Abstract
The differential diagnosis of autism spectrum disorders (ASDs) and attention deficit hyperactivity disorder (ADHD) based solely on symptomatic and behavioral assessments can be difficult, even for experts. Thus, the development of a neuroimaging marker that differentiates ASDs from ADHD would be an important contribution to this field. We assessed the differences in prefrontal activation between adults with ASDs and ADHD using an entirely non-invasive and portable neuroimaging tool, near-infrared spectroscopy. This study included 21 drug-naïve adults with ASDs, 19 drug-naïve adults with ADHD, and 21 healthy subjects matched for age, sex, and IQ. Oxygenated hemoglobin concentration changes in the prefrontal cortex were assessed during a stop signal task and a verbal fluency task. During the stop signal task, compared to the control group, the ASDs group exhibited lower activation in a broad prefrontal area, whereas the ADHD group showed underactivation of the right premotor area, right presupplementary motor area, and bilateral dorsolateral prefrontal cortices. Significant differences were observed in the left ventrolateral prefrontal cortex between the ASDs and ADHD groups during the stop signal task. The leave-one-out cross-validation method using mean oxygenated hemoglobin changes yielded a classification accuracy of 81.4% during inhibitory control. These results were task specific, as the brain activation pattern observed during the verbal fluency task did not differentiate the ASDs and ADHD groups significantly. This study therefore provides evidence of a difference in left ventrolateral prefrontal activation during inhibitory control between adults with ASDs and ADHD. Thus, near-infrared spectroscopy may be useful as an auxiliary tool for the differential diagnosis of such developmental disorders.
Keywords: Autism spectrum disorders, Attention deficit hyperactivity disorder, Near-infrared spectroscopy, Inhibitory control, Stop signal task
Highlights
-
•
We examined the prefrontal function in 2 developmental disorders and HC using NIRS.
-
•
NIRS signals were assessed during a stop signal task (SST) and a verbal fluency task.
-
•
ASDs showed significantly smaller activation during SST than ADHD in left VLPFC.
-
•
NIRS is a useful biomarker for the differential diagnosis of ASDs and ADHD.
1. Introduction
The differential diagnosis of the 2 commonest types of neurodevelopmental disorders, autism spectrum disorders (ASDs) and attention deficit hyperactivity disorder (ADHD), can be difficult. ASDs are characterized by impairments in social skills and communication, as well as repetitive interests and activities (American Psychiatric Association, 2000; Stigler et al., 2011). ADHD is characterized by symptoms of inattention, hyperactivity, and impulsivity (American Psychiatric Association, 2000). These conditions often share symptoms of inattention, hyperactivity, impulsivity, and neuropsychological deficits in inhibitory control (Willcutt et al., 2005; Corbett et al., 2009). Thus, misclassification between ASDs and ADHD may occur in clinical settings, particularly in cases of ASDs with comorbid ADHD symptoms. Because the clinical symptoms of high-functioning ASDs and ADHD of adulthood have been modified according to environmental and developmental factors (Nylander et al., 2013; Lehnhardt et al., 2012; Hofvander et al., 2009; Michielsen et al., 2013), it is more difficult to establish a differential diagnosis of ASDs and ADHD in adults.
These misclassifications may lead to a suboptimal treatment strategy. For example, the administration of methylphenidate (MPH), which is a common treatment for childhood ADHD (Whalen et al., 1989; Barkley et al., 2005; Newcorn et al., 2008; Jensen et al., 2007), to children with ASDs and comorbid ADHD symptoms is frequently associated with adverse effects (i.e., social withdrawal, irritability, and stereotypy) severe enough to warrant treatment discontinuation (Autism Network, 2005; Di Martino et al., 2004). Despite the insufficient study of the benefits and adverse effects of MPH in adults with ASDs and ADHD symptoms, MPH is often administered in these cases, even without evidence of its efficacy, because it is the first line of pharmacological treatment for adult ADHD (Kooij et al., 2010; Atkinson and Hollis, 2010). Other treatments are more appropriate for adult ASDs, such as selective serotonin reuptake inhibitors (Williams et al., 2010a), risperidone (McDougle et al., 1998), and cognitive behavioral therapy (White et al., 2009; Lang et al., 2010).
The inhibitory dysfunction observed in the two disorders may have different neurobiological bases, despite similar symptomatic and neuropsychological manifestations.
Stop signal and go/no-go tasks are commonly used tasks to detect inhibitory control in neuroimaging studies. The stop signal task creates a higher load on response inhibition processes compared to the go/no-go task, in that it involves the retraction of a response that has already been triggered by a go signal (Rubia et al., 2001). Go/no-go tasks have a higher load on response selection, due to the a priori knowledge about whether or not to respond to the presentation of specific categorical stimuli (Rubia et al., 2001).
Studies of children with ASDs have revealed no significant differences compared to healthy children in the stop signal task (Ozonoff and Strayer, 1997) but lower performance in the go/no-go task (Happé et al., 2006). Inhibitory motor control as assessed by the stop signal task has been studied extensively in ADHD. The meta-analysis revealed a significant difference in stop latency (stop signal reaction time) between ADHD patients and matched controls in both children and adults (Lijffijt et al., 2005), while children with ADHD had lower performance compared to healthy controls in the go/no-go task (Happé et al., 2006; Raymaekers et al., 2007).
Most previous neuropsychological and neuroimaging studies comparing ADHD with ASDs were performed in children. Neuropsychological studies have found that the ASDs group can have either better (Ozonoff and Jensen, 1999; Geurts et al., 2004; Happé et al., 2006) or poorer (Corbett et al., 2009) inhibitory control than the ADHD group. However, some studies showed little difference in executive function profiles between ADHD and ASDs (Goldberg et al., 2005; Verté et al., 2006). The only neuropsychological study performed in adult patients revealed significant differences between ADHD and ASDs in the Stroop task. However, using the Hayling Sentence Completion Test, which assesses verbal response inhibition, it was found that adults with ADHD did not exhibit more severe impairments compared to those with ASDs (Johnston et al., 2011).
Thus, the development of an inhibitory-task-related neurophysiological index as an auxiliary tool for the differential diagnosis of ASDs and ADHD in adults would be an important contribution to this field. Recent functional magnetic resonance imaging (fMRI) and event-related potential studies have revealed differences between children with ASDs and ADHD (Christakou et al., 2012; Malisza et al., 2011; Kemner et al., 1995; Groen et al., 2008). To date, however, no studies have directly compared adults with ASDs to those with ADHD using functional neuroimaging or neurophysiological indices.
Ideally, a diagnostic index should be developed using a neuroimaging tool that is suitable for application in clinical settings. Near-infrared spectroscopy (NIRS) is an optical neuroimaging technique that allows the non-invasive measurement of changes in the concentrations of oxygenated and deoxygenated hemoglobin ([oxy-Hb] and [deoxy-Hb], respectively), thus reflecting regional cerebral blood volume (Hoshi and Tamura, 1993; Villringer et al., 1993). NIRS is safe and portable and allows the examination of subjects in a natural sitting position. The resolution of NIRS for detecting time-course alterations in brain activation in the prefrontal cortex (PFC) is finer than that of fMRI. Therefore, NIRS could be applied as an auxiliary diagnosis tool in clinical psychiatry.
Our research aim was to determine whether prefrontal NIRS signals recorded during an inhibitory control task differed between adults with ASDs and those with ADHD. In this study, we used a stop signal task (SST) to detect brain activation associated with inhibitory control. We chose the letter version of the verbal fluency task (VFT) as a control index of prefrontal function to test whether the findings were task specific. We hypothesized that adults with ASDs and ADHD would exhibit differential prefrontal NIRS signals during the SST, and that both groups of patients would show activation of the PFC compared to the control group. Further, we hypothesized that during the VFT, both groups would show a similarly reduced activation of the PFC compared to the control group.
2. Materials and methods
2.1. Participants
Twenty-one adults with ASDs, 19 adults with ADHD, and 21 healthy control (HC) subjects participated in the study (Table 1). We recruited 26 adults with ASDs and 25 adults with ADHD from the outpatient clinic at the Department of Neuropsychiatry, University of Tokyo Hospital, Japan, and from community clinics. After recruitment of the patient group, some individuals were recruited in the control group in order to match patients for age, sex, and IQ. As a result, all participating subjects were matched for age, sex, and IQ (Table 1). All subjects gave written informed consent in accordance with the Declaration of Helsinki after a complete explanation of the study. The ethics committee of the University of Tokyo Hospital approved this study (approval no.: 630-6). The diagnoses of ASDs and ADHD were established in accordance with the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) based on comprehensive clinical assessments performed by at least 2 trained child psychiatrists (YK, HK, and AI). We included participants in this study only when at least 2 of the 3 child psychiatrists had seen patients and given consistent diagnoses. Current and lifetime DSM-IV diagnoses, other than ASDs/ADHD, were ruled out based on a consensus decision using information gained from independent clinical interviews, other available clinical data, and from the Mini-International Neuropsychiatric Interview (MINI). The exclusion criteria for all groups were as follows: full-scale IQ < 70, neurological illness, genetic disorders, traumatic brain injury with any known cognitive consequences or loss of consciousness for more than 5 min, a history of electroconvulsive therapy, a history of treatment with stimulants or other psychiatric medication, alcohol/substance abuse or addiction, bipolar disorder, and schizophrenia. An additional exclusion criterion for the control group was personal history of a psychiatric disease, as assessed using the MINI, or a family history of psychiatric disease among their first-degree relatives.
Table 1.
Characteristics and task performance.
| ASDs (n = 21) |
ADHD (n = 19) |
HC (n = 21) |
Comparison |
||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | P | Post-hoc P |
|||
| ASDs:HC | ADHD:HC | ASDs:ADHD | |||||
| Age, years | 30.8 (7.2) | 30.6 (7.4) | 28.8 (5.5) | 0.60 | |||
| Sex, men/women | 8 13 | 11 8 | 13 8 | 0.26 | |||
| IQ | 105.1 (14.6) | 102.6 (16.6) | 109.0 (5.6) | 0.25 | |||
| SST (all trials), % | 80.8 (11.6) | 78.9 (13.8) | 84.9 (9.3) | 0.26 | |||
| SST (stop trials), % | 49.2 (38.4) | 56.1 (26.3) | 65.6 (23.6) | 0.22 | |||
| SST (go trials), % | 94.3 (7.2) | 87.7 (20.2) | 92.7 (9.8) | 0.27 | |||
| MRT (SST), ms | 498.0 (102.7) | 539.1 (100.4) | 558.1 (75.8) | 0.11 | |||
| VFT, words | 16.0 (4.3) | 15.5 (4.5) | 16.9 (4.4) | 0.60 | |||
| ASRS | 3.2 (1.8) | 5.2 (0.8) | 1.3 (1.1) | < 0.01 | < 0.01 | < 0.01 | < 0.01 |
| WURS | 53.1 (23.2) | 62.1 (20.0) | 17.5 (9.3) | < 0.01 | < 0.01 | < 0.01 | 0.39 |
| AQ total score | 33.5 (7.9) | 27.6 (5.5) | 13.4 (4.2) | < 0.01 | < 0.01 | < 0.01 | 0.02 |
| GAF | 51.8 (13.2) | 58.8 (10.7) | 84.6 (3.1) | < 0.01 | < 0.01 | < 0.01 | 0.45 |
| Subtype | Asperger, 5; PDD NOS, 16 | ADHD, 11; ADD, 8 | |||||
ASDs, autism spectrum disorders; ADHD, attention deficit hyperactivity disorder; HC, healthy control subjects; Asperger, Asperger syndrome; PDD, pervasive developmental disorder; NOS, pervasive developmental disorder - not otherwise specified; IQ, intelligence quotient; SST, stop signal task; MRT, mean reaction time; VFT, verbal fluency task; ASRS, The World Health Organization (WHO) Adult ADHD Inhibitory-Report Scale; WURS, Wender Utah Rating Scale; AQ, autism spectrum quotient; GAF, Global Assessment of Functioning.
