Abstract
Patients with diabetes display a heightened propensity to use tobacco; however, it is unclear whether they experience enhanced rewarding effects of nicotine. Thus, this study examined the reinforcing effects of nicotine in a rodent model of diabetes involving administration of streptozotocin (STZ), a drug that is toxic to pancreatic insulin-producing cells. The first study compared STZ- and vehicle-treated rats that had 23-hour access to intravenous self-administration (IVSA) of nicotine or saline and concomitant access to food and water. In order to examine the contribution of dopamine to our behavioral effects, dopamine transporter (DAT), D1 and D2 receptor levels were compared in the nucleus accumbens (NAc) following 10 days of nicotine or saline IVSA. Dopamine levels in the NAc were also compared following nicotine administration. Lastly, nicotine metabolism and dose-dependent effects of nicotine IVSA were assessed. The results revealed that STZ-treated rats displayed enhanced nicotine intake and a robust increase in food and water intake relative to controls. Protein analysis revealed an increase in DAT and a decrease in D1 receptor levels in the NAc of STZ- versus vehicle-treated rats regardless of IVSA condition. STZ-treated rats also displayed suppressed NAc dopamine levels during baseline and in response to nicotine. STZ treatment did not alter our assessment of nicotine metabolism. Furthermore, STZ treatment increased nicotine IVSA in a dose-dependent manner. Our findings suggest that STZ-treatment increased the rewarding effects of nicotine. This suggests that strong reinforcing effects of nicotine may contribute to greater tobacco use in patients with diabetes.
Keywords: tobacco, abuse, self-administration, diabetes, dopamine, hypoinsulinemia, hyperglycemia
INTRODUCTION
Tobacco use remains a significant public health problem. Smoking is particularly concerning in patients with diabetes because they are twice as likely to experience mortality and various negative health outcomes if they use tobacco regularly (Scemama et al., 2006; Tonstad, 2009). The health care costs of patients with diabetes who smoke are 300% higher than non-smoking patients with this disease (Gilmer et al., 2005). Interestingly, smoking rates in adult patients with diabetes were shown to be greater than that of the general population (Bishop et al., 2009). Moreover, patients with diabetes are less likely to quit smoking and are more concerned about weight gain if they quit as compared to smokers without diabetes (Gill et al., 2005). Tobacco cessation rates are also lower among patients with diabetes, and they display higher rates of depression and anxiety during smoking abstinence (Eliasson et al., 1997). The strong linkage between diabetes and smoking behavior mostly reflects studies conducted with persons with Type 2 diabetes. Given the compounded health consequences of diabetes and smoking, an important question is whether patients with diabetes have a heightened propensity to use tobacco.
Several approaches have been used to study diabetes in animal models. One approach involves administration of streptozotocin (STZ), which is selectively toxic to insulin-producing beta cells of the pancreas and leads to hypoinsulinemia and a concomitant hyperglycemia. The STZ model has been widely used in rodents as a model of diabetes (Artinano and Castro, 2009; Bell and Hye, 1983; Lee et al., 2010). The hypoinsulinemia produced by STZ treatment reflects the underlying etiology of Type 1 diabetes and advanced stages of Type 2 diabetes when insulin production becomes compromised (Masiello, 2006).
The reinforcing effects of tobacco products are modulated, in large part, by the presence of nicotine, which is readily self-administered in rodents (Corrigall and Coen, 1989; Goldberg et al., 1981; Henningfield et al., 1985; Risner and Goldberg, 1983). Recent studies have utilized extended access procedures involving 23-hour access to nicotine intravenous self-administration (IVSA) in rats (Harris et al., 2007; LeSage et al., 2002; O'Dell et al., 2007; O'Dell and Koob, 2007; Valentine et al., 1997). Extended access procedures are advantageous because the rats are given access to nicotine in a chamber where operant responses for food and water can also be assessed. Rats in extended access procedures display average daily nicotine intake that approximates the levels observed in human smokers (0.18–1.5 mg/kg/day; LeSage et al., 2003).
The rewarding effects of nicotine are mediated, in large part, by dopamine neurotransmission in the mesolimbic pathway. The dopaminergic cell bodies of this pathway originate in the ventral tegmental area (VTA) and terminate in several forebrain structures including the nucleus accumbens (NAc; Aston-Jones and Harris, 2004; Carelli and Wightman, 2004). Nicotine stimulates nicotinic acetylcholine receptors in the VTA, causing the activation of dopaminergic neurons, which ultimately leads to the release of dopamine in the NAc (Mansvelder et al., 2003). The release of dopamine in the NAc and the concomitant activation of dopamine receptors in this region play an instrumental role in mediating the rewarding effects of nicotine.
Previous studies have demonstrated that the mesolimbic reward pathway is modulated by insulin. Insulin receptors have been detected in the NAc as well as on dopaminergic neurons in the VTA (Figlewicz et al., 2003; Havrankova et al., 1978). Activation of insulin receptors has been shown to negatively regulate reward. For example, intracerebroventricular administration of insulin results in attenuation of sucrose self-administration (Figlewicz et al., 2006) and preference for an environment paired with high fat food (Figlewicz et al., 2004). Insulin administered directly into the VTA decreases opioid-induced sucrose feeding behavior (Figlewicz et al., 2008). In contrast, decreased insulin signaling via an induction of a hypoinsulinemic state has been shown to increase reward functioning. Namely, hypoinsuliemic rats exhibit an increase in the rate of responding to lateral hypothalamic stimulation (Briese and Hernandez, 1970). Likewise, STZ-treated rats exhibit a decrease in intracranial self-stimulation thresholds that is reversed by insulin and naloxone (Carr, 1994; Carr et al., 2000). These findings suggest a negative modulatory effect of insulin receptors on the mesolimbic dopaminergic system that regulates reward.
To our knowledge, the mechanisms that modulate the rewarding effects of nicotine in rats with diabetes have not been studied. Given the involvement of the NAc in reward processing, the goal of this study is to examine how dopamine systems modulate the reinforcing effects of nicotine in the NAc of STZ- and vehicle-treated rats. These studies are a first step towards understanding enhanced vulnerability to tobacco abuse in patients with diabetes.
MATERIALS AND METHODS
Subjects
Sixty-day old male Wistar rats were handled for 3–5 days prior to the start of experimentation and received ad libitum access to food and water during this phase of the study. Rats were housed in groups of 2–3 per cage in a humidity- and temperature-controlled (20–22 °C) vivarium. The rats were bred from a fully out-bred stock from Harlan, Inc. (Indianapolis, IN). The UTEP Institutional Animal Care and Use Committee approved all procedures.
