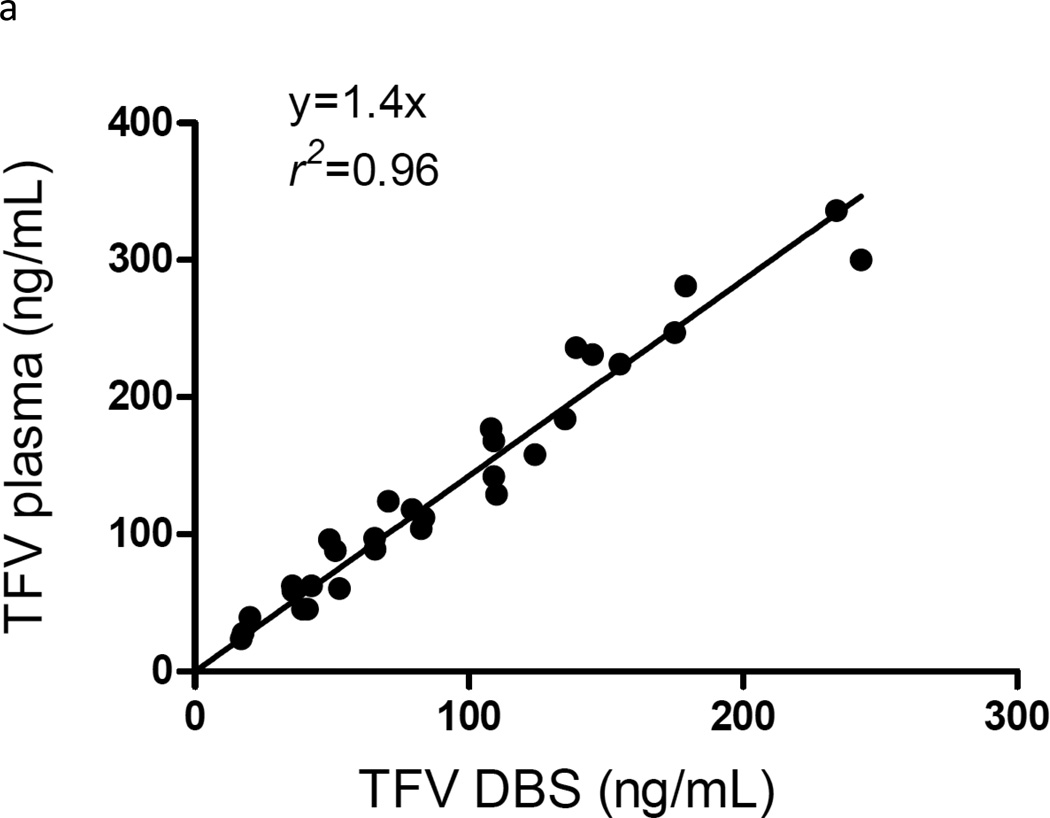
We previously described the relationship between plasma and DBS TFV and FTC in a communication about the potential clinical application of DBS for adherence monitoring [8]. Briefly, a total of 30 paired DBS and plasma samples from the same blood draw were evaluated to determine the relationship between TFV/FTC concentrations in DBS versus plasma, [8]. The samples were from two intensive pharmacokinetic assessments (5 time points each) in three HIV-negative subjects, one assessment after the first dose and the other after 30 days of daily TDF-FTC dosing. Linear regression analysis demonstrated coefficients of determinations (r2) ≥ 0.96 (Figure 5; reprinted from ref [8] with permission). The TFV plasma versus DBS relationship was defined as y=1.4x and that for FTC was y=0.8x. These results support the use of DBS as a suitable alternative for analysis of TFV/FTC concentrations in plasma. Additional studies will be needed to compare DBS derived from a finger-stick with that from pipetting blood from blood draw tubes, as was done in this analysis.
Figure 5.
DBS versus plasma from 2 intensive samplings (5 time points over 24 hours) in 3 subjects. One sampling was after the first dose and the other was after 30 days of daily dosing. TFV in DBS versus plasma (5a) and FTC in DBS versus plasma (5b) showed linear relationships defined by 1.4x, r2=0.96 for TFV and 0.8x, r2=0.99 for FTC. The regression did not account for repeated measures. Reprinted with permission from Castillo-Mancilla et al. AIDS Res Hum Retroviruses. 2013; 29(2):384–90.
5.0 Conclusion
This liquid chromatography-tandem mass spectrometry method for TFV/FTC measurement in dried blood spots was accurate, precise, and sensitive over a range of hematocrit concentrations, spot volumes, and punch locations, and samples were found to be stable in appropriate storage conditions for long periods of time. The use of clinical samples was critical to define appropriate stability conditions. Excellent correlation was demonstrated between TFV and FTC in DBS versus plasma, supporting the use of DBS as an alternative for plasma pharmacokinetic studies.
Highlights.