None of the adults with ASDs or ADHD had been treated with stimulants or other psychiatric medication. In Japan, MPH was approved only for treatment of children with ADHD in 2007, and it cannot be used for treating adult ADHD in Japan. Therefore, MPH cannot be used even for cases with severe ADHD symptoms.
The HC group was also free of medication. To the extent possible, we obtained childhood information from a person who knew the patient in childhood (usually the mother). At the time of the recruitment of the subjects, the usage of the Autism Diagnostic Interview, Revised (ADI-R), Autism Diagnostic Observation Schedule (ADOS), and Conners' Adult ADHD Diagnostic Interview for DSM-IV™ (CAADID) was extremely limited in Japan. Before we finished recruitment, we obtained permission to use the ADI-R, ADOS, and CAADID, which were administered by child psychiatrists and psychologists (HK, YK, and AI) to 6 participants (3 ASDs and 3 ADHD participants). The conventional diagnosis was coincident with the diagnosis obtained using ADI-R, ADOS, and CAADID in these 6 participants.
All participants were self-reported right-handers, as assessed using the Edinburgh score (> 70) (Oldfield, 1971). The IQ scores of subjects with ASDs and ADHD were obtained using the Wechsler Adult Intelligence Scale-Revised (WAIS-R), Japanese version. The IQ scores of the HCs were estimated using the Japanese version of the National Adult Reading Test (JART) (Matsuoka et al., 2006). Although the JART can measure IQ accurately in HC participants, the test is problematic for participants with ASDs and ADHD because of the well-known imbalances in their intellectual abilities.
A self-reported screening scale was used to assess ADHD symptoms; the World Health Organization (WHO) Adult ADHD Self-Report Scale (ASRS), which was developed in conjunction with the revision of the WHO Composite International Diagnostic Interview (CIDI) (cutoff > 3). The Japanese version of the Wender Utah Rating Scale (WURS), which is a self-reporting instrument that is used to identify childhood tendencies toward ADHD retrospectively (cutoff > 46) (Matsumoto et al., 2005), was also used. We used these 2 scales in the ASDs and ADHD groups.
We applied the Autism Spectrum Quotient (AQ), as obtained from a self-reported questionnaire, to quantify autistic symptoms in all participants (Baron-Cohen et al., 2001; Wakabayashi et al., 2006). The AQ comprises 50 questions, with 10 questions assessing each of 5 different areas: social skills, attention switching, attention to detail, communication, and imagination. Scores range from 0 to 50 (cutoff > 32).
2.2. Task procedure
Hemoglobin concentration ([Hb]) changes were measured during 2 cognitive activation tasks. Subjects sat on a comfortable chair with their eyes open throughout the NIRS measurements and were instructed to minimize movements such as head movements, strong biting, and eye blinking. The sequence of the following 2 tasks was counterbalanced across the subjects.
2.2.1. SST
The cognitive activation task included a 30 s pre-SST, an 81 s SST, and an 80 s post-SST period (Fig. 1A). We selected the block design for the cognitive activation task. In the pre- and post-SST periods (Fig. 1B), the participants were instructed to indicate the direction of an image of a dog (left or right) by pressing a button as quickly as possible. Participants performed 20 trials during the pre-task period and 30 trials during the post-task period. The mean reaction time was calculated automatically during the pre-task period. The image of a dog was displayed for 0.5 s. Between presentation of the images of dogs, a cross shape was shown for 0.4–1.0 s.
Fig. 1.
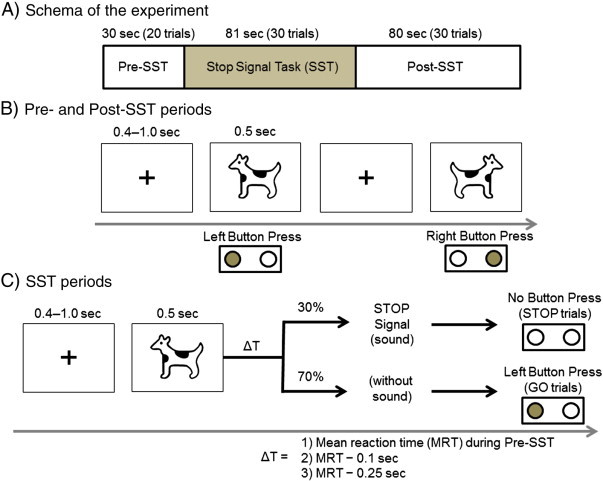
Schematic representation of the stop signal task. Auditory “STOP” signals were given under 3 conditions of delay (ΔT equal to the mean reaction time [MRT], MRT — 100 ms, and MRT — 250 ms) after the “GO” stimulus was presented.
During the SST (Fig. 1C), participants were instructed to respond to the “GO” stimulus as quickly as possible during the “GO” trials, and to try to withhold their response on the “STOP” trials (short beep). “STOP” signals were given under 3 conditions of delay after the “GO” stimulus was presented (ΔT equal to mean reaction time [MRT], MRT — 100 ms, and MRT — 250 ms). We used these 3 conditions of delay to avoid the usual tendency to delay the Go response. The subjects performed 21 “GO” trials and 9 “STOP” trials during the SST. We used the total number of correct responses (the number of correct inhibitions plus the number of correct responses to the direction of the dog) divided by the total number of trials as a measure of task performance. We also used the success rate of stop trials and go trials in SST period as a measure of task performance.
2.2.2. VFT
The VFT, which was presented as described previously (Takizawa et al., 2008, 2009), included a 30 s pre-VFT, a 60 s VFT (letter version), and a 70 s post-VFT period. In the pre- and post-task baseline periods, the subjects were instructed to repeat Japanese vowels (/a/, /i/, /u/, /e/, and /o/) aloud. This was intended to correct the data during the fluency task with regard to activation due to vocalization. During the VFT period, participants were instructed to generate as many Japanese words beginning with a designated syllable as possible. This approach is commonly used in the Japanese letter version of the VFT, as Japanese words inevitably begin with a vowel or a consonant–vowel syllable. The 3 initial syllables (first: /to/, /a/, or /na/; second: /i/, /ki/, or /se/; third: /ta/, /o/, or /ha/) were presented in an order that was counterbalanced among the subjects and changed every 20 s during the 60 s task period, to reduce the time during which the subjects remained silent. The subjects were instructed by an auditory cue at the start and end of the task and when the syllable was changed. The total number of correct words generated during the 60 s activation period was used as a measure of task performance.
2.3. NIRS measurement
Relative [oxy-Hb] and [deoxy-Hb] changes were monitored using a 52-channel NIRS machine (Hitachi ETG-4000) at 2 wavelengths of near-infrared light (695 and 830 nm) based on the modified Beer–Lambert law. The distance between pairs of light source and detector probes was set at 3 cm, and each measurement area between pairs of source/detector probes was defined as a “channel.” The probes of the NIRS machine were arranged in 3 × 11 shells and placed on the subject's frontal area. The lowest probes were positioned along the T3–Fpz–T4 line, according to the international 10/20 system. As described previously (Takizawa et al., 2008), this arrangement of the probes is able to detect [Hb] changes in the surface regions of the PFC on bilateral sides (dorsolateral PFC [DLPFC; Brodmann areas (BA) 9 and 46], ventrolateral PFC [VLPFC; BA44, 45, and 47], and frontopolar PFC [; BA10]), and the temporal cortex. To estimate the cortical localization of each channel, we used the virtual registration method (Tsuzuki et al., 2007; Tzourio-Mazoyer et al., 2002), which enables the probabilistic registration of NIRS data onto the Montreal Neurological Institute (MNI) coordinate space without data on magnetic resonance images or probe positions (Fig. 2).
Fig. 2.
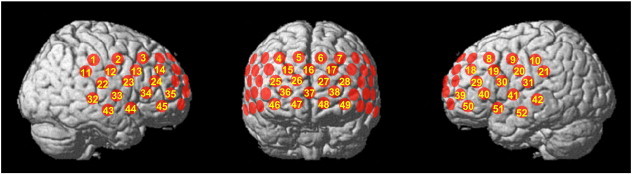
Locations of the near-infrared spectroscopy (NIRS) probes. The locations of NIRS measurements (channels) were estimated probabilistically and labeled anatomically in the standard brain space (LBPA40) according to Tsuzuki et al. (2007).
The sampling rate was set to 10 Hz. The pre-task period value was determined as the mean value over a 10 s period just prior to the task period, and the post-task period value was determined as the mean value over the last 10 s of the post-task period. Linear fitting was performed using data from the pre- and post-task periods. The moving average method was used to remove any short-term motion artifacts (moving average window, 5 s). The time resolution of the NIRS apparatus was set at 0.1 s and changes were analyzed using the first-order correction to exclude changes unrelated to the task, such as very slow oscillations or baseline drifts. To acquire a stable baseline, a 20 s non-measured period was included in the 30 s pre-task period; NIRS measurement started in the last 10 s of the pre-task period. Because the NIRS signal was sometimes unstable at the start of the pre-task period, the pre-task baseline was determined as the mean across the last 10 s of the this period, the post-task baseline was determined as the mean across the last 10 s of the post-task period, and a linear fitting was performed on the basis of data between the 2 baselines according to previous NIRS studies (Takizawa et al., 2008; Marumo et al., 2013).
Despite the application of these artifact-rejection methods, visible artifacts sometimes remained in the waveforms. We therefore used a computed rejection program (see Inline Supplementary material A.1) that automatically rejected channels that included waveforms with prominent artifacts. Because we excluded the rejected channels from further analyses, the number of available channels varied among individuals.