Materials
The drugs used in the IVSA experiments were: (−)nicotine hydrogen tartrate salt, d-amphetamine, STZ (Sigma Inc. St Louis, MO), Timentin and Brevitol (McKesson Inc., San Francisco, CA). All drugs were dissolved in 0.9% sterile saline and the doses were selected based on previous work in our laboratory (O'Dell et al., 2007; O'Dell and Koob, 2007). All chemicals for the dialysis procedures were purchased from (Sigma Inc. St Louis, MO). For Western Blot analysis, radioimmunoprecipitation assay (RIPA) lysis buffer (sc-24948), anti-alpha tubulin primary antibody (sc-8035), goat anti-rabbit IgG-HRP (sc-2004) and goat anti-mouse IgG-HRP (sc-2005) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Phosphatase inhibitor cocktail 1 (P2850) and cocktail 2 (P5726) were purchased from Sigma-Aldrich (St. Louis, MO). Bicinchoninic acid (BCA) protein assay kit and Supersignal west pico chemiluminescent substrate were produced by Thermo Scientific (Rockford, IL). Laemmli buffer, tris-glycine TGX gels and nitrocellulose membranes were purchased from Bio-Rad Laboratories (Hercules, CA). Anti-D1 receptor (MAB5290), anti-D2 receptor (AB5084P) and anti-DAT (AB15344) primary antibodies were purchased from Millipore (Billerica, MA). The anti-DAT antibody detects two bands corresponding to non-glycosylated (55KDa) and glycosylated (75KDa) proteins. Non-glycosylated DAT represents intracellular DAT levels, while glycosylated DAT represents cell surface DAT levels (Afonso-Oramas et al., 2009).
Procedural summary
The inset below depicts the timeline for the experimental procedures of this experiment. Study 1 compared the rewarding effects of nicotine using IVSA procedures in STZ- and vehicle-treated rats. Brain tissue from these rats was then analyzed for dopamine transporter (DAT), D1 and D2 receptor levels using Western Blot procedures. Study 2 compared dopamine levels in the NAc following systemic administration of nicotine and amphetamine in STZ- and vehicle-treated rats using in vivo microdialysis procedures.
![]()
![]()
Study 1: Self-administration procedures
The rats were tested in 23-hour sessions using operant chambers from Med Associates (St. Albans, VT). The animals were kept on a 12/12-hour light/dark cycle (lights on at 6 am) inside sound-attenuated chambers with continuous white noise, as previously described (O'Dell et al., 2007). A 28 Volt white cue light was illuminated above the active lever at the onset of the 1-second infusion and was terminated after a 20-second timeout period, during which time responses on the active lever had no scheduled consequence. All responses on the inactive lever were recorded without a time-out period. Each day, the rats were removed at 10 am from the operant chambers and placed into their home cages for 1 hour so the chambers could be cleaned and the water and food could be replenished. The rats were first trained for 5 days on a fixed ratio 1 (FR-1) schedule of reinforcement to obtain 45 mg food pellets (Bio-Serv; Frenchtown, NJ) from a dispenser with a swing door mounted between two levers on the front wall of the chamber. When the animal placed its head into the food hopper, the swing door broke a photo beam that then activated the delivery of a food pellet into the bottom of the food hopper. A nose-poke response was required in a separate hole positioned on the back of the chamber for delivery of 0.1 mL aliquots of water into an adjacent metal dipper cup.
After 5 days of food and water training sessions, the rats received an intraperitoneal injection of either vehicle (citrate buffer) or STZ (45 mg/kg). Beginning two days later, plasma glucose levels were monitored every other day for the remainder of the study. A 22-gauge needle was used at the base of the tail to excise a small drop of blood. Glucose values were then assessed using strips calibrated for rodent blood (AlphaTRAK, Abbott Laboratories, Abbott Park, IL). This dose of STZ produced two groups of rats that displayed glucose levels in a high (mean value=554±13 mg/dL; range=440–619 mg/dL) or intermediate (mean value=288±29 mg/dL; range=236–343 mg/dL) range. Vehicle-treated control rats displayed glucose levels in a normal range (124±2 mg/dL; range=116–129 mg/dL). STZ-treated rats that displayed glucose levels lower than 200 or higher than 650 mg/dL at any given time point were eliminated from the study. Most rats that receive this dose of STZ display glucose levels in the high range. Therefore, it is only possible to achieve glucose levels in an intermediate range in large cohorts of rats. A sufficient number of rats in the intermediate glucose range were only available in our behavioral experiments (Study 1). Thus, graded effects were only reported in the behavioral studies that required many animals. The rats were then sub-divided into separate groups that would receive saline or nicotine IVSA.
One week after the STZ injection, the rats were anesthetized with an isoflurane/oxygen vapor mixture (1–3%) and implanted with jugular venous catheters, as previously described (O'Dell et al., 2007). The catheters were then flushed daily for the duration of the experiment with a 0.3–0.5 ml infusion of a 100 mg/mL antibiotic solution containing Timentin that was diluted in saline containing heparin (30 USP units/mL). To verify patency of the catheters on the day before IVSA training, the rats received a 0.1 mL infusion of a 10 mg/mL solution of the ultra short-acting barbiturate anesthetic Brevital® sodium (1% methohexital sodium) through the catheter. Rats with non-patent catheters were excluded from the study. This barbiturate produces a rapid anesthesia that dissipates within less than a minute of drug administration. Thus, we suspect that there were no carry-over effects to the next day of IVSA testing.
Following 3 days of recovery from surgery, the rats were re-introduced into the chambers by connecting the exit port of the catheter fittings to the metal spring that was attached to the swivel and balance arm. Rats performed nose-poke responses for food and water (FR-1) in 23-hour sessions for 3 days in the absence of any levers. This was done in order to re-establish stable levels of food and water intake prior to the introduction of the levers in the next phase of the study.
Following food and water reinstatement, rats were presented with the active and inactive levers beginning on the first day at 10 am. The rats were removed each day for 1 hour from the operant chambers from 10–11 am to clean the boxes and replenish the food and water. Rats were then given access to saline or nicotine IVSA (0.03 mg/kg/0.1 mL infusion; expressed as base) on an FR-1 schedule of reinforcement. The nicotine solutions were prepared daily based on the rats' weights from the previous day. The dose of nicotine was selected based on previous studies comparing 23-hour nicotine IVSA in adult Wistar rats (O'Dell et al., 2007; O'Dell and Koob, 2007). Food and water were available throughout the entire 23-hour sessions. IVSA behavior was only assessed for 10 days. The short period of time was employed to avoid the side effects of STZ that emerge after 4 weeks of STZ treatment, such as tactile allodynia and cataract formation (Morrow 2004; Wei et al., 2003). Thus, we employed fewer days of IVSA but extended the daily access to 23 hours. This allowed a comparison of the present data to previously published results showing robust and stable nicotine IVSA behavior within 5 days of extended access to this drug (O'Dell et al., 2007). To examine whether longer periods of IVSA could be done with STZ-treated rats, a study was conducted with a separate cohort of vehicle-and STZ-treated rats (n=12 per group) that were given a total of 4 weeks of nicotine IVSA. The results revealed that 4 weeks after STZ treatment, the rates of nicotine IVSA drop to negligible levels given the emergence of lethargy and inactivity produced by long-term hypoinsulinemia (data not shown).
Study 1: Protein analyses
Two hours after the last IVSA session, vehicle- and STZ-treated rats displaying high glucose levels were decapitated and the brains were removed, flash frozen and maintained at −80°C. Only the brains of rats displaying high glucose levels (mean 554±13 mg/dL) were included in the protein analysis. This was done in order to compare changes in protein levels in groups of rats that displayed the largest changes in blood glucose levels. At a later time, the NAc was dissected and homogenized using a handheld homogenizer in a RIPA lysis buffer containing EDTA (1 mM), PMSF (1%), sodium orthovanadate (1%), protease inhibitor cocktail (2%) and phosphatase inhibitor cocktails 1 and 2 (1%). Homogenized samples were incubated in the buffer for 30 minutes on ice and then centrifuged at 14,000 rpm for 10 minutes. The lysates were then used for protein analysis. Protein concentration in the lysate was quantified using a BCA protein assay kit.
