Abstract
Objective
To determine the prevalence of specific micronutrient (iron, zinc, magnesium, phosphorus, selenium, copper, folate, vitamins A, D, E and B12) deficiencies in children with intestinal failure (IF), and identify risk factors associated with developing these deficiencies.
Study design
A retrospective review of prospectively collected data from 178 children with IF managed by the intestinal rehabilitation program at Cincinnati Children’s Hospital Medical Center, Ohio, USA between August1st 2007 and July 31st 2012. Transition to full enteral nutrition (FEN) was defined as the period during which the patient received between 20%–100% of estimated required nutrition enterally. FEN was defined as the patient tolerating all of the estimated required nutrition (100%) enterally for > 2 weeks.
Results
Necrotizing enterocolitis (NEC) was the most common cause of IF (27.5 %). Iron was the most common micronutrient deficiency identified during (83.9%) and after (61%) successful transition to FEN with significant reduction in the percentage of patients with iron deficiency between the two periods (P=0.003). Predictors of micronutrient deficiency after successful transition to FEN include birth weight (P=0.03), weight percentiles (P=0.02), height percentiles (P=0.04) and PN duration (P=0.013). After multivariate adjustments, only PN duration remained statistically significant (P=0.03).
Conclusions
Micronutrient deficiencies persist in patients with IF during and after transition to enteral nutrition. These data support the need for routine monitoring and supplementation of these patients especially those on prolonged PN.
Keywords: Prevalence, Micronutrient deficiency, Intestinal failure
Intestinal failure (IF) is defined as intestinal dysfunction severe enough to mandate chronic dependence upon parenteral nutrition (PN) for adequate growth, hydration, or micronutrient balance [1]. Normally over 95% of vitamins and minerals in food are absorbed in the proximal small bowel with the exception of vitamin B12 which is bound to intrinsic factor and absorbed in the terminal ileum [2]. Specific regions of the small intestine may be resected in some gastrointestinal disorders thereby resulting in both loss of bowel and malabsorption. In children with IF, the risk of developing nutrient deficiencies depends on the postsurgical anatomy, amount of bowel resected and degree of malabsorption [3]. In addition, increased inflammation from bacterial overgrowth, impaired motility and chronically damaged bowel mucosa further contributes to malabsorption [4]. Occult blood loss may also take place, contributing to iron deficiency.
Micronutrient deficiencies in patients with IF can occur during transition to full enteral nutrition (FEN), and may persist after successful transition [5]. Deficiencies of fat-soluble vitamins (A, D, E, and K) and certain minerals such as zinc are likely to develop because of uncertain absorption if parenteral nutrition is discontinued [6]. Micronutrient deficiencies can result in an increased risk of infections, anemia, growth failure, and (in extreme cases) death [7, 8, 9, 10]. In this study, the prevalence of iron, zinc, magnesium, phosphorus, selenium, copper, folate, vitamins A, D, E and B12 deficiency during and after successful transition to enteral nutrition (EN) is reported. Risk factors for developing deficiencies of these nutrients were evaluated.
Methods
This is a single-center retrospective study involving review of electronic medical records and database of patients with IF seen between August1, 2007, and July 31, 2012. Two hundred and fifty six patients were identified, and 178 met inclusion criteria, which were: (1) diagnosis of IF, defined by the need for PN support for greater than 30 days; (2) measurement of at least one micronutrient during or after transition to enteral nutrition; and (3) follow-up in the Intestinal Care Center of Cincinnati Children’s Hospital Medical Center during the study period. Patients with liver, small bowel, combined or multi-visceral transplant (MVT) history were excluded.
Approval was obtained from the Institutional Review Board of the Cincinnati Children’s Hospital Medical Center; both caregiver consent and patient assent were waived because patients were deidentified and this study did not involve any direct patient contact, relying only upon analysis of data obtained during routine patient care.
Electronic data of eligible patients were retrieved: birth, age, birth weight, sex, ethnicity, anthropometric percentiles (weight, height), gestational age, PN duration, duration off PN, residual bowel length, resected part of small bowel, history of cholestasis, short gut syndrome and MVT. Serum levels of iron, total iron binding capacity, ferritin, zinc, magnesium, phosphorus, selenium, copper, folate, vitamins A, D, E and B12 during transition from PN to FEN and after successful transition to FEN were retrieved. Data were obtained longitudinally for every visit where available throughout the follow up period. Patient follow up in the out-patient clinic depended on the severity and clinical status of the patient. Chronic patients who are weaned off PN are typically seen for follow up visits every six months and monitored for micronutrient deficiencies annually. Patients on PN are seen in clinic weekly in the initial post hospital period and this frequency is decreased to monthly when stable and up to every 6 months if the patient is a long distance referral and clinically stable. Micronutrient supplementations include multivitamin plus minerals for patients on TPN and oral/tube multivitamins for patients fully transitioned to EN.