Finally, the [oxy-Hb] and [deoxy-Hb] data obtained for each channel were averaged for the task period and the [task + post-task] period, respectively. We chose these 2 NIRS signals to detect the time course of [oxy-Hb] and [deoxy-Hb] changes during cognitive tasks.
2.4. Statistical analyses
All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) 18 software (SPSS Inc., Tokyo, Japan).
2.4.1. Clinical scale and task performance
Between-group differences in the scores for clinical scales and task performance were tested using a 1-way analysis of variance (ANOVA) and Tukey's honestly significant difference (HSD) test as a post-hoc analysis. A chi-squared test was used for testing sex differences. Clinical and behavioral results were considered significant at P < 0.05.
2.4.2. NIRS data
We focused on the mean [oxy-Hb], as the [oxy-Hb] increase is assumed to reflect cognitive activation more directly than does the [deoxy-Hb] decrease, as shown by a stronger correlation of [oxy-Hb] with the blood-oxygenation-level-dependent signal measured by fMRI (Strangman et al., 2002) and by results of animal studies (Hoshi et al., 2001).
For the SST and VFT, mean [oxy-Hb] values were analyzed via one-way ANOVA using the mean [oxy-Hb] as the dependent variable and the diagnosis (ASDs, ADHD, or HC) as the independent variable for each period (the task period or the [task + post-task] period) and for each channel. A false-discovery rate (FDR) correction for multiple comparisons (52 channels) was applied. We set the value for the maximum FDR to 0.05, to allow no more than 5% false positives on average (Singh and Dan, 2006). The mean [oxy-Hb] values were then analyzed using Tukey's HSD test, as a post-hoc analysis, for each period and for each channel.
2.4.3. Classification and cross-validation
The individual expression values were defined as the mean [oxy-Hb] values during the task (or task + post-task) period. We submitted the resulting individual expression values to a parametric Fisher's linear discriminant analysis classification algorithm (Ponseti et al., 2012) in order to discriminate the ASDs from the ADHD group, the ASDs from the HC group, and the ADHD from the HC group.
First, we used stepwise analysis for all channels that showed a significant difference (P < 0.05) between the ASDs and ADHD group, the ASDs and HC group, or the ADHD and HC group to select the channels to be used in Fisher's linear discriminant analysis. A stepwise analysis was performed in a forward direction using P values for entry (P = 0.05) and removal (P = 0.10).
Second, we classified each participant according to these values using Fisher's linear discriminant analysis. We cross validated the classification method using a leave-one-out procedure 40–42 times, to account for each participant. We determined the predictive power of the classification procedure by calculating specificity and sensitivity, as well as average sensitivity and specificity values and mean classification accuracy.
2.4.4. Additional analyses
We evaluated whether [deoxy-Hb] changes had tendencies similar to the [oxy-Hb] changes among the 3 groups, although, overall, we focused on [oxy-Hb] changes. We also addressed prefrontal [oxy-Hb] changes in ASDs adults with ADHD symptoms, who are difficult to differentiate clinically from ADHD adults. We defined a subgroup of ASDs adults who exceeded the ASRS cutoff (> 3) (n = 10). A different activation between participants with a diagnosis of ASDs who also had ADHD symptoms and the ADHD group would represent an ASDs-specific brain dysfunction. The 3 diagnostic groups (the ASDs subgroup with ADHD symptoms, the entire ADHD group, and the entire HC group) were then compared using the statistical procedures described above. Furthermore, we compared [oxy-Hb] changes between male and female subjects in each group, because a previous NIRS study demonstrated that [oxy-Hb] changes were affected by sex (Kameyama et al., 2004). In addition, we performed a correlation analysis to examine the relationship between [oxy-Hb] changes and clinical conditions and demographic data, such as age, symptom severity, and task performance.
3. Results
3.1. Clinical characteristics and behavioral results
All patients with ADHD and 10 patients with ASDs exceeded the cutoff of the ASRS (Table 1). The ASRS scores of all HC subjects were below the cutoff value. The mean WURS scores of the patients exceeded the threshold (> 46), suggesting a difficulty in distinguishing these 2 disorders based on retrospective inhibitory-reporting assessments in their childhood. The ASDs group had significantly higher AQ scores than did the other groups. No statistically significant differences were observed among the 3 groups in any of the task-performance indices.
3.2. NIRS data results
3.2.1. SST
The typical grand-averaged waveforms for [oxy-Hb] in the left VLPFC (ch50) in the HC, ASDs, and ADHD groups are shown in Fig. 3. During the SST period, we found significant main effects of the group in 29 channels (ch1–3, 10–13, 18, 20, 22, 24, 28, 29, 31, 32, 35–39, 41, 42, and 45–51; F [df = 2, 53–58] = 3.911–15.448; FDR-corrected P ≤ 0.001–0.026).
Fig. 3.
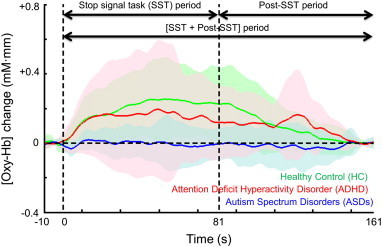
Time courses of the hemodynamic responses in the left ventrolateral prefrontal cortex (ch50) for the 3 diagnostic groups. The [oxy-Hb] changes for the healthy control (HC) group (black, n = 21), attention deficit hyperactivity disorder (ADHD) group (pink, n = 19), and autism spectrum disorders (ASDs) group (blue, n = 21) during the activation stop signal task (SST) and post-SST conditions are presented as grand-averaged waveforms in ch50. The shaded color indicates the standard deviation.
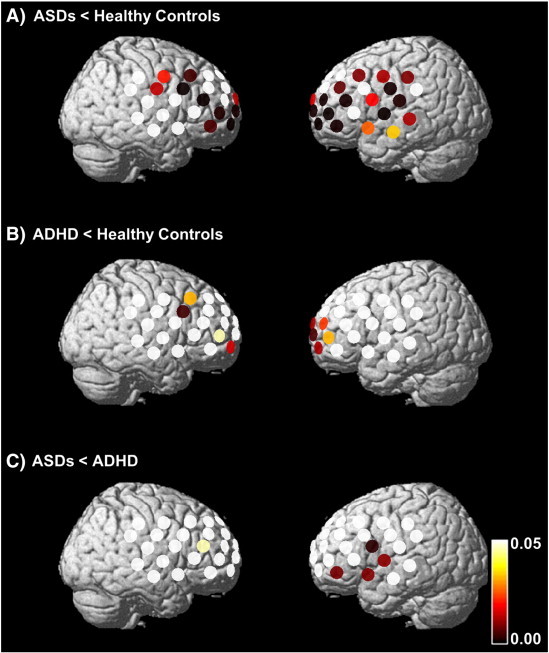
The [oxy-Hb] changes in the ASDs group were significantly smaller than those in the ADHD group in 2 channels corresponding to the left VLPFC (ch50 and 51; post-hoc P = 0.030–0.034) (Figs. 3 and 4, see Inline Supplementary Table S1). Tukey's HSD test indicated that [oxy-Hb] changes in the ASDs group were significantly smaller than those in the HC group in 28 channels corresponding to the bilateral DLPFC, left VLPFC, left premotor area (PMA), left presupplementary motor area (SMA), and frontal pole (ch2, 3, 10–13, 18, 20, 22, 24, 28, 29, 31, 32, 35–39, 41, 42, and 45–51; post-hoc P ≤ 0.001–0.046) (Figs. 3 and 4, see Inline Supplementary Table S1). [oxy-Hb] changes in the ADHD group were significantly smaller than those in the HC group in 17 channels corresponding to the frontal pole, the bilateral DLPFC, the right PMA, and the right pre-SMA (ch1–3, 11–13, 18, 22, 28, 29, 36, 38, 39, and 46–49; post-hoc P = 0.001–0.047) (Fig. 4, see Inline Supplementary Table S1).
Fig. 4.
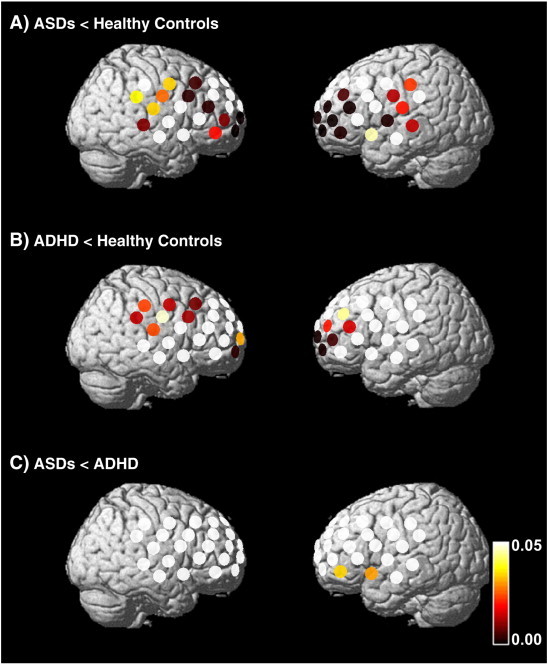
Differences in [oxy-Hb] changes during the SST between the HC and ADHD groups (A), between the HC and ASDs groups (B), and between the ASDs and ADHD groups (C) (A: post-hoc P ≤ 0.001–0.046; B: post-hoc P = 0.001–0.047: and C: post-hoc P = 0.030–0.034). The colored bar represents the P value.
Inline Supplementary Table S1.
Table S1.
The differences in channels during SST periods.
| Channel | Location | |
|---|---|---|
| ADHD, ASDs < HC | ch2, 3, 11–13, 18, 22, 28, 29, 36, 38, 39, and 46–49 | The frontal pole, both the DLPFC, the right PMA |
| ADHD < HC | ch1–3, 11–13, 18, 22, 28, 29, 36, 38, 39, and 46–49 | The frontal pole, both the DLPFC, the right PMA, and the right pre-SMA |
| ASDs < HC | ch2, 3, 10–13, 18, 20, 22, 24, 28, 29, 31, 32, 35–39, 41, 42, and 45–51 | Both the DLPFC, left VLPFC, left PMA, left pre-SMA and frontal pole |
| Only ADHD < HC | ch1 | The right pre-SMA |
| Only ASDs < HC | ch10, 20, 24, 31, 32, 35, 37, 41, 42, 45, 50, and 51 | Left VLPFC, left PMA, left pre-SMA, and frontal pole |
| ASDs < ADHD | ch50, 51 | Left VLPFC |
| ADHD, ASDs < HC | ch2, 3, 11–13, 18, 22, 28, 29, 36, 38, 39, and 46–49 | The frontal pole, both the DLPFC, the right PMA |
ADHD, attention deficit hyperactivity disorder; ASDs, autism spectrum disorders; DLPFC, dorsolateral prefrontal cortex; PMA, premotor area; pre-SMA, presupplementary motor area; VLPFC, ventrolateral prefrontal cortex.