Western blots were performed using SDS-PAGE. Specifically, 10 μg of sample was combined with Laemmli buffer containing beta-mercaptoethanol (5%). Samples were then boiled for 3 minutes, centrifuged, and the supernatant loaded onto Tris-Glycine TGX gels and electrophoresed at 100 V constant. Proteins were then transferred to nitrocellulose membranes. Membranes were blocked for 1 hour at room temperature in 5% non-fat dry milk in tris-buffered saline with tween-20 (TBST) or in 5% bovine serum albumin (BSA) in TBST. For primary antibody incubation, membranes were incubated in 5% BSA containing antibodies for D1 receptors (1:1000), D2 receptors (1:1000), overnight at 4°C. For detection of DAT (1:1000), and alpha tubulin (1:1000) membranes were incubated in 5% non-fat dry milk in TBST overnight at 4°C. After several washes in TBST, membranes were then incubated in their corresponding secondary antibodies at a 1:1000 concentration for 2 hours at room temperature. Membranes were then washed and visualized using Supersignal West Pico chemiluminescent substrate using a Kodak digital imaging system. For quantitating band intensities, densitometry was conducted using the Carestream software (Woodbridge, CT).
Study 2: Neurochemistry procedures
Separate groups of rats (n=5–6 per group) received an intraperitoneal injection of either vehicle (citrate buffer) or STZ (45 mg/kg). The rats were then returned to their home cages for 22 days, in order to approximate the time frame in which the brain tissues were collected in Study 1. Glucose levels were monitored every other day after the STZ injection. STZ-treated rats displayed glucose levels in a similar high range as was observed in Study 1. On Day 23, the rats were anesthetized with an isoflurane/oxygen mixture and then were stereotaxically implanted with a dialysis probe aimed at the NAc using the following coordinates relative to bregma (AP=+1.7, ML=±1.4, DV=−8.1). The probes were purchased from CMA-Microdialysis (model CMA 11; Solna, Sweden) with an active membrane length of 2 mm in the NAc. The probes were perfused for at least 1 hour prior to implantation at a rate of 0.5 μL/minute with artificial cerebral spinal fluid composed of 145 mM NaCl, 2.8 mM KCl, 1.2 mM CaCl2, 1.2 mM MgCl2, 5.4 mM d-glucose, and 0.25 mM ascorbic acid and adjusted to a pH of 7.2–7.4. Following surgery, the animals were transferred to Plexiglas® cages (24 cm long × 24 cm wide × 31 cm high) and food and water were available throughout dialysis testing.
The dialysis collection was done the following day (Day 24) because our experience with these procedures has shown that this time period is optimal for tissue equilibration and detecting pharmacological effects across different treatment conditions (O'Dell and Parsons, 2004). The day after probe implantation, the samples were collected in 20-minute intervals for 1 hour to establish a baseline period, and then for 4 separate 1-hour collection periods consisting of an intraperitoneal injection of saline, 2 doses of nicotine in increasing order (0.4 and 0.8 mg/kg) and a final injection of amphetamine (1 mg/kg). The injection of amphetamine was included as a positive control to examine robust neurochemical effects and to assess whether the effects are specific to nicotine. All dialysate samples collected from the NAc probe were diluted with 10 μl of a perchloric-acid solution (0.05 N) in order to preserve the samples and prevent degradation of dopamine. After sample collection, the dialysates were immediately frozen on dry ice then stored in a −70°C freezer until analysis. At the end of the experiment, all rats were sacrificed and the probe placements were verified in 40 μm coronal sections of the NAc.
Dopamine levels were quantified from a 10-μl sample injected into a HPLC system equipped with an ESA HR-80 80×4.6 mm column (3 μm BetaBasic packing material, C-18 stationary phase, Chelmsford, MA) and eluted using a mobile phase composed of a 75 mM NaH2PO4 (monohydrate, monobasic) buffer (pH 3.75) with 10% acetonitrile, 0.025 mM sodium-EDTA, 0.4% (v/v) triethylamine and 1.7 mM 1-octanesulfonic acid sodium salt delivered at 1 mL/minute by an ESA model 580 syringe pump (Chelmsford, MA). Quantification was achieved via an ESA Coulochem II detector equipped with a coulometric sensor containing dual glassy carbon working electrodes (Chelmsford, MA) set at −150 mV. The extracellular levels of dopamine were estimated using external calibration curves with standards containing known concentrations of dopamine.
At the end of the experiment, all rats were euthanized with pentobarbital (100 mg/kg, salt; IP) and the brains were extracted. Probe placements was verified during tissue sectioning using a rat brain atlas (Paxinos and Watson, 1998). As a final elimination criterion, baseline dopamine concentration for an individual subject had to fall within a range that was less than two standard deviations from the group mean in order to be included in the final analysis.
Study 3: Nicotine pharmacokinetics
The purpose of this study was to compare nicotine metabolism in vehicle- and STZ-treated rats. Nicotine metabolism was assessed indirectly by comparing plasma cotinine levels in vehicle- and STZ-treated rats (n=10–14 per group). Rats received an intraperitoneal injection of vehicle or STZ (45 mg/kg) and were returned to their home cages for 22 days in order to approximate the time frame in which the tissues were collected from the previous studies. Glucose levels were monitored every other day after the STZ injection. STZ-treated rats displayed glucose levels in a similar high range as was observed in the previous studies. On Day 23, the rats received a subcutaneous injection of nicotine (0.4 mg/kg) and blood plasma samples were collected from tail blood 30 and 60 minutes after the injection. After the second sampling period, the rats received another injection of a higher dose of nicotine (0.8 mg/kg) and blood samples were collected again 30 and 60 minutes later. Plasma cotinine levels were analyzed using commercially available 96-well plate ELISA kits (OraSure Technologies, Inc., Bethlehem, PA). Standard curves were used to estimate plasma cotinine levels using a Spectra Maxplus spectrophotometer (Molecular Devices Inc, Sunnyvale, CA).
Study 4: Nicotine dose-response IVSA study
The purpose of this study was to compare the sensitivity of the rewarding effects of nicotine in vehicle- and STZ-treated rats (n=5). A dose-response curve for nicotine IVSA was first generated in naïve rats. Then, the nicotine IVSA dose-response curve was re-assessed following STZ-treatment in the same rats. A within subjects design was conducted based on our previous finding that rats administer the same amount of nicotine when given access a second time to the same escalating dose regimen (O'Dell and Koob, 2007). Therefore, if the rats displayed an increase in nicotine intake following STZ treatment, the results would imply an increase in the rewarding effects of nicotine. Rats were trained to perform operant responses for food and water for 3 days. Upon completion of food and water training, the rats were implanted with jugular catheters and then given access to nicotine IVSA in 3-day intervals of increasing doses of nicotine (0.03, 0.06, 0.09 mg/kg/0.1 mL infusion) on an FR-1 schedule of reinforcement. Following the last IVSA session, the rats were given an injection of STZ (45 mg/kg) and were returned to their home cages. The rats displayed glucose levels that were initially normal (133±7.2 mg/dL), but remained in a high range following STZ administration (500±32.5 mg/dL). The day after STZ administration, the rats were given re-access to the same escalating dose regimen of nicotine given that they immediately displayed glucose levels in a high range (450–563 mg/dL).