Transition to FEN was defined as the period during which the patient received between 20%–100% of estimated required nutrition enterally. FEN was defined as the patient tolerating all of the estimated required nutrition (100%) enterally for > 2 weeks. This definition of > 2 weeks has been used previously to define enteral independence [5, 11].
Low serum levels were used to define deficiencies. Serum 25(OH) D concentrations was measured by radioimmunoassay with I125-labeled tracer (DiaSorin, Stillwater, MN). Values less than 20ng/ml and between 20 and 30ng/ml were defined as vitamin D deficiency and insufficiency, respectively. Abnormal total and direct bilirubin were > 1.9 mg/dL and 0.3 mg/dL, respectively. Normal ranges for vitamin A, E and B12 were 200–490 mcg/L, 5.0 – 20.0 mcg/mL and 211 – 911 pg/mL, respectively. Serum folate greater than or equal to 5.38 ng/mL was normal. Normal ranges for serum copper and iron were 80 – 155 mcg/dL and 50 – 175 mcg/dL, respectively. Iron deficiency was defined as serum iron <50 mcg/dL or an iron saturation of <20% or a serum ferritin of <30 ng/mL for males, and <15 ng/mL for postmenarchal females. The normal ranges for serum selenium, magnesium and phosphorus were 23 – 190 mcg/L, 1.5 – 2.3 mg/dL and 2.9 – 4.6 mg/dL, respectively. Normal serum zinc is 60 – 120 mcg/dL. Iron deficiency anemia was suspected if the mean corpuscular hemoglobin (MCV) was <79 fL. saturation was <20% or a serum ferritin was <30 ng/mL for males, and <15 ng/mL for postmenarchal females in the presence of age defining anemia.
The percentage of expected residual bowel length was calculated using criteria described by Struijs et al based on patient length [12]. Bowel length percentages of 10% and 50% were used in the analysis based on previous definition of short bowel syndrome and outcome data [13]. The weight and height variables were converted to sex-and age-specific Z-scores using the National center for health statistics reference and the WHO standard [14, 15]. Stunting and wasting were defined as Z-scores less than −2 SDs below the age-and sex-specific reference mean [14, 15].
Statistical Analyses
The data were analyzed using SPSS version 19. Chi-square and Fisher Exact were used to test significant association of the categorical variables. The distributions of all continuous variables were checked for normality using Shapiro Wilk test. T-tests were used to compare the means of normally distributed continuous variables, and Mann U Whitney test was used if the data were not normally distributed. Univariate analysis of the following independent variables and micronutrient deficiency during and after successful transition to EN was done: age, ethnicity, sex, birth weight, health insurance, primary gastrointestinal diagnosis, history of short gut syndrome, presence of cholestasis, absence of ileocecal valve, ileal resection, residual bowel length, weight and height for age z-scores, PN duration, duration off PN, route of feeding, formula and micronutrient supplementation. Logistic regressions were used in adjusting for all significant independent variables. A P-value < 0.05 was significant and all reported P-values were 2-sided.
Results
Detailed characteristics of the patients are shown in Table I. Among patients whose weight and height values were available at the beginning of transition to EN; 53 of 118 (44.9%) patients had height for age z-score (HAZ) of <-2. The HAZ of 57 out of 136 patients (41.9%) were <-2 by the time of FEN. This difference in HAZ between the two periods was not significant (P=0.27). Fifty-three of 120 patients (44.2%) had weight for age z-score (WAZ) <-2 at transition to EN, and 52 of 139 patients (37.4%) had WAZ of <-2 when fully transitioned to EN. There was no difference in the WAZ between the two periods (P=0.63).
Table 1.