The [oxy-Hb] changes in the ASDs group were significantly smaller than those in the ADHD group in 2 channels corresponding to the left VLPFC (ch50 and 51; post-hoc P = 0.030–0.034) (Figs. 3 and 4, see Inline Supplementary Table S1). Tukey's HSD test indicated that [oxy-Hb] changes in the ASDs group were significantly smaller than those in the HC group in 28 channels corresponding to the bilateral DLPFC, left VLPFC, left premotor area (PMA), left presupplementary motor area (SMA), and frontal pole (ch2, 3, 10–13, 18, 20, 22, 24, 28, 29, 31, 32, 35–39, 41, 42, and 45–51; post-hoc P ≤ 0.001–0.046) (Figs. 3 and 4, see Inline Supplementary Table S1). [oxy-Hb] changes in the ADHD group were significantly smaller than those in the HC group in 17 channels corresponding to the frontal pole, the bilateral DLPFC, the right PMA, and the right pre-SMA (ch1–3, 11–13, 18, 22, 28, 29, 36, 38, 39, and 46–49; post-hoc P = 0.001–0.047) (Fig. 4, see Inline Supplementary Table S1).
Inline Supplementary Table S1 can be found online at http://dx.doi.org/10.1016/j.nicl.2013.10.002.
During the [SST + post-SST] period, there was a main effect of the group in 31 channels (ch2, 3, 8–10, 12, 13, 18, 20, 24, 26–31, 35–39, 41, 42, and 45–52; F [df = 2, 53–58] = 4.291–12.721; FDR-corrected P ≤ 0.001–0.019).
The [oxy-Hb] increases observed in the ADHD group were significantly larger than those recorded in the ASDs group in 5 channels corresponding to the left VLPFC and frontal pole (ch24, 30, 41, 50, and 51; post-hoc P = 0.003–0.046) (Figs. 3 and 5, Supplementary Table A. 2). The number of channels that exhibited significantly different NIRS signals between the ASDs and ADHD groups were larger for the [SST + post SST] analysis than that for the SST analysis, which was mainly driven by a post-SST reascending in [oxy-Hb] in the ADHD group (Fig. 3). The post-hoc Tukey's HSD test revealed that the [oxy-Hb] increases observed during the [SST + post-SST] period in the ASDs group were significantly smaller than those in the HC group in 31 channels corresponding to the bilateral DLPFC, bilateral VLPFC, bilateral PMA, bilateral pre-SMA, and frontal pole (ch2, 3, 8–10, 12, 13, 18, 20, 24, 26–31, 35–39, 41, 42, and 45–52; post-hoc P ≤ 0.001–0.033) (Figs. 3 and 5, see Inline Supplementary Table S2). The [oxy-Hb] increases observed in the ADHD group were significantly smaller than those in the HC group in 11 channels corresponding to the frontal pole, bilateral DLPFC, right PMA, and right pre-SMA (ch3, 13, 27, 28, 35, 38, 39, and 46–49; post-hoc P = 0.005–0.046) (Fig. 5, see Inline Supplementary Table S2).
Fig. 5.
Differences in [oxy-Hb] changes during the [SST + post-SST] period between the HC and ADHD groups (A), between the HC and ASDs groups (B), and between the ASDs and ADHD groups (C) (A: post-hoc P ≤ 0.001–0.033; B: post-hoc P = 0.005–0.046; and C: post-hoc P = 0.003–0.046). The colored bar represents the P value.
Inline Supplementary Table S2.
Table S2.
Differences in channels during [SST + post-SST] periods.
| Channel | Location | |
|---|---|---|
| ADHD, ASDs < HC | ch3, 13, 27, 28, 35, 38, 39, and 46–49 | The frontal pole, bilateral DLPFC, right PMA, and right pre-SMA |
| ADHD < HC | ch3, 13, 27, 28, 35, 38, 39, and 46–49 | The frontal pole, bilateral DLPFC, right PMA, and right pre-SMA |
| ASDs < HC | ch2, 3, 8–10, 12, 13, 18, 20, 24, 26–31, 35–39, 41, 42, and 45–52 | Both DLPFC, both VLPFC, both PMA, both pre-SMA, left temporal, and the frontal pole |
| Only ADHD < HC | None | None |
| Only ASDs < HC | ch2, 8–10, 12, 18, 20, 24, 26, 29, 30, 31, 36, 37, 41, 42, 45, and 50–52 | Left VLPFC, right PMA, left pre-SMA, and frontal pole |
| ASDs < ADHD | ch24, 30, 41, 50, and 51 | Left VLPFC and frontal pole |
ADHD, attention deficit hyperactivity disorder; ASDs, autism spectrum disorders; DLPFC, dorsolateral prefrontal cortex; PMA, premotor area; pre-SMA, presupplementary motor area; VLPFC, ventrolateral prefrontal cortex.
The [oxy-Hb] increases observed in the ADHD group were significantly larger than those recorded in the ASDs group in 5 channels corresponding to the left VLPFC and frontal pole (ch24, 30, 41, 50, and 51; post-hoc P = 0.003–0.046) (Figs. 3 and 5, Supplementary Table A. 2). The number of channels that exhibited significantly different NIRS signals between the ASDs and ADHD groups were larger for the [SST + post SST] analysis than that for the SST analysis, which was mainly driven by a post-SST reascending in [oxy-Hb] in the ADHD group (Fig. 3). The post-hoc Tukey's HSD test revealed that the [oxy-Hb] increases observed during the [SST + post-SST] period in the ASDs group were significantly smaller than those in the HC group in 31 channels corresponding to the bilateral DLPFC, bilateral VLPFC, bilateral PMA, bilateral pre-SMA, and frontal pole (ch2, 3, 8–10, 12, 13, 18, 20, 24, 26–31, 35–39, 41, 42, and 45–52; post-hoc P ≤ 0.001–0.033) (Figs. 3 and 5, see Inline Supplementary Table S2). The [oxy-Hb] increases observed in the ADHD group were significantly smaller than those in the HC group in 11 channels corresponding to the frontal pole, bilateral DLPFC, right PMA, and right pre-SMA (ch3, 13, 27, 28, 35, 38, 39, and 46–49; post-hoc P = 0.005–0.046) (Fig. 5, see Inline Supplementary Table S2).
Inline Supplementary Table S2 can be found online at http://dx.doi.org/10.1016/j.nicl.2013.10.002.
3.2.2. VFT
During the VFT period, we found no significant main effects of the group. There were no significant differences in [oxy-Hb] changes between the ASDs and ADHD groups during the VFT. During the [VFT + post-VFT] period, we found significant main effects of the group in 2 channels (ch13 and 34; F [df = 2, 51–55] = 7.277–8.056; FDR-corrected P = 0.001–0.002).
There were no significant differences for any channels between the ASDs and ADHD groups during the [VFT + post-VFT] period. Post-hoc Tukey's HSD tests showed that the [oxy-Hb] changes in the ASDs group were significantly smaller than those in the HC group in 2 channels corresponding to the left VLPFC and DLPFC (ch13 and 34; post-hoc P = 0.002–0.003) during the [VFT + post-VFT] period. The [oxy-Hb] changes in the ADHD group were significantly smaller than those recorded in the HC group in 2 channels corresponding to the left VLPFC and DLPFC (ch13 and 34; post-hoc P = 0.004–0.019) during the [VFT + post-VFT] period.
3.3. Classification and cross-validation
3.3.1. Discrimination between the ASDs and ADHD groups
ANOVA revealed the presence of significant differences between patients with ASDs and those with ADHD in the left VLPFC. Individual brain responses were characterized by expression values in 2 channels with significant differences in the mean [oxy-Hb] during the SST period between ASDs and ADHD adults (ch50 and 51). The stepwise analysis selected 1 channel (ch50: left VLPFC) using the mean [oxy-Hb] during the SST period (P = 0.02). The leave-one-out classification algorithm using the mean [oxy-Hb] during the SST period had an accuracy of 72.9% (sensitivity, 85.7%; specificity, 57.9%).
Individual brain responses were characterized by expression values in 5 channels with significant differences in the mean [oxy-Hb] during the [SST + post-SST] period between adults with ASDs and those with ADHD (ch24, 30, 41, 50, and 51). The stepwise regression analysis selected 1 channel (ch30: left VLPFC) using the mean [oxy-Hb] during the [SST + post-SST] period (P = 0.002). The algorithm that used the mean [oxy-Hb] during the [SST + post-SST] period had high accuracy (81.4%; sensitivity, 90.0%; specificity, 70.6%).
3.3.2. Discrimination of the ASDs from the HC group
ANOVA revealed the presence of significant differences between patients with ASDs and HC individuals in the broad prefrontal area. Individual brain responses were characterized by expression values in 34 channels that were significantly different regarding the mean [oxy-Hb] during the SST period between patients with ASDs and healthy adults (ch2, 3, 7–13, 17, 18, 20, 22, 24, 28, 29, 31, 32, 35–39, 41, 42, and 45–52). The stepwise regression analysis selected 1 channel (ch39: left VLPFC) using the mean [oxy-Hb] during the SST period (P < 0.001). The leave-one-out classification algorithm had an accuracy of 81.1% (sensitivity, 90.5%; specificity, 71.4%) using the mean [oxy-Hb] during the SST period.
Individual brain responses were characterized by expression values in 36 channels that showed significant differences in the mean [oxy-Hb] during the [SST + post-SST] period between patients with ASDs and healthy adults (ch2, 3, 7–10, 12, 13, 18–20, 24–32, 35–39, 41, 42, and 45–52). The stepwise regression analysis selected 2 channels (ch47 and 50: frontal pole and left VLPFC) using the mean [oxy-Hb] during the [SST + post-SST] period (P < 0.001). The algorithm that used the mean [oxy-Hb] during the [SST + post-SST] period had high accuracy (89%; sensitivity, 90.0%; specificity, 80.0%).
3.3.3. Discrimination of the ADHD from the HC group
ANOVA revealed the presence of significant differences between patients with ADHD and HC individuals in the right pre-SMA, right PMA, and bilateral DLPFC. Individual brain responses were characterized by expression values in 17 channels with significant differences in the mean [oxy-Hb] during the SST period between patients with ADHD and healthy adults (ch1–3, 11–13, 18, 22, 28, 29, 36, 38, 39, and 46–49). The stepwise regression analysis selected 1 channel (ch11: right pre-SMA and right PMA) using the mean [oxy-Hb] during the SST period. The leave-one-out classification algorithm using the mean [oxy-Hb] during the SST period had a mean accuracy of 78.8% (sensitivity, 84.2%; specificity, 76.2%).
Individual brain responses were characterized by expression values in 11 channels with significant differences in the mean [oxy-Hb] during the [SST + post-SST] period between patients with ADHD and healthy adults (ch3, 13, 27, 28, 35, 38, 39, and 46–49).