Statistical analyses
Statistical analyses included overall ANOVAs followed by post-hoc testing where appropriate. The initial analyses compared the effect of various behavioral and biological markers using ANOVA across treatment (vehicle versus STZ), drug condition (nicotine versus saline) and time (IVSA sessions in Study 1 or sampling period during dialysis testing in Study 2). Separate analyses were conducted for each measure that focused only on nicotine IVSA groups of rats with varying glucose levels (control, intermediate and high). In instances where significant interaction effects were not observed, main effects are reported with appropriate group comparisons using Fisher's LSD tests (p < 0.05). The type of analysis that was performed is denoted by different symbols on the figures. For Western blot analysis, one-way ANOVA or t-tests were performed to detect statistical significance.
RESULTS
Study 1: Glucose levels and nicotine, food and water intake
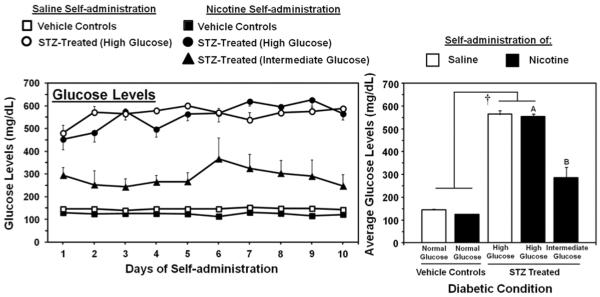
Figure 1 denotes glucose levels in STZ- and vehicle-treated rats from Study 1. STZ administration produced a graded increase in glucose levels as compared to vehicle-treated controls. The initial analysis revealed a main effect of STZ treatment (F1,28=1191.2; p < 0.001), with STZ producing higher glucose levels than vehicle-treated controls regardless of IVSA condition (p < 0.05). A separate analysis of glucose levels in nicotine IVSA rats with varying glucose levels revealed a main effect of STZ treatment (F2,23=175.3; p < 0.01), with STZ producing a graded increase in glucose levels. Specifically, STZ treatment produced a group of rats displaying higher glucose levels (554±13 mg/dL) as compared to all other nicotine IVSA groups, and a group of rats displaying intermediate levels (288±29 mg/dL) that were significantly higher than vehicle-treated controls with glucose levels in a normal range (124±2 mg/dL; p < 0.05).
Figure 1.
Mean daily glucose levels (± SEM) during each day (left panel) and averaged across the 10 days of IVSA (right panel). The group numbers for saline IVSA rats were vehicle controls (n=6) and STZ-treated (high glucose; n=7). The group numbers for nicotine IVSA rats were vehicle controls (n=7), STZ-treated (high glucose; n=12), and STZ-treated (intermediate glucose; n=7). The dagger (†) denotes a significant effect of STZ treatment, the A symbol denotes significantly higher glucose levels as compared to all other nicotine IVSA groups and the B symbol denotes significantly higher glucose levels as compared to the vehicle-treated nicotine IVSA group (p < 0.05).
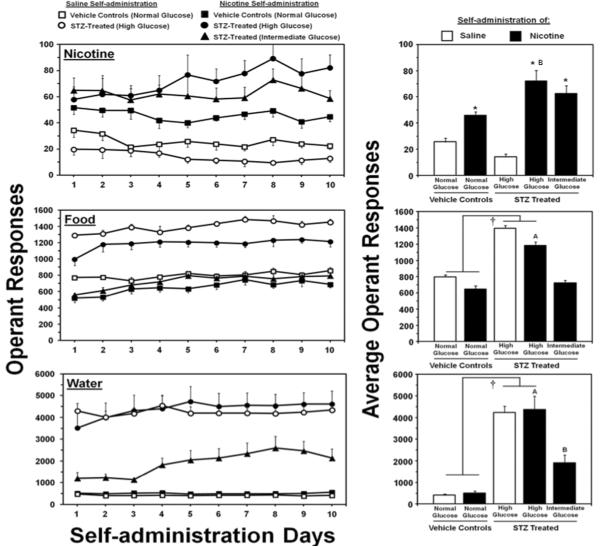
Figure 2 denotes nicotine, food, and water intake in STZ- and vehicle-treated rats from Study 1. Overall, the results revealed that STZ-treated rats displayed a graded increase in the level of nicotine, food, and water intake versus vehicle-treated controls. The initial analysis revealed an interaction of drug condition (nicotine or saline) and STZ treatment (F1,28=7.7; p < 0.01). A main effect of drug condition was also observed (F1,28=1191.2; p < 0.001), with all rats displaying higher nicotine versus saline intake regardless of STZ treatment (p < 0.05). A separate analysis of nicotine intake in groups of rats with varying glucose levels revealed a significant main effect of STZ treatment (F2,23=3.3; p < 0.05), with rats displaying high glucose levels exhibiting higher levels of nicotine IVSA as compared to vehicle-treated nicotine IVSA rats (p < 0.05). Our analyses of the inactive lever revealed that there were no differences in operant responses on the inactive lever across STZ treatment (F1,28=0.6; p = 0.4) or drug conditions (F1,28=0.07; p = 0.8; data not shown).
Figure 2.
Mean daily operant responses (± SEM) for nicotine or saline (top row), food pellets (middle row) or water deliveries (bottom row) during each day (left column) and averaged across the 10 days of IVSA (right column). The daggers (†) denote a main effect of STZ treatment, the asterisks (*) denotes a significant increase from saline IVSA rats, the letter symbol (A) denotes significantly higher intake as compared to all other nicotine IVSA groups, and the letter symbol (B) denotes higher intake as compared to the vehicle-treated nicotine IVSA group (p < 0.05).
The initial analysis comparing food intake revealed a main effect of STZ treatment (F1,28=160.1; p < 0.001), with STZ-treated rats displaying higher food intake regardless of drug condition (p < 0.05). This analysis also revealed a main effect of drug condition (F1,28=16.2; p < 0.001), with rats given nicotine IVSA access displaying less food intake regardless of STZ treatment. A separate analysis of food intake in groups of rats with varying glucose levels revealed a main effect of STZ treatment (F2,23=47.3; p < 0.05), with rats displaying high glucose levels exhibiting higher levels of food intake as compared to all other nicotine IVSA groups (p < 0.05).
The initial analyses of water intake revealed a main effect of STZ treatment (F1,28=57.8; p < 0.001), with all STZ-treated rats displaying higher water intake as compared to vehicle-treated controls regardless of drug condition (p < 0.05). The effect of STZ on water intake was not influenced by nicotine IVSA, as expected. A separate analysis of water intake in groups of rats with varying glucose levels revealed a main effect of STZ treatment (F2,23=16.7; p < 0.001), with rats displaying high glucose levels exhibiting higher levels of water intake as compared to all other nicotine IVSA groups (p < 0.05). Rats displaying intermediate glucose levels also exhibited higher levels of water intake as compared to vehicle-treated controls (p < 0.05).