Measurement of TFV and FTC in dried blood spot to 2.5 ng/mL
DBS storage requires −20°C or below because phosphate anabolites degrade to TFV
Measurement was accurate and precise for hematocrit spanning the normal range
Measurement was not impacted by punch location and spot volumes ( 10µL to 50µL)
DBS can be used as a plasma alternative for pharmacokinetic analyses in vivo
Acknowledgments
The authors thank the NIH AIDS Research and Reference Reagent Program for the reference standards used for the assays; the study personnel and nursing staff who assisted with the clinical protocol; Gilead for donating study drug for clinical studies; and the subjects who participated. This work was supported by grants from the NIH: U01 AI84735 (P.L.A.) and UL1 RR025780 (University of Colorado Clinical and Translational Sciences Institute).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; [Accessed, May 22, 2013]. Panel on Antiretroviral Guidelines for Adults and Adolescents. Updated Feb 12, 2013. Available at: http://www.aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. [Google Scholar]
- 2.Gilead Sciences. Foster City, CA: 2012. Nov, Viread® (tenofovir disoproxil fumarate) product information. [Google Scholar]
- 3.Gilead Sciences. Foster City, CA: 2012. Jul, Truvada® (tenofovir disoproxil fumarate and emtricitabine) product information. [Google Scholar]
- 4.Flynn PM, Mirochnick M, Shapiro DE, Bardeguez A, Rodman J, Robbins B, et al. Pharmacokinetics and Safety of Single-Dose Tenofovir Disoproxil Fumarate and Emtricitabine in HIV-1-Infected Pregnant Women and Their Infants. Antimicrob. Agents Chemother. 2011;55:5914–5922. doi: 10.1128/AAC.00544-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirt D, Ekouévi DK, Pruvost A, Urien S, Arrivé E, Blanche S, et al. Plasma and Intracellular Tenofovir Pharmacokinetics in the Neonate (ANRS 12109 Trial, Step 2) Antimicrob. Agents Chemother. 2011;55:2961–2967. doi: 10.1128/AAC.01377-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King JR, Yogev R, Jean-Philippe P, Graham B, Wiznia A, Britto P, et al. Steady-State Pharmacokinetics of Tenofovir-Based Regimens in HIV-Infected Pediatric Patients. Antimicrob. Agents Chemother. 2011;55:4290–4294. doi: 10.1128/AAC.01334-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li W, Tse FL. Dried blood spot sampling in combination with LC-MS/MS for quantitative analysis of small molecules. Biomed. Chromatogr. 2010;24:49–65. doi: 10.1002/bmc.1367. [DOI] [PubMed] [Google Scholar]
- 8.Castillo-Mancilla JR, Zheng JH, Rower JE, Meditz A, Gardner EM, Predhomme J, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res. Hum. Retroviruses. 2013;29:384–390. doi: 10.1089/aid.2012.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bushman LR, Kiser JJ, Rower JE, Klein B, Zheng JH, Ray ML, et al. Determination of nucleoside analog mono-, di-, and tri-phosphates in cellular matrix by solid phase extraction and ultra-sensitive LC-MS/MS detection. J. Pharm. Biomed. Anal. 2011;56:390–401. doi: 10.1016/j.jpba.2011.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delahunty T, Bushman LR, Robbins B, Fletcher CV. The simultaneous assay of tenofovir and emtricitabine in plasma using LC/MS/MS and isotopically labeled internal standards. J. of Chromatog. B. 2009;877:1907–1914. doi: 10.1016/j.jchromb.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viswanathan CT, Bansal S, Booth B, DeStefano AJ, Rose MJ, Sailstad J, et al. Quantitative bioanalytical methods validation and implementation: best practices for chromatographic and ligand binding assays. Pharm. Res. 2007;24:1962–1973. doi: 10.1007/s11095-007-9291-7. [DOI] [PubMed] [Google Scholar]
- 12.Holub M, Tuschl K, Ratschmann R, Strnadova KA, Muhl A, Heinze G, et al. Influence of hematocrit and localisation of punch in dried blood spots on levels of amino acids and acylcarnitines measured by tandem mass spectrometry. Clin. Chim. Acta. 2006;373:27–31. doi: 10.1016/j.cca.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Liu G, Snapp HM, Ji QC. Internal standard tracked dilution to overcome challenges in dried blood spots and robotic sample preparation for liquid chromatography/tandem mass spectrometry assays. Rapid Commun. Mass Spectrom. 2011;25:1250–1256. doi: 10.1002/rcm.4990. [DOI] [PubMed] [Google Scholar]
- 14.ter Heine R, Rosing H, van Gorp EC, Mulder JW, van der Steeg WA, Beijnen JH, et al. Quantification of protease inhibitors and non-nucleoside reverse transcriptase inhibitors in dried blood spots by liquid chromatography-triple quadrupole mass spectrometry. J Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008;867:205–212. doi: 10.1016/j.jchromb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal. Chem. 2003;75:3019–3030. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 16.Bennetto-Hood C, Long MC, Acosta EP. Development of a sensitive and specific liquid chromatography/mass spectrometry method for the determination of tenofovir in human plasma. Rapid Commun. Mass Spectrom. 2007;21:2087–2094. doi: 10.1002/rcm.3056. [DOI] [PubMed] [Google Scholar]
- 17.Gomes NA, Vaidya VV, Pudage A, Joshi SS, Parekh SA. Liquid chromatography–tandem mass spectrometry (LC–MS/MS) method for simultaneous determination of tenofovir and emtricitabine in human plasma and its application to a bioequivalence study. J. Pharm. Biomed. Anal. 2008;48:918–926. doi: 10.1016/j.jpba.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 18.Durand-Gasselin L, Da Silva D, Benech H, Pruvost A, Grassi J. Evidence and possible consequences of the phosphorylation of nucleoside reverse transcriptase inhibitors in human red blood cells. Antimicrob. Agents Chemother. 2007;51:2105–2111. doi: 10.1128/AAC.00831-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson PL, Meditz A, Kiser JJ, Zheng JH, Predhomme J, Gardner EM, et al. The single dose pharmacokinetic profile of intracellular tenofovir-diphosphate (TFV-DP) and emtricitabine-triphosphate (FTC-TP) in HIV-negative volunteers. Paper presented at the 18th Conference on Retroviruses and Opportunistic Infections; 2011 Feb 27–Mar 2; Boston, MA, USA. [Google Scholar]