The stepwise regression analysis selected 1 channel (ch13: right pre-SMA and right PMA) using the mean [oxy-Hb] during the [SST+post-SST] period (P = 0.006). The algorithm that used the mean [oxy-Hb] during the [SST + post-SST] period had a mean accuracy of 72.5% (sensitivity, 72.2%; specificity, 71.4%).
3.3.4. Additional analyses
There were no significant main effects in the results obtained for [deoxy-Hb] during the task or the [task + post-task] period, for either the SST or VFT tasks (see inline Supplementary material A. 2). In addition, the secondary group comparison (the ASDs subgroup with ADHD symptoms, the entire ADHD group, and the entire HC group) yielded statistical conclusions regarding clinical characteristics, behavioral results, and NIRS data results that were similar to those of the original group comparisons (see Inline Supplementary Table S3 and Supplementary material A. 3). There were no significant differences in NIRS data between male and female subjects during the task or the [task + post-task] period. Finally, for both the SST and VFT, there were no significant correlations between [oxy-Hb] changes during the task or the [task + post-task] period and clinical symptoms or task performance for either the ASDs or the ADHD group.
Inline Supplementary Table S3.
Table S3.
Characteristics and task performance of the secondary groups.
| ASDs with ADHD symptoms (n = 10) |
ADHD (n = 19) |
HC (n = 21) |
Comparison |
||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | P | Post-hoc P |
|||
| ASDs:HC | ADHD:HC | ASDs:ADHD | |||||
| Age, years | 29.8 (7.7) | 30.6 (7.4) | 28.8 (5.4) | 0.71 | |||
| Sex, men/women | 6/4 | 11/8 | 8/13 | 0.17 | |||
| IQ | 99.7 (1.6) | 102.6 (16.6) | 109.0 (5.6) | 0.21 | |||
| SST (all trials), % | 78.3 (13.2) | 78.9 (13.8) | 84.9 (9.3) | 0.26 | |||
| SST (stop trials), % | 45.6 (38.4) | 56.1 (26.3) | 65.6 (23.6) | 0.22 | |||
| SST (go trials), % | 92.4 (6.4) | 87.7 (20.2) | 92.7 (9.8) | 0.50 | |||
| MRT (SST), ms | 483.2 (116.6) | 539.1 (100.4) | 558.1 (75.8) | 0.13 | |||
| VFT, words | 13.7 (3.6) | 15.5 (4.5) | 16.9 (4.4) | 0.16 | |||
| ASRS | 4.9 (0.9) | 5.2 (0.8) | 1.3 (1.1) | < 0.01 | < 0.01 | < 0.01 | 0.78 |
| WURS | 61.1 (2.3) | 62.1 (20.0) | 17.5 (9.3) | < 0.01 | < 0.01 | < 0.01 | 0.99 |
| AQ total score | 34.0 (9.8) | 27.6 (5.5) | 13.4 (4.2) | < 0.01 | < 0.01 | < 0.01 | 0.03 |
| GAF | 49.3 (1.4) | 58.8 (10.7) | 84.6 (3.1) | < 0.01 | < 0.01 | < 0.01 | 0.03 |
| Subtype | Asperger, 2; | ADHD, 11; | |||||
| PDD NOS, 8 | ADD, 8 | ||||||
ASDs, autism spectrum disorders; ADHD, attention deficit hyperactivity disorder; HC, healthy control subjects; Asperger, Asperger syndrome; PDD, pervasive developmental disorder; NOS, pervasive developmental disorder – not otherwise specified; IQ, intelligence quotient; SST, stop signal task; MRT, mean reaction time; VFT, verbal fluency task; ASRS, The World Health Organization (WHO) Adult ADHD Inhibitory-Report Scale; WURS, Wender Utah Rating Scale; AQ, autism spectrum quotient; GAF, Global Assessment of Functioning.
There were no significant main effects in the results obtained for [deoxy-Hb] during the task or the [task + post-task] period, for either the SST or VFT tasks (see inline Supplementary material A. 2). In addition, the secondary group comparison (the ASDs subgroup with ADHD symptoms, the entire ADHD group, and the entire HC group) yielded statistical conclusions regarding clinical characteristics, behavioral results, and NIRS data results that were similar to those of the original group comparisons (see Inline Supplementary Table S3 and Supplementary material A. 3). There were no significant differences in NIRS data between male and female subjects during the task or the [task + post-task] period. Finally, for both the SST and VFT, there were no significant correlations between [oxy-Hb] changes during the task or the [task + post-task] period and clinical symptoms or task performance for either the ASDs or the ADHD group.
Inline Supplementary Table S3 can be found online at http://dx.doi.org/10.1016/j.nicl.2013.10.002.
4. Discussion
To our knowledge, this is the first study showing differences in prefrontal activation associated with inhibitory control between adults with ASDs and those with ADHD. We found more profound abnormalities in the PFC during inhibitory control in drug-naïve individuals with ASDs than in drug-naïve individuals with ADHD, despite similar performance levels. Although the ASDs group showed underactivation in the left VLPFC compared to the HC group, the ADHD group did not exhibit a significant decrease in VLPFC activation compared to the HC group. Significant differences between the ASDs and ADHD groups were found during the SST, even in comparisons between the ASDs subgroup with ADHD symptoms and the ADHD group. These differences in activation were localized to the left VLPFC. In contrast, there were no significant differences in [oxy-Hb] increases during the VFT between the ASDs and ADHD groups. The use of NIRS, a portable neuroimaging device, represented a strong advantage of our study. Therefore, our findings may be a step toward the development of a clinically useful biomarker for the differential diagnosis of the 2 commonest neurodevelopmental disorders, which has been difficult when based on clinical and neuropsychological measures.
4.1. Activation of [Oxy-Hb] in the left VLPFC
This study found significantly reduced activation in the left VLPFC in drug-naïve adults with ASDs compared to drug-naïve adults with ADHD (Figs. 4 and 5), which is consistent with the previously reported structural abnormalities in the left VLPFC of patients with ASDs, including reduced gray matter density (Yamasaki et al., 2010; Abell et al., 1999). The right dominant abnormalities observed in the ADHD group were consistent with the results of previous structural (Makris et al., 2007; Overmeyer et al., 2001) and fMRI studies performed during go/no-go (Casey et al., 2007) and stop tasks (Rubia et al., 1999; Hart et al., 2013). Our findings are also consistent with the results of fMRI studies showing dysfunction in this region during facial imitation (Dapretto et al., 2006; Bookheimer et al., 2008). Action mirroring is assumed to underlie the imitation of an observed action, social understanding, and communication with other people, and the mirroring system is a function of the VLPFC (Dapretto et al., 2006).
The reduced [oxy-Hb] increase observed in the left VLPFC of the ASDs group was inconsistent with the results of previous studies showing increased activation (Schmitz et al., 2006) or similar activation (Xiao et al., 2012) in this region for the ASDs group compared to that for the HC group during a go/no-go task. Thus, the SST load, as an inhibition task, may be higher than that of the go/no-go task (Rubia et al., 2001).
Furthermore, our previous study using NIRS during a go/no-go task found that activation under the no-go condition was lower than that under the go condition in the HC group (Nishimura et al., 2011), unlike the results of the present study. Therefore, the pattern of activation observed in the PFC during the SST may be different from that observed in a go/no-go task. The present results for the SST showed that the ASDs group might have a greater abnormality in the left VLPFC than the ADHD group. This result is consistent with the findings of a neuropsychological study reporting that children with ASDs had a more profound disability in inhibitory control than did children with ADHD (Corbett et al., 2009). An fMRI study performed in children showed that the DLPFC was significantly less activated in boys with ADHD than in those with ASDs during a sustained attention task (Christakou et al., 2012), which is in contrast with the activation pattern observed here. This might be because the task used in that study did not involve inhibitory controls.
The meaning of the [oxy-Hb] reascending observed in the left VLPFC in the ADHD group during the post-SST period remains unclear. However, patients with schizophrenia also showed a similarly robust [oxy-Hb] reascending during the post-task period of the VFT in previous NIRS studies (Suto et al., 2004; Takizawa et al., 2008). These results may be explained by a common dysfunction of the monoamine system in ADHD and schizophrenia, as the repertoire of ADHD-related genes resembles that of schizophrenia-related genes (Williams et al., 2010b; Burbach, 2010). In the present study, NIRS signal analyses that included the post-SST period distinguished the ASDs and ADHD groups better (Fig. 5). The classification accuracy observed between the ASDs and ADHD groups using [oxy-Hb] changes during the [SST + post-SST] period was also higher than the accuracy obtained using [oxy-Hb] changes only during the SST period. The high time resolution of NIRS enables detailed measurements of time-course changes, thus providing important insights into differences in inhibitory control during the SST between patients with ASDs and those with ADHD.
4.2. Differences between the HC and patient groups
Our results support the hypothesis that the ASDs group has lower activation in a broad prefrontal area (Figs. 4 and 5) relative to the HC group. The classification accuracy obtained using [oxy-Hb] changes between the ASDs and HC groups during both the SST and the [SST + post-SST] period was highest in the channels for the left VLPFC. These results are consistent with those of previous studies (Yamasaki et al., 2010; Abell et al., 1999). A previous fMRI study showed that compared to healthy adults, adults with ASDs had significantly lower task-related activation in the DLPFC during a spatial working memory task (Luna et al., 2002; Ohnishi et al., 2000; Smith et al., 2004; Rinehart et al., 2002). Further, compared to healthy children, children with autism also showed reduced activation in the right DLPFC and VLPFC during a novelty detection task (Gomot et al., 2006).
The fMRI study revealed that pre-SMA and PMA would show functional interconnectivity via the basal ganglia circuitry to mediate response execution or inhibition, whereas the VLPFC would influence the basal ganglia circuitry via connectivity with pre-SMA (Duann et al., 2009). The DLPFC, with its direct connections to the basal ganglia (Alexander et al., 1986), is part of a distributed neural network supporting the selection and suppression of motor responses (Garavan et al., 2002). The fMRI study on ASDs children showed that when using a go/no-go task, there was a significant negative correlation between age and 2 right VLPFC correlation pairs: right VLPFC–bilateral pre-SMA and right VLPFC–right caudate (Lee et al., 2009). Our study detected a dysfunction in the neural basis of inhibition in adults with ASDs in areas including the bilateral VLPFC, DLPFC, Pre-SMA, and PMA, when compared to healthy controls using the NIRS, although it was difficult to detect a dysfunction of the basal ganglia.