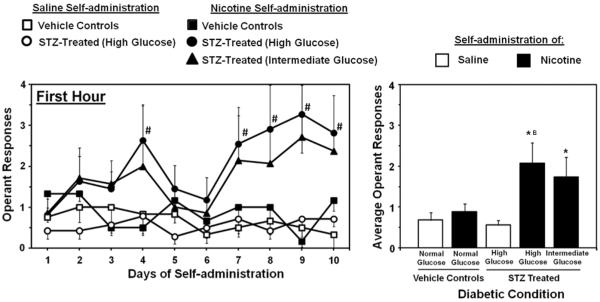
Figure 3 denotes nicotine intake during the first hour of access in STZ- and vehicle-treated rats from Study 1. Overall, the results revealed that STZ-treated rats displayed an increase in nicotine intake during the first hour of nicotine IVSA as compared to vehicle-treated controls that did not change across the 10 days of testing. The initial analysis revealed a significant interaction between time and STZ treatment (F9,252=2.5; p < 0.01). Subsequent analyses revealed that nicotine intake during the first hour of drug access was significantly higher in STZ-treated rats displaying high glucose levels on the 4th, 7th, 8th, 9th and 10th days of IVSA as compared to the 1st day of testing (p < 0.05). A main effect of drug condition was observed such that nicotine intake was generally higher than saline intake (F1,28=4.6; p < 0.05), and this effect was statistically significant in high and intermediate glucose nicotine IVSA rats (p < 0.05). A separate analysis of nicotine intake in groups of rats with varying glucose levels revealed a main effect of STZ treatment (F2,23=3.3; p < 0.05), with rats displaying high glucose levels exhibiting higher levels of nicotine intake as compared to vehicle-treated nicotine IVSA rats (p < 0.05).
Figure 3.
Mean daily operant responses (± SEM) for nicotine or saline during the first hour of access plotted across each day (left panel) and averaged across the 10 days of IVSA (right panel). Asterisks (*) denote a significant difference from saline IVSA rats and the letter symbol (B) denotes higher intake as compared to the vehicle-treated nicotine IVSA group (p < 0.05).
Study 1: Protein analysis
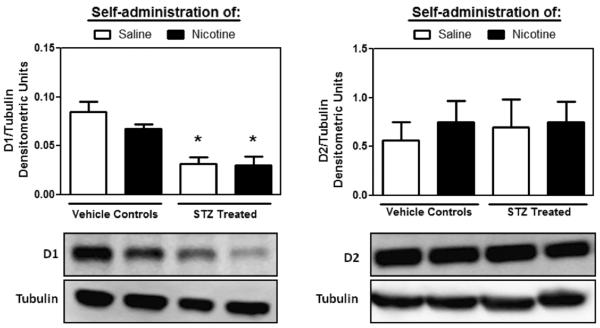
Figure 4 denotes D1 and D2 receptor protein densities in the NAc of STZ-treated rats displaying high glucose levels and vehicle-treated rats from Study 1. STZ-treated rats displayed a significant reduction in dopamine D1 receptor levels in the NAc, regardless of IVSA condition (F3,17=12.8; p < 0.001). On the other hand, STZ treatment or IVSA condition did not change D2 receptor levels in the NAc.
Figure 4.
Dopamine D1 (left panel) and D2 (right panel) receptor levels (±SEM) in STZ-treated and control rats following saline or nicotine IVSA. Tubulin was used as a loading control for each individual sample and was similar across all treatment groups. Asterisks (*) denote a significant difference from vehicle-treated controls that received saline IVSA (p<0.05).
Figure 5 denotes cytoplasmic (55 KDa) and membrane bound (75 KDa) DAT levels in the NAc of STZ- and vehicle-treated rats. The changes in DAT levels were primarily detected as a function of STZ treatment. STZ treated rats displayed higher DAT levels at both molecular weights as compared to controls (t15=3.17; p < 0.05 for 55 KDa and t15=2.19; p < 0.05 for 75 KDa). Nicotine IVSA did not significantly affect DAT levels in STZ-treated and control rats. A previous study indicated a reduction in tubulin mRNA in sensory neurons upon STZ treatment (Scott et al., 1999). However, in our study no changes in tubulin protein levels were detected in the NAc between control and STZ-treated rat (data not shown).
Figure 5.
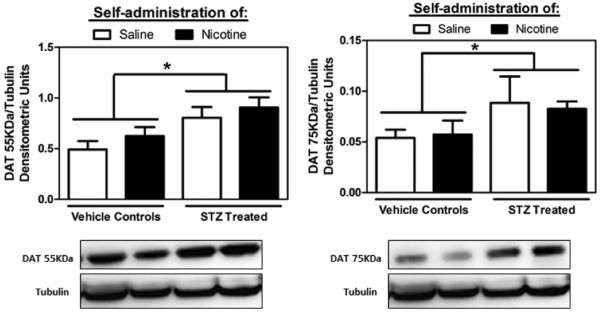
Levels of cytoplasmic (55KDa; left panel) and membrane bound (75KDa; right panel) DAT (±SEM) in the NAc of STZ-treated and control rats following saline or nicotine IVSA. Tubulin levels were similar across all treatment groups. Asterisks (*) denote a main effect of STZ treatment, with STZ-treated rats displaying higher DAT levels regardless of IVSA condition (p<0.05).
Study 2: Dopamine levels
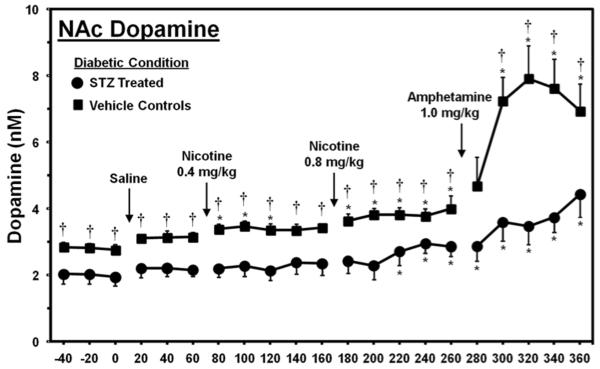
Figure 6 denotes dopamine levels in the NAc of vehicle- and STZ-treated rats that displayed high glucose levels similar to Study 1. Overall, the results revealed that dopamine levels were suppressed in the NAc of STZ- versus vehicle-treated controls. Statistical analysis revealed a significant interaction of time and STZ treatment (F20,180=4.3, p < 0.001). Subsequent analyses revealed that STZ-treated rats displayed lower dopamine levels versus controls during all sampling periods (p < 0.05), with the exception of the 280-minute sampling period where significant differences were not observed. STZ-treated rats displayed a significant increase in dopamine levels relative to baseline only during the 220–360 minute sampling periods (p < 0.05). On the other hand, vehicle-treated rats displayed robust increases in dopamine levels relative to baseline that were significant during the 80–260 minute sampling periods after nicotine administration, and during the 300–360 minute sampling periods after amphetamine administration (p < 0.05).
Figure 6.
Dopamine levels (nM± SEM) in the NAc of STZ-treated and control rats. The group numbers were controls (n=5) and STZ (n=6). Asterisks (*) denote a significant difference relative to baseline and daggers (†) reflect a significant difference between STZ- and vehicle-treated control rats (p < 0.05).