Characteristics of the subjects
| Age at study entry (years) | |
| Mean (SD) | 5.7 (4.8) |
| Median | 4.0 |
| Mean birth weight in kg (SD) | 2.1 (3.1) |
| Mean gestational age in weeks (SD) | 32.8 (4.6) |
| n (%) | |
| Boys | 100 (56.2) |
| Girls | 78 (43.8) |
| Race/Ethnicity | |
| Caucasian | 132 (74.2) |
| African American | 28 (15.7) |
| Hispanic | 10 (5.6) |
| Others | 8 (4.5) |
| Primary GI diagnosis | |
| NEC | 49 (27.5) |
| Atresia | 25 (14.1) |
| Malrotation with volvulus | 21 (11.8) |
| Gastroschisis | 20 (11.2) |
| Hirschsprung disease | 15 (8.4) |
| Dysmotility | 15 (8.4) |
| Gastroschisis with atresia | 10 (5.6) |
| Others | 23 (13.0) |
| Type of surgery and complications | |
| Small bowel resection | 109 (65.3) |
| History of cholestasis | 39 (21.9) |
| Ileal resection | 93 (52.2) |
| ICV resection | 60 (33.7) |
| Mortality | 3 (1.7) |
| PN | Mean (SD) |
| Mean PN duration in months | 20.1 (26.5) |
| Mean duration off PN in months | 35.5 (38.7) |
SD=standard deviation, NEC=necrotizing enterocolitis, ICV=ileo-cecal valve, PN=parenteral nutrition.
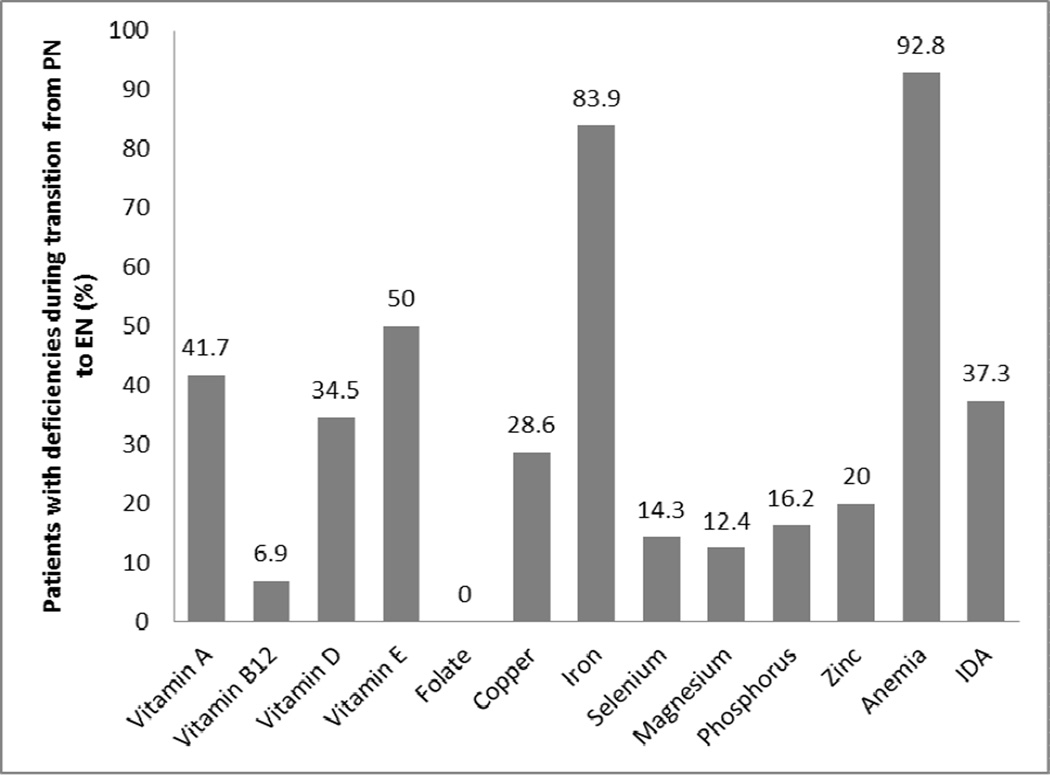
The prevalence of various micronutrient deficiencies during transition to EN is shown in Figure 1. Sixty four of 120 patients (53.3%) were on micronutrient supplementations during transition from PN to EN compared with 73.2% (101/138) after successful enteral nutrition has been achieved (P<0.001). Fifty-six percent had ≥1 mineral deficiency, and 35.4% and 33.1% had ≥1 vitamin or multiple micronutrients deficiencies, respectively.