As hypothesized, compared to the HC group, the ADHD group showed underactivation of the right SMA, pre-SMA, and bilateral DLPFC during the SST. The classification accuracy obtained using [oxy-Hb] changes between the ADHD and the HC group during both the SST and the [SST + post-SST] period was highest in the channels for the right pre-SMA and right PMA. These results are consistent with those of previous studies on drug-naïve adult patients with ADHD (Cubillo et al., 2010; Rubia et al., 2011), which used fMRI to show reduced activation in the right PFC during the SST. We also found that the ADHD group had lesser activation than the HC group in the left DLPFC, which has been directly implicated in attention switching in normal adults (Smith et al., 2004). A recent meta-analysis showed that patients with ADHD have consistent functional abnormalities in 2 distinct domain-dissociated right hemispheric fronto-basal ganglia networks, the VLPFC, supplementary motor area, and anterior cingulate cortex for inhibition and the DLPFC, parietal, and cerebellar areas for attention (Hart et al., 2013). Regarding cortical surface areas that NIRS could measure, these results are consistent with our current study and a recent NIRS study of adults with ADHD, which used the SST to show reduced bilateral activation of the inferior frontal cortex in these individuals compared to healthy adults (Schecklmann et al., 2013).
Our observation of decreased frontal activation during the SST was in contrast to previous studies showing no abnormality of activation in patients with ADHD during a go/no-go task (Dibbets et al., 2009; Kooistra et al., 2010). This inconsistency may be related to task differences and MPH treatment history. Most of the patients included in those 2 previous studies (Dibbets et al., 2009; Kooistra et al., 2010) had been chronically medicated with MPH, and there is evidence of long-term effects of MPH on brain structure (Shaw et al., 2009) and brain function (Konrad et al., 2007).
4.3. Limitations
This study had several limitations. First, trying to differentiate two behaviorally defined psychiatric disorders by using biological markers may not be the final goal for the psychiatry, since the current diagnostic system is solely based on the categorization by behavior. Rather, future psychiatry should pursue comprehensive recapturing of the association between various dimensions of behavior and their biological basis. The importance of identified biomarkers in the current study should be interpreted in this context. For example, neuroimaging biomarkers may be more useful in making decision on the use of a certain pharmacological intervention for an individual patient, compared with the behaviorally categorized diagnosis per se, which should be clarified in future studies. Second, only 6 patients underwent structured interviews using the ADI-R, ADOS, and CAADID. Although we included other participants in this study only when at least 2 of the 3 trained child psychiatrists had seen patients and given consistent diagnoses, the inter-rater reliability for their evaluating psychiatrists was not established. Third, our study focused on adult subjects; thus, it is unclear whether our results can be extended to children with ASDs and ADHD. Fourth, the application of our results in clinical practice requires the replication of the findings in an independent sample. Fifth, the number of patients included in the subsample (patients having ASDs with ADHD symptoms: 10 participants) was smaller than the optimal sample size for neuroimaging (Carter et al., 2008). However, we found a significant difference in prefrontal activation between patients having ASDs with ADHD symptoms and those having ADHD. Although we also analyzed the correlation between [oxy-Hb] changes during the task or the [task + post-task] period and ASRS scores in addition to the analysis of this subsample using cut off score of ASRS, there were no significant correlations for either the ASDs or the ADHD group. Finally, because subjects were matched for IQ in each group and since IQ scores were relatively high, participants in our study may not be representative of all general patients. It is necessary that our data be replicated in a larger sample of participants.
4.4. Conclusions
In conclusion, the present study provides evidence of functional differences in activation in the left VLPFC between drug-naïve patients with ASDs and those with ADHD. Thus, the signal time course in the left VLPFC may be a diagnostic marker for distinguishing ADHD from ASDs. NIRS may be a candidate for an auxiliary diagnostic tool that is useful for both clinicians and patients.
Competing interests
Dr. Kiyoto Kasai reports the following financial relationship. The University of Tokyo and the Research and Developmental Center, Hitachi Medical Corporation, have had an official contract for a collaborative study on the clinical applications of near-infrared spectroscopy in psychiatric disorders, which has been approved by the Research Promotion Office, University of Tokyo Hospital. For the present study, the Hitachi Medical Corporation provided a project grant (JPY 300,000/year). Drs. Ayaka Ishii-Takahashi, Ryu Takizawa, Yuki Kawakubo, Hitoshi Kuwabara, and Kiyoto Kasai at the University of Tokyo and Shingo Kawasaki at the Hitachi Medical Corporation developed the “stimulus presentation device and stimulus task presentation method for optional measurement apparatus” described (patent no. 2008-146721, Japan; patent no. 12996190, United States of America; patent no. 09758336.3, European Union; and patent no. 20090120823.5, the People's Republic of China). The University of Tokyo transferred this patent to the Hitachi Medical Corporation, and the Hitachi Medical Corporation paid a transfer fee (JPY 100,000) to the University of Tokyo. The other authors report no conflicts of interest.
Role of the funding source
This study was supported in part by Grants-in-Aid for Scientific Research (innovative areas nos. 23118001 & 23118004 [Adolescent Mind & Self-Regulation] to KK; no. 23791309 to RT) and for the “Development of Biomarker Candidates for Social Behavior” study carried out under the Strategic Research Program for Brain Sciences (to KK) by the MEXT. This study was also supported in part by Health and Labor Sciences Research Grants for Comprehensive Research on Disability Health and Welfare (H23-seishin-Ippan-002 to RT&YN&AI), the Japan Society for the Promotion of Science, KAKENHI (Grant-in-Aid for Young Scientists (B) no. 2479201 to AI)), an Intramural Research Grant for Neurological and Psychiatric Disorders of NCNP (no. 23-10 to RT&YN), and by the Japan Research Foundation for Clinical Pharmacology (to RT).
Author contributions
Ishii-Takahashi, M.D., Ph.D., had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Ishii-Takahashi, M.D., Ph.D., Kawakubo, Ph.D., Kasai, M.D., Ph.D. Acquisition of data: Ishii-Takahashi, M.D., Ph.D., Kawakubo, Ph.D., Kuwabara, M.D., Ph.D., Okuhata, Ph.D., Hamada, Med. Analysis and interpretation of data: Ishii-Takahashi, M.D., Ph.D., Hamada, Bed, Nishimura, Ph.D., Takizawa, M.D., Ph.D., Kawasaki, MS. Drafting of the manuscript: Ishii-Takahashi, M.D., Ph.D. Critical revision of the manuscript for important intellectual content: Nishimura, Ph.D. Takizawa, M.D., Ph.D., Matsubayashi, Ph.D., Yamasue, M.D., Ph.D., Kasai, M.D., Ph.D. Statistical analysis: Ishii-Takahashi, M.D., Ph.D., Nishimura, Ph.D., Takizawa, M.D., Ph.D., Kawasaki, MS. Obtained funding: Ishii-Takahashi, M.D., Ph.D., Takizawa, M.D., Ph.D., Nishimura, Ph.D., Kawakubo, Ph.D., Kasai, M.D., Ph.D. Administrative, technical, or material support: Kawasaki, MS. Study supervision: Kano, M.D., Ph.D., Kasai, M.D., Ph.D., Igarashi M.D., Ph.D. All contributors have approved the final version of the manuscript.
Acknowledgments
The authors would like to thank all the participants in this study.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Appendix A. Supplementary data
Supplementary material
References
- Abell F., Krams M., Ashburner J., Passingham R., Friston K., Frackowiak R., Happe F., Frith C., Frith U. The neuroanatomy of autism: a voxel-based whole brain analysis of structural scans. Neuroreport. 1999;10:1647–1651. doi: 10.1097/00001756-199906030-00005. [DOI] [PubMed] [Google Scholar]
- Alexander G.E., DeLong M.R., Strick P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . 4th edn. American Psychiatric Association; Washington, DC: 2000. Diagnostic and Statistical Manual of Mental Disorders. Text Revision; pp. 69–93. [Google Scholar]
- Atkinson M., Hollis C. NICE guideline: attention deficit hyperactivity disorder. Arch. Dis. Child. Educ. Pract. Ed. 2010;95:24–27. doi: 10.1136/adc.2009.175943. [DOI] [PubMed] [Google Scholar]
- Autism Network (RUPP) Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Arch. Gen. Psychiatry. 2005;62:1266–1274. doi: 10.1001/archpsyc.62.11.1266. [DOI] [PubMed] [Google Scholar]
- Barkley R.A., Murphy K.R., O'Connell T., Connor D.F. Effects of two doses of methylphenidate on simulator driving performance in adults with attention deficit hyperactivity disorder. J. Safety Res. 2005;36:121–131. doi: 10.1016/j.jsr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S., Skinner R., Martin J., Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J. Autism Dev. Disord. 2001;31:5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Bookheimer S.Y., Wang A.T., Scott A., Sigman M., Dapretto M. Frontal contributions to face processing differences in autism: evidence from fMRI of inverted face processing. J. Int. Neuropsychol. Soc. 2008;14:922–932. doi: 10.1017/S135561770808140X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbach J.P. Neuropsychiatric connections of ADHD genes. Lancet. 2010;376:1367–1368. doi: 10.1016/S0140-6736(10)61192-0. [DOI] [PubMed] [Google Scholar]
- Carter C.S., Heckers S., Nichols T., Pine D.S., Strother S. Optimizing the design and analysis of clinical functional magnetic resonance imaging research studies. Biol. Psychiatry. 2008;15:842–849. doi: 10.1016/j.biopsych.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Epstein J.N., Buhle J., Liston C., Davidson M.C., Tonev S.T., Spicer J., Niogi S., Millner A.J., Reiss A., Garrett A., Hinshaw S.P., Greenhill L.L., Shafritz K.M., Vitolo A., Kotler L.A., Jarrett M.A., Glover G. Frontostriatal connectivity and its role in cognitive control in parent–child dyads with ADHD. Am. J. Psychiatry. 2007;164:1729–1736. doi: 10.1176/appi.ajp.2007.06101754. [DOI] [PubMed] [Google Scholar]