Study 3: Nicotine pharmacokinetics
Table 1 denotes cotinine values in vehicle- and STZ-treated rats 30 and 60 minutes after administration of two separate injections of nicotine. The results revealed that cotinine levels were similar in vehicle- and STZ-treated rats across both time points and doses of nicotine (F1,22=1.5; p = 0.23).
Table 1.
Plasma cotinine values (ng/ml) in control and STZ-treated rats
| Nicotine Dose | ||||
|---|---|---|---|---|
|
|
||||
| 0.4 mg/kg | 0.8 mg/kg | |||
| Time after inj: | 30 min | 60 min | 30 min | 60 min |
|
| ||||
| Control | 16 ± 1.7 | 10 ± 1.4 | 8 ± 1.6 | 8 ± 0.7 |
|
| ||||
| STZ-treated | 16 ± 1.8 | 8.3 ± 2.4 | 10 ± 1.2 | 11.8 ± 1.5 |
Study 4: Nicotine intake across doses
Figure 7 denotes intake of escalating doses of nicotine in rats given extended access to IVSA before and after STZ treatment. Overall, a dose-dependent increase in nicotine IVSA was observed, consistent with our previous observations in naïve rats given extended access to an escalating dose regimen of nicotine IVSA (O'Dell and Koob, 2007). After STZ treatment, nicotine intake was increased across all doses. Statistical analysis revealed a significant main effect of STZ treatment (F1,4=10.3, p < 0.05) and nicotine dose (F1.8=11.4 p < 0.001).
Figure 7.
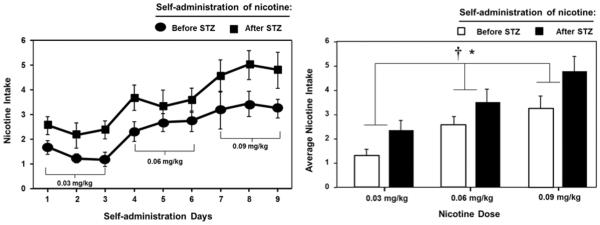
Nicotine intake (± SEM) during each day (left panel) and averaged across the 3 days of nicotine IVSA (right panel) prior to and following STZ administration (n=5). STZ treatment increased intake across all doses of nicotine. The asterisk (*) denotes a main effect of nicotine dose and the dagger (†) denotes a significant effect of STZ treatment (p < 0.05).
DISCUSSION
The main finding of this report is that STZ-treated rats displayed significantly higher nicotine IVSA compared to controls. One strength of our approach is that the IVSA procedure was able to concomitantly measure nicotine, food and water intake. The finding that food and water intake were higher in STZ-treated rats is confirmation of the induction of a hyperglycemic state. The levels of food intake were also lower in STZ-treated rats that pressed for nicotine as compared to STZ-treated rats that pressed for saline. The latter effect is consistent with the appetite-suppressant effects of nicotine (Mangubat et al., 2012). Our data also show that STZ treatment produced a suppression of mesolimbic dopamine systems at both pre- and post-synaptic levels. Lastly, our effects do not appear to be related to pharmacokinetic differences produced by STZ given that cotinine levels were similar in response to nicotine in vehicle- and STZ-treated rats.
The present study demonstrated that STZ-treated rats displayed higher levels of nicotine IVSA as compared to controls. STZ-treated rats also displayed an escalation of nicotine intake during the first hour of drug access, suggesting strong motivational effects of nicotine. Previous studies have revealed that rats given extended access to nicotine IVSA display an increase in intake in the first hour of drug access across repeated testing (O'Dell et al., 2007). This rise in nicotine IVSA during the first hour of access is believed to reflect an increase in the motivational effects of nicotine that emerge with the development of nicotine dependence. Our interpretation that STZ treatment enhances the rewarding effects of nicotine is consistent with our finding that nicotine intake was dose-dependently greater in STZ- versus vehicle-treated rats. Moreover, the increase in nicotine intake was augmented as a function of glucose levels. Importantly, nicotine intake was also increased following STZ treatment across a range of escalating nicotine doses. It is unlikely that this effect was due to re-exposure to nicotine, given our previous finding that rats administer the same amount of nicotine when given access a second time to the same escalating dose regimen (O'Dell and Koob, 2007). Therefore, the increase in the level of nicotine intake following STZ treatment, is believe to reflect an increase in the rewarding effects of nicotine. Taken together, these results suggest that hypoinsulinemia produced by STZ administration enhances the rewarding effects of nicotine.
Nicotine reward is modulated via the mesolimbic dopamine pathway, which originates in the VTA and terminates in the NAc (Corrigall and Coen, 1991; Corrigall et al., 1992). The present study demonstrated that STZ treatment produced a decrease in dopamine levels in the NAc during baseline and in response to nicotine and amphetamine administration. These findings are consistent with previous studies showing reduced basal and amphetamine-evoked dopamine release in the striatum of STZ- versus vehicle-treated rats (Lim et al., 1994; Murzi et al., 1996; Owens et al., 2005; Saller, 1984; Williams et al., 2007). The attenuated dopamine levels are either a direct effect of hyperglycemia on dopamine neuronal firing rates (Saller et al., 1980), a decrease in presynaptic D2 receptor signal transduction (Owens et al., 2012), or an indirect effect via opioid systems that suppress dopamine activity (Berman et al., 1997; Carr, 1994; Wolinsky et al., 1996). Considering that DAT levels were increased in the NAc of STZ-treated rats, it is also possible that the reduced levels of DA in the NAc is due to an increase in DA clearance. The reduced dopamine release observed in the present study may lead to the increased nicotine IVSA. This suggestion is consistent with the finding in human studies showing that a decrease in dopamine levels is associated with increased nicotine intake (Dagher et al., 2001).
The present study also revealed that STZ treatment produced a decrease in D1 receptor levels in the NAc. One might predict that lower extracellular levels of dopamine may have led to an up-regulation of post-synaptic D1 and D2 receptors. However, lower D1 receptor expression and no changes in D2 receptors may reflect a deficiency in the regulation of the dopamine system produced by a hypoinsulinemic state. Similar changes in D1 and D2 receptor levels have been reported in STZ-treated rodents (Saitoh et al., 1998; Salkovic et al., 1995; Sumiyoshi et al., 1997). It is possible that the decrease in D1 receptors contributes to the enhanced nicotine IVSA in STZ-treated rats. This suggestion is based on the finding that blockade of D1 receptors in the NAc increased the rewarding effects of nicotine (Laviolette et al., 2008). Since receptor antagonism and a decrease in receptor levels both lead to diminished receptor signaling, the possibility exists that lower levels of D1 receptors in the NAc contribute to the increase in nicotine IVSA observed in the present study.