Figure 1.
Prevalence of micronutrient deficiencies during transition from parenteral nutrition to enteral nutrition Bar showing percentage of patients with specific micronutrient deficiency during transition from parenteral to enteral nutrition.
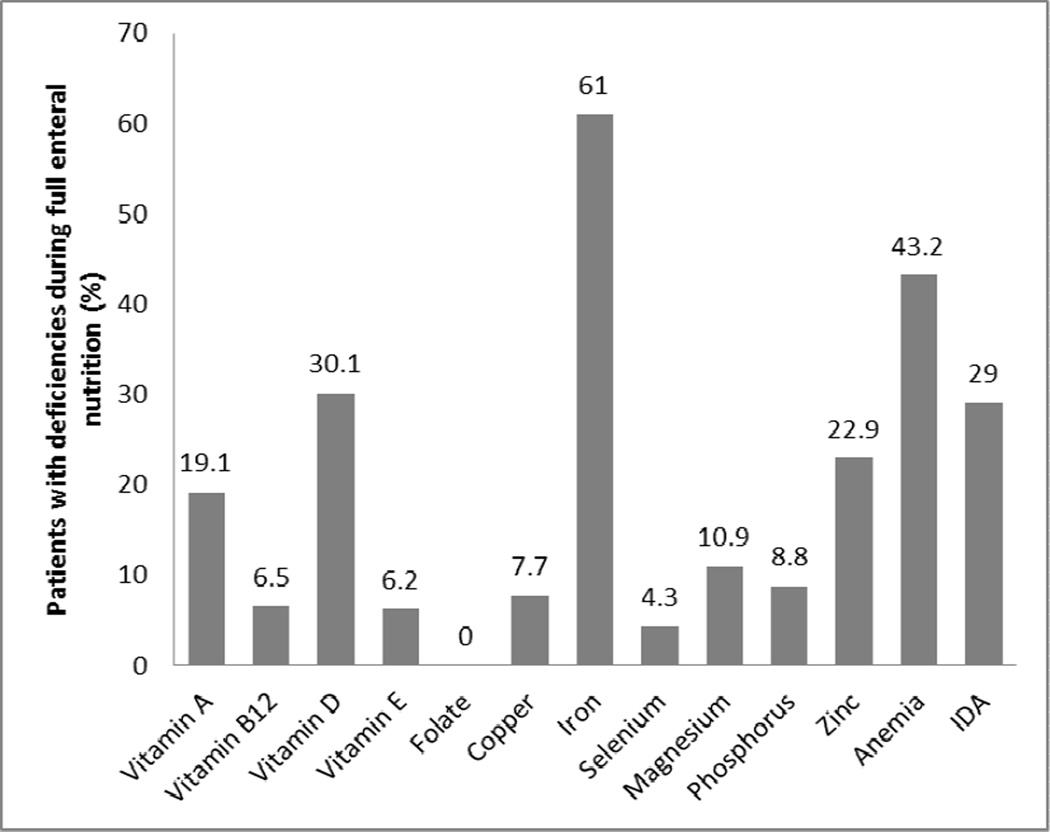
The prevalence of micronutrient deficiencies during FEN is shown in Figure 2. The proportion of patients that had ≥1 vitamin, ≥1mineral and multiple micronutrients deficiencies were 42.5%, 25% and 27.5%, respectively. The proportion of patients deficient in micronutrients decreased or remained stable during FEN. The proportion of patients deficient in the following nutrients decreased when successfully transitioned to FEN: vitamin A from 41.7 to 19.1% (P=0.046), vitamin E from 50% to 6.2% (P<0.001) and iron from 83.9 to 61% (P=0.0.003). The proportion of anemic patients also significantly decreased from 92.8 to 43.2% (P<0.001) after successful transition to FEN. A comparison of micronutrient deficiencies during and after transition to FEN is shown in Table II.
Figure 2.
Prevalence of micronutrient deficiencies during full enteral nutrition. Bar showing percentage of patients with specific micronutrient deficiency full enteral nutrition.
Table 2.