- Christakou A., Murphy C.M., Chantiluke K., Cubillo A.I., Smith A.B., Giampietro V., Dal Y.E., Ecker C., Robertson D., Murphy D.G., Rubia K. Disorder-specific functional abnormalities during sustained attention in youth with attention deficit hyperactivity disorder (ADHD) and with Autism. Mol. Psychiatry. 2012;18:236–244. doi: 10.1038/mp.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett B.A., Constantine L.J., Hendren R., Rocke D., Ozonoff S. Examining executive functioning in children with autism spectrum disorder, attention deficit hyperactivity disorder and typical development. Psychiatry Res. 2009;166:210–222. doi: 10.1016/j.psychres.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillo A., Halari R., Ecker C., Giampietro V., Taylor E., Rubia K. Reduced activation and inter-regional functional connectivity of fronto-striatal networks in adults with childhood Attention-Deficit Hyperactivity Disorder (ADHD) and persisting symptoms during tasks of motor inhibition and cognitive switching. J. Psychiatr. Res. 2010;44:629–639. doi: 10.1016/j.jpsychires.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Dapretto M., Davies M.S., Pfeifer J.H., Scott A.A., Sigman M., Bookheimer S.Y., Iacoboni M. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat. Neurosci. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A., Melis G., Cianchetti C., Zuddas A. Methylphenidate for pervasive developmental disorders: safety and efficacy of acute single dose test and ongoing therapy: an open-pilot study. J. Child Adolesc. Psychopharmacol. 2004;14:207–218. doi: 10.1089/1044546041649011. [DOI] [PubMed] [Google Scholar]
- Dibbets P., Evers L., Hurks P., Marchetta N., Jolles J. Differences in feedback- and inhibition-related neural activity in adult ADHD. Brain Cogn. 2009;70:73–83. doi: 10.1016/j.bandc.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Duann J.R., Ide J.S., Luo X., Li C.S. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J. Neurosci. 2009;29:171–179. doi: 10.1523/JNEUROSCI.1300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H., Ross T.J., Murphy K., Roche R.A., Stein E.A. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Geurts H.M., Verté S., Oosterlaan J., Roeyers H., Sergeant J.A. How specific are executive functioning deficits in attention deficit hyperactivity disorder and autism? J. Child Psychol. Psychiatry. 2004;45:836–854. doi: 10.1111/j.1469-7610.2004.00276.x. [DOI] [PubMed] [Google Scholar]
- Goldberg M.C., Mostofsky S.H., Cutting L.E., Mahone E.M., Astor B.C., Denckla M.B., Landa R.J. Subtle executive impairment in children with autism and children with ADHD. J. Autism Dev. Disord. 2005;35:279–293. doi: 10.1007/s10803-005-3291-4. [DOI] [PubMed] [Google Scholar]
- Gomot M., Bernard F.A., Davis M.H., Belmonte M.K., Ashwin C., Bullmore E.T., Baron-Cohen S. Change detection in children with autism: an auditory event-related fMRI study. Neuroimage. 2006;29:475–484. doi: 10.1016/j.neuroimage.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Groen Y., Wijers A.A., Mulder L.J., Waggeveld B., Minderaa R.B., Althaus M. Error and feedback processing in children with ADHD and children with autistic spectrum disorder: an EEG event-related potential study. Clin. Neurophysiol. 2008;119:2476–2493. doi: 10.1016/j.clinph.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Happé F., Booth R., Charlton R., Hughes C. Executive function deficits in autism spectrum disorders and attention-deficit/hyperactivity disorder: examining profiles across domains and ages. Brain Cogn. 2006;61:25–39. doi: 10.1016/j.bandc.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Hart H., Radua J., Nakao T., Mataix-Cols D., Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70:185–198. doi: 10.1001/jamapsychiatry.2013.277. [DOI] [PubMed] [Google Scholar]
- Hofvander B., Delorme R., Chaste P., Nydén A., Wentz E., Ståhlberg O., Herbrecht E., Stopin A., Anckarsäter H., Gillberg C., Råstam M., Leboyer M. Psychiatric and psychosocial problems in adults with normal-intelligence autism spectrum disorders. BMC Psychiatry. 2009;9:35. doi: 10.1186/1471-244X-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi Y., Tamura M. Dynamic changes in cerebral oxygenation in chemically induced seizures in rats: study by near-infrared spectrophotometry. Brain Res. 1993;603:215–221. doi: 10.1016/0006-8993(93)91240-s. [DOI] [PubMed] [Google Scholar]
- Hoshi Y., Kobayashi N., Tamura M. Interpretation of near-infrared spectroscopy signals: a study with a newly developed perfused rat brain model. J. Appl. Physiol. 2001;90:1657–1662. doi: 10.1152/jappl.2001.90.5.1657. [DOI] [PubMed] [Google Scholar]
- Jensen P.S., Arnold L.E., Swanson J.M., Vitiello B., Abikoff H.B., Greenhill L.L., Hechtman L., Hinshaw S.P., Pelham W.E., Wells K.C., Conners C.K., Elliott G.R., Epstein J.N., Hoza B., March J.S., Molina B.S., Newcorn J.H., Severe J.B., Wigal T., Gibbons R.D., Hur K. 3-year follow-up of the NIMH MTA study. J. Am. Acad. Child Adolesc. Psychiatry. 2007;46:989–1002. doi: 10.1097/CHI.0b013e3180686d48. [DOI] [PubMed] [Google Scholar]
- Johnston K., Madden A.K., Bramham J., Russell A.J. Response inhibition in adults with autism spectrum disorder compared to attention deficit/hyperactivity disorder. J. Autism Dev. Disord. 2011;41:903–912. doi: 10.1007/s10803-010-1113-9. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Fukuda M., Uehara T., Mikuni M. Sex and age dependencies of cerebral blood volume changes during cognitive activation: a multichannel near-infrared spectroscopy study. Neuroimage. 2004;22:1715–1721. doi: 10.1016/j.neuroimage.2004.03.050. [DOI] [PubMed] [Google Scholar]
- Kemner C., Verbaten M.N., Cuperus J.M., Camfferman G., van Engeland H. Auditory event-related brain potentials in autistic children and three different control groups. Biol. Psychiatry. 1995;38:150–165. doi: 10.1016/0006-3223(94)00247-Z. [DOI] [PubMed] [Google Scholar]
- Konrad K., Neufang S., Fink G.R., Herpertz-Dahlmann B. Long-term effects of methylphenidate on neural networks associated with executive attention in children with ADHD: results from a longitudinal functional MRI study. J. Am. Acad. Child Adolesc. Psychiatry. 2007;46(12):1633–1641. doi: 10.1097/chi.0b013e318157cb3b. [DOI] [PubMed] [Google Scholar]
- Kooij S.J., Bejerot S., Blackwell A., Caci H., Casas-Brugue M., Carpentier P.J., Edvinsson D., Fayyad J., Foeken K., Fitzgerald M., Gaillac V., Ginsberg Y., Henry C., Krause J., Lensing M.B., Manor I., Niederhofer H., Nunes-Filipe C., Ohlmeier M.D., Oswald P., Pallanti S., Pehlivanidis A., Ramos-Quiroga J.A., Rastam M., Ryffel-Rawak D., Stes S., Asherson P. European consensus statement on diagnosis and treatment of adult ADHD: the European Network Adult ADHD. BMC Psychiatry. 2010;10:67. doi: 10.1186/1471-244X-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooistra L., van der Meere J.J., Edwards J.D., Kaplan B.J., Crawford S., Goodyear B.G. Preliminary fMRI findings on the effects of event rate in adults with ADHD. J. Neural Transm. 2010;117:655–662. doi: 10.1007/s00702-010-0374-y. [DOI] [PubMed] [Google Scholar]
- Lang R., Regester A., Lauderdale S., Ashbaugh K., Haring A. Treatment of anxiety in autism spectrum disorders using cognitive behaviour therapy: a systematic review. Dev. Neurorehabil. 2010;13:53–63. doi: 10.3109/17518420903236288. [DOI] [PubMed] [Google Scholar]
- Lee P.S., Yerys B.E., Della Rosa A., Foss-Feig J., Barnes K.A., James J.D., VanMeter J., Vaidya C.J., Gaillard W.D., Kenworthy L.E. Functional connectivity of the inferior frontal cortex changes with age in children with autism spectrum disorders: a fcMRI study of response inhibition. Cereb. Cortex. 2009;19:1787–1794. doi: 10.1093/cercor/bhn209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnhardt F.G., Gawronski A., Volpert K., Schilbach L., Tepest R., Vogeley K. Psychosocial functioning of adults with late diagnosed autism spectrum disorders—a retrospective study. Fortschr. Neurol. Psychiatr. 2012;80:88–97. doi: 10.1055/s-0031-1281642. [DOI] [PubMed] [Google Scholar]
- Lijffijt M., Kenemans J.L., Verbaten M.N., van Engeland H. A meta-analytic review of stopping performance in attention-deficit/hyperactivity disorder: deficient inhibitory motor control? J. Abnorm. Psychol. 2005;114:216–222. doi: 10.1037/0021-843X.114.2.216. [DOI] [PubMed] [Google Scholar]
- Luna B., Minshew N.J., Garver K.E., Lazar N.A., Thulborn K.R., Eddy W.F., Sweeney J.A. Neocortical system abnormalities in autism: an fMRI study of spatial working memory. Neurology. 2002;59:834–840. doi: 10.1212/wnl.59.6.834. [DOI] [PubMed] [Google Scholar]
- Makris N., Biederman J., Valera E.M., Bush G., Kaiser J., Kennedy D.N., Caviness V.S., Faraone S.V., Seidman L.J. Cortical thinning of the attention and executive function networks in adults with attention-deficit/hyperactivity disorder. Cereb. Cortex. 2007;17:1364–1375. doi: 10.1093/cercor/bhl047. [DOI] [PubMed] [Google Scholar]
- Malisza K.L., Clancy C., Shiloff D., Holden J., Jones C., Paulson K., Yu D.C., Summers R., Chudley A.E. Functional magnetic resonance imaging of facial information processing in children with autistic disorder, attention deficit hyperactivity disorder and typically developing controls. Int. J. Adolesc. Med. Health. 2011;23:269–277. doi: 10.1515/ijamh.2011.055. [DOI] [PubMed] [Google Scholar]
- Marumo K., Takizawa R., Kinou M., Kawasaki S., Kawakubo Y., Fukuda M., Kasai K. Functional abnormalities in the left ventrolateral prefrontal cortex during a semantic fluency task, and their association with thought disorder in patients with schizophrenia. Neuroimage. 2013 doi: 10.1016/j.neuroimage.2013.04.050. (S1053-8119(13)00397-2) [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Yamaguchi A., Asami T., Kamijo A., Iseki E., Hirayasu Y., Wada K. Drug preferences in illicit drug abusers with a childhood tendency of attention deficit/hyperactivity disorder: a study using the Wender Utah Rating Scale in a Japanese prison. Psychiatry Clin. Neurosci. 2005;59:311–318. doi: 10.1111/j.1440-1819.2005.01376.x. [DOI] [PubMed] [Google Scholar]