Behavioral studies have extensively investigated the effects of various psychostimulants and selective dopaminergic compounds in STZ-treated rats. STZ treatment has been shown to decrease D2 receptor sensitivity in behavioral assays assessing catalepsy, yawning, and locomotor activity (Sevak et al., 2005, 2007); although no change in sensitivity of D2 receptors to food reward have been found (Sevak et al, 2008). Studies evaluating the rewarding effects of amphetamine in STZ-treated rats have produced mixed results. For example, Galici et al. (2003) demonstrated that STZ-treated rats displayed a reduction in amphetamine IVSA, whereas Sevak et al. (2008) found that STZ did not alter place preference produced by amphetamine. These results contrast our finding that STZ-treated rats displayed an increase in the rewarding effects of nicotine. This may be related to the distinct mechanisms of action of amphetamine versus nicotine and/or a different period of delay following STZ treatment. Specifically, the Gallici and Sevak studies assessed behavioral effects 7 days after STZ treatment, whereas the present study assessed behavioral effects 14 days after STZ administration. Future studies may be warranted to systematically examine the effects of diabetes on reward processing produced by different drugs of abuse.
In the present study, STZ-treated rats displayed an increase in nicotine IVSA and reduced NAc dopamine systems. It is possible that low brain insulin levels may increase susceptibility of nicotine use to compensate for attenuated dopaminergic system functioning. This is based on our finding that STZ treatment produced a general suppression of mesolimbic dopaminergic systems. Also, a recent report demonstrated that insulin produced an increase in dopaminergic neuron firing in the VTA that was abolished in mice lacking insulin receptor signaling (Könner et al., 2011). A compensation hypothesis is consistent with the finding that blockade of dopamine receptors increases amphetamine IVSA (Yokel and Wise, 1976). Another possibility is that STZ treatment enhanced nicotine IVSA via changes in other neurochemical systems, such as cholinergic systems that modulate nicotine reward. Future studies are needed to explore the contribution of dopamine and other systems in modulating the rewarding effects of nicotine in diabetic rats.
It is acknowledged that STZ produces an array of biological effects that could have influenced our findings. For example, STZ-treated rats may self-administer more nicotine in an attempt to increase their fluid levels. However, STZ-treated rats that were pressing for saline displayed low levels of saline IVSA. Another potential concern is that chronic administration of STZ produces tactile allodynia and cataract formation that could influence IVSA behavior (Morrow, 2004; Wei et al., 2003). These effects; however, do not emerge until after our testing period. Furthermore, STZ-treated rats displayed high levels of operant responding for nicotine, food, and water that do not appear to be influenced by the emergence of side effects. Lastly, the results from the present study are unable to rule out the effects of STZ on other pancreatic peptides, such as amylin that modulate glycemic regulation. Amylin is synthesized by pancreatic beta islet cells, and is co-packaged and secreted with insulin (Johnson et al., 1988; Lukinius et al., 1989; Ogawa et al., 1990). STZ is known to reduce amylin levels via a destruction of beta cells. Amylin receptors have been identified in numerous brain regions, including the midbrain and the NAc (Aiyar et al., 1995; Christopoulos et al., 1995; Sexton et al., 1994; Van Rossum et al., 1994). Therefore, changes in amylin levels may have contributed to the enhanced nicotine IVSA and the suppressed dopamine systems in our study. STZ treatment involves a physiological stress that dysregulates glucose homeostasis. We believe that the dysregulation produced by STZ, in part, changes the brain reward system in a manner that enhances the rewarding effects of nicotine.
The present findings are an important first step in understanding the interaction between insulin, the mesolimbic dopamine system, and the behavioral effects of nicotine. Future studies are needed to more fully examine the underlying mechanisms by which STZ treatment enhances the rewarding effects of nicotine. The recent U.S. Food and Drug Administration approval of Cycloset (bromocriptine) (2010), a dopamine agonist for the treatment of insulin resistance may attest to the importance of understanding the role of dopamine in diabetes and co-morbid conditions such as tobacco addiction.
ACKNOWLEDGEMENTS
This project was supported by a grant from the American Diabetes Association (7-12-BS-135) and The National Institute of Minority Health Disparities (G12MD007592) as part of the UTEP Border Biomedical Research Center. Research funds were also provided by the Western University of Health Sciences. This project was also supported by training grants provided by The National Institute on Drug Abuse (R24-DA029989 and R25-DA033613; LEO). Dr. Friedman received salary support from MIDARP grant R24DA017298. Dr. Luis Natividad received a pre-doctoral fellowship from the NIH Ruth L. Kirschstein Program (F31-DA021133). Undergraduate student stipend support was also provided though the UTEP Bridges to the Baccalaureate Program (5R25GM049011-12) and the Research Initiative for Scientific Advancement Program.
Footnotes
Conflict of interest: The authors have no relevant conflicts of interest to disclose.
References
- Bromocriptine (Cycloset) for type 2 diabetes. The Medical letter on drugs and therapeutics. 2010;52:97–98. [PubMed] [Google Scholar]
- Afonso-Oramas D, Cruz-Muros I, Alvarez de la Rosa D, Abreu P, Giraldez T, Castro-Hernandez J, Salas-Hernandez J, Lanciego JL, Rodriguez M, Gonzalez-Hernandez T. Dopamine transporter glycosylation correlates with the vulnerability of midbrain dopaminergic cells in Parkinson's disease. Neurobiology of disease. 2009;36:494–508. doi: 10.1016/j.nbd.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Aiyar N, Baker E, Martin J, Patel A, Stadel JM, Willette RN, Barone FC. Differential calcitonin gene-related peptide (CGRP) and amylin binding sites in nucleus accumbens and lung: potential models for studying CGRP/amylin receptor subtypes. J Neurochem. 1995;65:1131–1138. doi: 10.1046/j.1471-4159.1995.65031131.x. [DOI] [PubMed] [Google Scholar]
- Artinano A, Castro M. Experimental rat models to study the metabolic syndrome. Br J Nutr. 2009;102:1246–1253. doi: 10.1017/S0007114509990729. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Harris GC. Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology. 2004;47(Suppl 1):167–179. doi: 10.1016/j.neuropharm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Berman Y, Devi L, Spangler R, Kreek MJ, Carr KD. Chronic food restriction and streptozotocin-induced diabetes differentially alter prodynorphin mRNA levels in rat brain regions. Brain Res Mol Brain Res. 1997;46:25–30. doi: 10.1016/s0169-328x(96)00175-1. [DOI] [PubMed] [Google Scholar]
- Bell RH, Jr., Hye RJ. Animal models of diabetes mellitus: physiology and pathology. J Surg Res. 1983;35:433–460. doi: 10.1016/0022-4804(83)90034-3. [DOI] [PubMed] [Google Scholar]
- Bishop FK, Maahs DM, Snell-Bergeon JK, Ogden LG, Kinney GL, Rewers M. Lifestyle risk factors for atherosclerosis in adults with type 1 diabetes. Diab Vasc Dis Res. 2009;6:269–275. doi: 10.1177/1479164109346359. [DOI] [PubMed] [Google Scholar]