A comparison of prevalence of micronutrient deficiencies during and after transition to full enteral nutrition
| Micronutrients | Deficiency during transition to EN (%) |
Deficiency during full EN (%) |
P-value |
|---|---|---|---|
| Vitamin A | 41.7 | 19.1 | 0.046* |
| Vitamin B12 | 6.9 | 12.4 | 0.41 |
| Vitamin D | 34.5 | 30.1 | 0.56 |
| Vitamin E | 50 | 6.2 | <0.001* |
| Folate | 0 | 0 | 1 |
| Copper | 28.6 | 7.7 | 0.14 |
| Iron | 83.9 | 61 | 0.003 |
| Selenium | 14.3 | 4.3 | 0.26 |
| Magnesium | 12.4 | 10.9 | 0.71 |
| Phosphorus | 16.2 | 8.8 | 0.08 |
| Zinc | 20 | 22.9 | 0.73 |
| Anemia | 92.8 | 43.2 | <0.001* |
| Iron deficiency anemia | 37.3 | 29 | 0.21 |
During transition to EN, the mean age was significantly higher in the micronutrient deficient group (5.4 years) than in children without deficiencies (3.2 years) (P=0.02). Patients with micronutrient deficiency also had significantly higher mean PN duration (31.4 months) than those without deficiency (12.4 months) (P<0.001). Thirty-nine of 59 patients (66.1%) with WAZ ≥ −2 compared with 25 of 51 (49%) with WAZ <-2 had micronutrient deficiency (P=0.08). Thirty-seven of 60 (61.7%) with HAZ ≥ −2 compared with 27 of 53 (50.9%) with HAZ <-2 had micronutrient deficiency (P=0.17). However, 24 of 66 patients (36.4%) with HAZ-score <2212;2 had mineral deficiency compared with 28 of 50 patients (56%) with HAZ score ≥ −2 (P=0.04), and 22 of 68 patients (32.4%) with WAZ-score <-2 also had mineral deficiency compared with 30 of 49 patients (61.2%) with WAZ score ≥ −2 (P=0.003). Primary gastrointestinal diagnosis of Hirschsprung disease, dysmotility and complex gastroschisis (gastroschisis with atresia) were significantly associated with deficiencies (P=0.003). Only the duration off PN (P<0.001) remained significant after multivariate adjustments.
After successful transition to EN, higher birth weight (P=0.03) and longer PN duration (P=0.01) were significantly associated with micronutrients deficiency. Residual bowel lengths less than 50% of expected (P=0.06) and history of cholestasis (P=0.07) although not significant showed a trend toward poorer micronutrient status. The mean residual small bowel length was 70 cm for the micronutrient deficient patients compared with 81 cm in the non-deficient group (P=0.31). After multivariate adjustments, only longer PN duration remained statistically significant (P=0.03).
Following successful transition to EN, vitamin B12 deficiency was significantly associated with WAZ score <-2 (P=0.02), HAZ score <-2 (P=0.03) and ileocecal valve resection (P=0.03). Iron deficiency (P=0.02) and anemia (P<0.001) were significantly higher in patients without history of small bowel resection. Residual bowel length <50% of expected (P=0.02) was associated with vitamin D deficiency (P<0.001).
Discussion
In this study, we report that multiple micronutrient deficiencies occur both during transition from PN to FEN and after successful transition to FEN. During the transition period, multiple deficiencies were documented for key micronutrients, vitamins and minerals. After successful enteral transition, the prevalence of these deficiencies remained high. The most common micronutrient deficiencies during both periods were iron, copper, and fat soluble vitamins.
Our findings are similar to those reported by Yang et al on the micronutrient status in patients with IF in whom the levels of vitamins A, B12, D, E; folate, Cu, Fe, Sn and Zn were assessed during and after successful transition to FEN [5]. They documented that during transition from PN to EN, children with greater height for age z-score were less likely to have mineral deficiency. Our study showed that children with WAZ and HAZ <-2 had significantly higher likelihood of experiencing mineral deficiencies during the same period. The same group had previously reported multiple micronutrient deficiencies (vitamins A, D, E, and B12 and zinc) and anemia in a child with short bowel syndrome and normal growth [16]. Leonberg et al also reported micronutrient deficiencies and growth failure in children with IF after being transitioned to FEN [17]. These findings underscore the need for aggressive biochemical monitoring of children with IF as they are being weaned off PN and achieve FEN even if they are achieving normal growth.