- Matsuoka K., Uno M., Kasai K., Koyama K., Kim Y. Estimation of premorbid IQ in individuals with Alzheimer's disease using Japanese ideographic script (Kanji) compound words: Japanese version of National Adult Reading Test. Psychiatry Clin. Neurosci. 2006;60:332–339. doi: 10.1111/j.1440-1819.2006.01510.x. [DOI] [PubMed] [Google Scholar]
- McDougle C.J., Holmes J.P., Carlson D.C., Pelton G.H., Cohen D.J., Price L.H. A double-blind, placebo-controlled study of risperidone in adults with autistic disorder and other pervasive developmental disorders. Arch. Gen. Psychiatry. 1998;55:633–641. doi: 10.1001/archpsyc.55.7.633. [DOI] [PubMed] [Google Scholar]
- Michielsen M., Comijs H.C., Semeijn E.J., Beekman A.T., Deeg D.J., Kooij J.J. The comorbidity of anxiety and depressive symptoms in older adults with attention-deficit/hyperactivity disorder: a longitudinal study. J. Affect. Disord. 2013;148:220–227. doi: 10.1016/j.jad.2012.11.063. [DOI] [PubMed] [Google Scholar]
- Newcorn J.H., Kratochvil C.J., Allen A.J., Casat C.D., Ruff D.D., Moore R.J., Michelson D. Atomoxetine and osmotically released methylphenidate for the treatment of attention deficit hyperactivity disorder: acute comparison and differential response. Am. J. Psychiatry. 2008;165:721–730. doi: 10.1176/appi.ajp.2007.05091676. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Takizawa R., Muroi M., Marumo K., Kinou M., Kasai K. Prefrontal cortex activity during response inhibition associated with excitement symptoms in schizophrenia. Brain Res. 2011;1370:194–203. doi: 10.1016/j.brainres.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Nylander L., Holmqvist M., Gustafson L., Gillberg C. Attention-deficit/hyperactivity disorder (ADHD) and autism spectrum disorder (ASD) in adult psychiatry. A 20-year register study. Nord. J. Psychiatry. 2013;67:344–350. doi: 10.3109/08039488.2012.748824. [DOI] [PubMed] [Google Scholar]
- Ohnishi T., Matsuda H., Hashimoto T., Kunihiro T., Nishikawa M., Uema T., Sasaki M. Abnormal regional cerebral blood flow in childhood autism. Brain. 2000;123:1838–1844. doi: 10.1093/brain/123.9.1838. [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Overmeyer S., Bullmore E.T., Suckling J., Simmons A., Williams S.C., Santosh P.J., Taylor E. Distributed grey and white matter deficits in hyperkinetic disorder: MRI evidence for anatomical abnormality in an attentional network. Psychol. Med. 2001;31:1425–1435. doi: 10.1017/s0033291701004706. [DOI] [PubMed] [Google Scholar]
- Ozonoff S., Jensen J. Brief report: specific executive function profiles in three neurodevelopmental disorders. J. Autism Dev. Disord. 1999;29:171–177. doi: 10.1023/a:1023052913110. [DOI] [PubMed] [Google Scholar]
- Ozonoff S., Strayer D.L. Inhibitory function in nonretarded children with autism. J. Autism Dev. Disord. 1997;27:59–77. doi: 10.1023/a:1025821222046. [DOI] [PubMed] [Google Scholar]
- Ponseti J., Granert O., Jansen O., Wolff S., Beier K., Neutze J., Deuschl G., Mehdorn H., Siebner H., Bosinski H. Assessment of pedophilia using hemodynamic brain response to sexual stimuli. Arch. Gen. Psychiatry. 2012;69:187–194. doi: 10.1001/archgenpsychiatry.2011.130. [DOI] [PubMed] [Google Scholar]
- Raymaekers R., Antrop I., van der Meere J.J., Wiersema J.R., Roeyers H. HFA and ADHD: a direct comparison on state regulation and response inhibition. J. Clin. Exp. Neuropsychol. 2007;29:418–427. doi: 10.1080/13803390600737990. [DOI] [PubMed] [Google Scholar]
- Rinehart N.J., Bradshaw J.L., Brereton A.V., Tonge B.J. Lateralization in individuals with high-functioning autism and Asperger's disorder: a frontostriatal model. J. Autism Dev. Disord. 2002;32:321–331. doi: 10.1023/a:1016387020095. [DOI] [PubMed] [Google Scholar]
- Rubia K., Overmeyer S., Taylor E., Brammer M., Williams S.C., Simmons A., Bullmore E.T. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study with functional MRI. Am. J. Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- Rubia K., Russell T., Overmeyer S., Brammer M.J., Bullmore E.T., Sharma T., Simmons A., Williams S.C., Giampietro V., Andrew C.M., Taylor E. Mapping motor inhibition: conjunctive brain activations across different versions of go/no-go and stop tasks. Neuroimage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- Rubia K., Halari R., Cubillo A., Smith A.B., Mohammad A.-M., Brammer M., Taylor E. Methylphenidate normalizes fronto-striatal underactivation during interference inhibition in medication-naïve boys with attention-deficit hyperactivity disorder. Neuropsychopharmacology. 2011;36:1–12. doi: 10.1038/npp.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecklmann M., Ehlis A.C., Plichta M.M., Dresler T., Heine M., Boreatti-Hummer A., Romanos M., Jacob C., Pauli P., Fallgatter A.J. Working memory and response inhibition as one integral phenotype of adult ADHD? A behavioral and imaging correlational investigation. J. Atten. Disord. 2013;17:470–482. doi: 10.1177/1087054711429702. [DOI] [PubMed] [Google Scholar]
- Schmitz N., Rubia K., Daly E., Smith A., Williams S., Murphy D.G. Neural correlates of executive function in autistic spectrum disorders. Biol. Psychiatry. 2006;59:7–16. doi: 10.1016/j.biopsych.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Shaw P., Sharp W.S., Morrison M., Eckstrand K., Greenstein D.K., Clasen L.S., Evans A.C., Rapoport J.L. Psychostimulant treatment and the developing cortex in attention deficit hyperactivity disorder. Am. J. Psychiatry. 2009;166:58–63. doi: 10.1176/appi.ajp.2008.08050781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.K., Dan I. Exploring the false discovery rate in multichannel NIRS. Neuroimage. 2006;33:542–549. doi: 10.1016/j.neuroimage.2006.06.047. [DOI] [PubMed] [Google Scholar]
- Smith A.B., Taylor E., Brammer M., Rubia K. Neural correlates of switching set as measured in fast, event-related functional magnetic resonance imaging. Hum. Brain Mapp. 2004;21:247–256. doi: 10.1002/hbm.20007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stigler K.A., McDonald B.C., Anand A., Saykin A.J., McDougle C.J. Structural and functional magnetic resonance imaging of autism spectrum disorders. Brain Res. 2011;1380:146–161. doi: 10.1016/j.brainres.2010.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strangman G., Boas D.A., Sutton J.P. Non-invasive neuroimaging using near-infrared light. Biol. Psychiatry. 2002;52:679–693. doi: 10.1016/s0006-3223(02)01550-0. [DOI] [PubMed] [Google Scholar]
- Suto T., Fukuda M., Ito M., Uehara T., Mikuni M. Multichannel near-infrared spectroscopy in depression and schizophrenia: cognitive brain activation study. Biol. Psychiatry. 2004;55:501–511. doi: 10.1016/j.biopsych.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Takizawa R., Kasai K., Kawakubo Y., Marumo K., Kawasaki S., Yamasue H., Fukuda M. Reduced frontopolar activation during verbal fluency task in schizophrenia: a multi-channel near-infrared spectroscopy study. Schizophr. Res. 2008;99:250–262. doi: 10.1016/j.schres.2007.10.025. [DOI] [PubMed] [Google Scholar]
- Takizawa R., Tochigi M., Kawakubo Y., Marumo K., Sasaki T., Fukuda M., Kasai K. Association between catechol-O-methyltransferase Val108/158Met genotype and prefrontal hemodynamic response in schizophrenia. PLoS ONE. 2009;4:e5495. doi: 10.1371/journal.pone.0005495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzuki D., Jurcak V., Singh A.K., Okamoto M., Watanabe E., Dan I. Virtual spatial registration of stand-alone fNIRS data to MNI space. Neuroimage. 2007;34:1506–1518. doi: 10.1016/j.neuroimage.2006.10.043. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Verté S., Geurts H.M., Roeyers H., Oosterlaan J., Sergeant J.A. The relationship of working memory, inhibition, and response variability in child psychopathology. J. Neurosci. Methods. 2006;151:5–14. doi: 10.1016/j.jneumeth.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Villringer A., Planck J., Hock C., Schleinkofer L., Dirnagl U. Near infrared spectroscopy (NIRS): a new tool to study hemodynamic changes during activation of brain function in human adults. Neurosci. Lett. 1993;154:101–104. doi: 10.1016/0304-3940(93)90181-j. [DOI] [PubMed] [Google Scholar]
- Wakabayashi A., Baron-Cohen S., Wheelwright S., Tojo Y. The Autism-Spectrum Quotient (AQ) in Japan: a cross-cultural comparison. J. Autism Dev. Disord. 2006;36:263–270. doi: 10.1007/s10803-005-0061-2. [DOI] [PubMed] [Google Scholar]
- Whalen C.K., Henker B., Buhrmester D., Hinshaw S.P., Huber A., Laski K. Does stimulant medication improve the peer status of hyperactive children? J. Consult. Clin. Psychol. 1989;57:545–549. doi: 10.1037//0022-006x.57.4.545. [DOI] [PubMed] [Google Scholar]
- White S.W., Ollendick T., Scahill L., Oswald D., Albano A.M. Preliminary efficacy of a cognitive-behavioral treatment program for anxious youth with autism spectrum disorders. J. Autism Dev. Disord. 2009;39:1652–1662. doi: 10.1007/s10803-009-0801-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt E.G., Doyle A.E., Nigg J.T., Faraone S.V., Pennington B.F. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol. Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Williams K., Wheeler D.M., Silove N., Hazell P. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD) Cochrane Database Syst. Rev. 2010:CD004677. doi: 10.1002/14651858.CD004677.pub2. [DOI] [PubMed] [Google Scholar]
- Williams N.M., Zaharieva I., Martin A., Langley K., Mantripragada K., Fossdal R., Stefansson H., Stefansson K., Magnusson P., Gudmundsson O.O., Gustafsson O., Holmans P., Owen M.J., O'Donovan M., Thapar A. Rare chromosomal deletions and duplications in attention-deficit hyperactivity disorder: a genome-wide analysis. Lancet. 2010;376:1401–1408. doi: 10.1016/S0140-6736(10)61109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao T., Xiao Z., Ke X., Hong S., Yang H., Su Y., Chu K., Xiao X., Shen J., Liu Y. Response inhibition impairment in high functioning autism and attention deficit hyperactivity disorder: evidence from near-infrared spectroscopy data. PLoS ONE. 2012;7:e46569. doi: 10.1371/journal.pone.0046569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki S., Yamasue H., Abe O., Suga M., Yamada H., Inoue H., Kuwabara H., Kawakubo Y., Yahata N., Aoki S., Kano Y., Kato N., Kasai K. Reduced gray matter volume of pars opercularis is associated with impaired social communication in high-functioning autism spectrum disorders. Biol. Psychiatry. 2010;68:1141–1147. doi: 10.1016/j.biopsych.2010.07.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material