Yang et al [5] also reported that after weaning off PN, 70% of patients had vitamin deficiency. All 13 patients (100%) not taking multivitamin supplementation in their study developed at least one vitamin deficiency compared with 8 of 17 (47%) who were taking multivitamins (P =0.003). Multivariate logistic regression analysis revealed that an intact ileocecal valve and vitamin supplementation were predictors of vitamin sufficiency. Our study also found that patients on micronutrient supplementations had lower risk of vitamin D deficiency after successful transition to EN. Resection of the ICV was a significant predictor of vitamin B12 deficiency. This is due to the loss of part or all of the ileum. However, we did not find any significant association between ileal resection and other micronutrient deficiencies. The ileum is the site of preferential absorption of vitamin B12 and bile salts. Satisfactory bile acid salvage is necessary for optimal fat and fat-soluble vitamin absorption. Furthermore, the ileum has a far greater ability to adapt than does the jejunum [18]. Thus significant ileal resections may result in fat soluble vitamin and vitamin B12 deficiencies after a successful transition to EN [6, 7]. We documented a decrease in the prevalence of vitamin (A, D, E) deficiencies after successful transition to EN likely due to an increase in the number of patients on micronutrient supplementation when on FEN compared with during the transition period (P<0.001).
Our findings support the existing literature that most mineral deficiencies (iron, copper, selenium) decrease in prevalence, but zinc deficiency increases in prevalence after successful transition to FEN [4, 5]. Our evaluation of additional micronutrients in this study showed no folate deficiency during or after successful enteral transition. Presumably, intraluminal bacterial folate synthesis played a role in maintaining sufficiency [19]. We also found that patients with intestinal resection were significantly less likely to develop iron deficiency and anemia. The reason for this could be because iron absorption occurs mostly in the duodenum which is spared in most patients with surgical resection. In addition 15 (8.4%) of our patients had dysmotility disorders that do not require small bowel resection but can lead to significant malabsorption of micronutrients distally in the small bowel.
This is study examined patients over a long period. It adds to the literature on the micronutrient status of children with IF as they transition to FEN. The major limitations are that it was retrospective in nature and that patients were not controlled with regard to specific micronutrient intake. In clinical practice, patients with borderline or overt micronutrient deficiencies are more likely to be started on supplementation.
There are important considerations from this data. Children with normal height and weight z-scores unexpectedly had more significant micronutrient deficiencies than those with abnormal anthropometric measurements. Although this might be a reflection of more aggressive nutritional (micro and macronutrients) management in the latter, it also points out the limitations of anthropometric measurements and the need to rely on biochemical assessment of micronutrients in identifying deficiencies [5]. Overall, mineral deficiencies were more common than vitamins both during the transition to EN and after successful transition. Providers may need to consider specific mineral supplementations in children with IF. The significantly higher mean age for the micronutrient deficient group than in the non-deficient children might be a reflection of the severity of the disease that predisposes these children to be on PN for a longer period. Children who adapt at a younger age are likely to have less severe disease. Significant micronutrient deficiencies occurred during and after transition to FEN in patients with longer PN duration and may be a reflection of delayed intestinal adaptation and disease severity. This is supported by our finding that patients with less than 50% of expected small bowel length showed a trend for micronutrient deficiency after successful transition to FEN (P=0.06).
Acknowledgments
The authors are grateful to Dr M.B. Rao (Department of Environmental Health, University of Cincinnati), Misty Troutt (Program manager), and Justina Dunigan (program administrative assistant) for their assistance during this study. We also are grateful to the families of our patients, whose data were used in the study.
Funded by the Global Health Center, Cincinnati Children’s Hospital Medical Center and the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (NIH; 8 UL1 TR000077-04). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Soden JS. Clinical assessment of the child with intestinal failure. Seminars in Pediatric Surgery. 2010;19:10–19. doi: 10.1053/j.sempedsurg.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Shulman RJ, Phillips S. Parenteral nutrition in infants and children. J Pediatr Gastroenterol and Nutr. 2003;36:587–607. doi: 10.1097/00005176-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Mziray-Andrew CH, Sentongo TA. Nutritional deficiencies in intestinal failure. Pediatr Clin N Am. 2009;56:1185–1200. doi: 10.1016/j.pcl.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Beattie LM, Barclay AR, Wilson DC. Short bowel syndrome and intestinal failure in infants and children. Pediartic and child health. 2010;20:485–491. [Google Scholar]
- 5.Yang CF, Duro D, Zurakowski D, Lee M, Jaksic T, Duggan C. High Prevalence of Multiple Micronutrient Deficiencies in Children with Intestinal Failure: A Longitudinal Study. J Pediatr. 2011;159:39–44. doi: 10.1016/j.jpeds.2010.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu J, Tang Q, Feng Y, Huang J, Tao Y, Wang Y, Cai W, Shi C. Nutrition assessment in children with short bowel syndrome weaned off parenteral nutrition: a long-term follow-up study. J Pediatr Surg. 2007;42:1372–1376. doi: 10.1016/j.jpedsurg.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 7.Goulet O, Olieman J, Ksiazyk J, Spolidoro J, Tibboe D, Köler H, Yagci VR, Falconer J, Grimble G, Beattie RM. Neonatal short bowel syndrome as a model of intestinal failure: physiological background for enteral feeding. Clinical Nutrition. 2012;30:1–10. doi: 10.1016/j.clnu.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Vanderhoof JA, Langnas AN. Short-bowel syndrome in children and adults. Gastroenterology. 1997;113:1767–1778. doi: 10.1053/gast.1997.v113.pm9352883. [DOI] [PubMed] [Google Scholar]
- 9.Snow CF. Laboratory diagnosis of vitamin B12 and folate deficiency: a guide for primary care physicians. Arch Intern Med. 1999;159:1289–1298. doi: 10.1001/archinte.159.12.1289. [DOI] [PubMed] [Google Scholar]
- 10.Poskitt EM. Early history of iron deficiency. Br J Haematology. 2003;122:554–562. doi: 10.1046/j.1365-2141.2003.04529.x. [DOI] [PubMed] [Google Scholar]
- 11.Squires RH, Duggan C, Teitelbaum DH, Wales PW, Balint J, Venick R, et al. Natural history of pediatric intestinal failure: initial report from the Pediatric Intestinal Failure Consortium. J Pediatr. 2012;161:723.e2–728.e2. doi: 10.1016/j.jpeds.2012.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Struijs M, Diamond IR, de Silva N, Wales PW. Establishing norms for intestinal length in children. Journal of Pediatric Surgery. 2009;44:933–938. doi: 10.1016/j.jpedsurg.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 13.Spencer AU, Neaga A, West B, Safran J, Brown P, Btaiche I, et al. Pediatric short bowel syndrome: redefining predictors of success. Ann Surg. 2005;242:403–409. doi: 10.1097/01.sla.0000179647.24046.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geneva: World Health Organization; 2006. WHO Multicentre Growth Reference Study Group: WHO Child Growth Standards: Length/ height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. [Google Scholar]
- 15.Wang F, Cheng H. Use of percentiles and Z-scores in anthropometry. In: Preedy VR, editor. Handbook of anthropometry: physical measures of human form in health and disease. USA: Springer; 2012. pp. 29–41. [Google Scholar]
- 16.Duro D, Jaksic T, Duggan C. Multiple micronutrient deficiencies in a child with short bowel syndrome and normal somatic growth. J Pediatr Gastroenterol and Nutr. 2008;46:461–464. doi: 10.1097/MPG.0b013e3181373b91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leonberg BL, Chuang E, Eicher P, Tershakovec AM, Leonard L, Stallings VA, et al. Long-term growth and development in children after home parental nutrition. J Pediatr. 1998;132:461–466. doi: 10.1016/s0022-3476(98)70021-6. [DOI] [PubMed] [Google Scholar]
- 18.Dehmer JJ, Fuller MK, Helmrath MA. Management of pediatric intestinal failure. Advances in Pediatrics. 2011;58:181–194. doi: 10.1016/j.yapd.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Camilo E, Zimmerman J, Mason JB, et al. Folate synthesized by bacteria in the human upper small intestine is assimilated by the host. Gastroenterology. 1996;110:991–998. doi: 10.1053/gast.1996.v110.pm8613033. [DOI] [PubMed] [Google Scholar]
- 20.Stoltzfus RJ. Iron deficiency: Global prevalence and consequences. Food & Nutrition Bulletin. 2003;24:99–103. doi: 10.1177/15648265030244S206. [DOI] [PubMed] [Google Scholar]
- 21.Brown KH, Peerson JM, Baker SJ, Hess SY. Preventive zinc supplementation among infants, preschoolers, and older pre-pubertal children. Food Nutr Bull. 2009;30:S12–S40. doi: 10.1177/15648265090301S103. [DOI] [PubMed] [Google Scholar]