- Briese E, Hernández L. Self-stimulation enhancement in diabetic rats. Acta Physiol Lat Am. 1970;20:24–29. [PubMed] [Google Scholar]
- Carelli RM, Wightman RM. Functional microcircuitry in the accumbens underlying drug addiction: insights from real-time signaling during behavior. Curr Opin Neurobiol. 2004;14:763–768. doi: 10.1016/j.conb.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Carr KD. Streptozotocin-induced diabetes produces a naltrexone-reversible lowering of self-stimulation threshold. Brain Res. 1994;664:211–214. doi: 10.1016/0006-8993(94)91973-9. [DOI] [PubMed] [Google Scholar]
- Carr KD, Kim G, Cabeza de Vaca S. Hypoinsulinemia may mediate the lowering of self-stimulation thresholds by food restriction and streptozotocin-induced diabetes. Brain research. 2000;863:160–168. doi: 10.1016/s0006-8993(00)02143-0. [DOI] [PubMed] [Google Scholar]
- Christopoulos G, Paxinos G, Huang XF, Beaumont K, Toga AW, Sexton PM. Comparative distribution of receptors for amylin and the related peptides calcitonin gene related peptide and calcitonin in rat and monkey brain. Can J Physiol Pharmacol. 1995;73:1037–1041. doi: 10.1139/y95-146. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Selective dopamine antagonists reduce nicotine self-administration. Psychopharmacology (Berl) 1991;104:171–176. doi: 10.1007/BF02244174. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 1992;107:285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Dagher A, Bleicher C, Aston JA, Gunn RN, Clarke PB, Cumming P. Reduced dopamine D1 receptor binding in the ventral striatum of cigarette smokers. Synapse. 2001;42:48–53. doi: 10.1002/syn.1098. [DOI] [PubMed] [Google Scholar]
- Eliasson B, Attvall S, Taskinen MR, Smith U. Smoking cessation improves insulin sensitivity in healthy middle-aged men. Eur J Clin Invest. 1997;27:450–456. doi: 10.1046/j.1365-2362.1997.1330680.x. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain research. 2003;964:107–115. doi: 10.1016/s0006-8993(02)04087-8. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Bennett J, Evans SB, Kaiyala K, Sipols AJ, Benoit SC. Intraventricular insulin and leptin reverse place preference conditioned with high-fat diet in rats. Behav Neurosci. 2004;118:479–487. doi: 10.1037/0735-7044.118.3.479. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Bennett JL, Naleid AM, Davis C, Grimm JW. Intraventricular insulin and leptin decrease sucrose self-administration in rats. Physiol Behav. 2006;89:611–616. doi: 10.1016/j.physbeh.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Bennett JL, Aliakbari S, Zavosh A, Sipols AJ. Insulin acts at different CNS sites to decrease acute sucrose intake and sucrose self-administration in rats. American journal of physiology Regulatory, integrative and comparative physiology. 2008;295:R388–394. doi: 10.1152/ajpregu.90334.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galici R, Galli A, Jones DJ, Sanchez TA, Saunders C, Frazer A, Gould GG, Lin RZ, France CP. Selective decreases in amphetamine self-administration and regulation of dopamine transporter function in diabetic rats. Neuroendocrinology. 2003;77:132–140. doi: 10.1159/000068650. [DOI] [PubMed] [Google Scholar]
- Gill GV, Morgan C, MacFarlane IA. Awareness and use of smoking cessation treatments among diabetic patients. Diabet Med. 2005;22:658–660. doi: 10.1111/j.1464-5491.2005.01471.x. [DOI] [PubMed] [Google Scholar]
- Gilmer TP, O'Connor PJ, Rush WA, Crain AL, Whitebird RR, Hanson AM, Solberg LI. Predictors of health care costs in adults with diabetes. Diabetes Care. 2005;28:59–64. doi: 10.2337/diacare.28.1.59. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD, Goldberg DM. Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science. 1981;214:573–575. doi: 10.1126/science.7291998. [DOI] [PubMed] [Google Scholar]
- Harris AC, Pentel PR, Lesage MG. Prevalence, magnitude, and correlates of an extinction burst in drug-seeking behavior in rats trained to self-administer nicotine during unlimited access (23 h/day) sessions. Psychopharmacology (Berl) 2007;194:395–402. doi: 10.1007/s00213-007-0848-2. [DOI] [PubMed] [Google Scholar]
- Havrankova J, Roth J, Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature. 1978;272:827–829. doi: 10.1038/272827a0. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Miyasato K, Jasinski DR. Abuse liability and pharmacodynamic characteristics of intravenous and inhaled nicotine. J Pharmacol Exp Ther. 1985;234:1–12. [PubMed] [Google Scholar]
- Johnson KH, O'Brien TD, Hayden DW, Jordan K, Ghobrial HK, Mahoney WC, Westermark P. Immunolocalization of islet amyloid polypeptide (IAPP) in pancreatic beta cells by means of peroxidase-antiperoxidase (PAP) and protein A-gold techniques. Am J Pathol. 1988;130:1–8. [PMC free article] [PubMed] [Google Scholar]
- Könner AC, Hess S, Tovar S, Mesaros A, Sanchez-Lasheras C, Evers N, Verhagen LA, Bronneke HS, Kleinridders A, Hampel B, Kloppenburg P, Bruning JC. Role for insulin signaling in catecholaminergic neurons in control of energy homeostasis. Cell metabolism. 2011;13:720–728. doi: 10.1016/j.cmet.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Lauzon NM, Bishop SF, Sun N, Tan H. Dopamine signaling through D1-like versus D2-like receptors in the nucleus accumbens core versus shell differentially modulates nicotine reward sensitivity. J Neurosci. 2008;28:8025–8033. doi: 10.1523/JNEUROSCI.1371-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Yang SH, Oh JM, Lee MG. Pharmacokinetics of drugs in rats with diabetes mellitus induced by alloxan or streptozocin: comparison with those in patients with type I diabetes mellitus. J Pharm Pharmacol. 2010;62:1–23. doi: 10.1211/jpp.62.01.0001. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Shoeman D, Raphael D, Collins G, Pentel PR. Continuous nicotine infusion reduces nicotine self-administration in rats with 23-h/day access to nicotine. Pharmacol Biochem Behav. 2002;72(1–2):279–289. doi: 10.1016/s0091-3057(01)00775-4. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Collins G, Pentel PR. Effects of continuous nicotine infusion on nicotine self-administration in rats: relationship between continuously infused and self-administered nicotine doses and serum concentrations. Psychopharmacology (Berl) 2003;170:278–286. doi: 10.1007/s00213-003-1539-2. [DOI] [PubMed] [Google Scholar]
- Lim DK, Lee KM, Ho IK. Changes in the central dopaminergic systems in the streptozotocin-induced diabetic rats. Arch Pharm Res. 1994;17:398–404. doi: 10.1007/BF02979114. [DOI] [PubMed] [Google Scholar]
- Lukinius A, Wilander E, Westermark GT, Engström U, Westermark P. Co-localization of islet amyloid polypeptide and insulin in the B cell secretory granules of the human pancreatic islets. Diabetologia. 1989;32:240–244. doi: 10.1007/BF00285291. [DOI] [PubMed] [Google Scholar]
- Mangubat M, Lutfy K, Lee ML, Pulido L, Stout D, Davis R, Shin CS, Shahbazian M, Seasholtz S, Sinha-Hikim A, Sinha-Hikim I, O'Dell LE, Lyzlov A, Liu Y, Friedman TC. Effect of nicotine on body composition in mice. J Endocrinol. 2012;212:317–326. doi: 10.1530/JOE-11-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, De Rover M, McGehee DS, Brussaard AB. Cholinergic modulation of dopaminergic reward areas: upstream and downstream targets of nicotine addiction. Eur J Pharmacol. 2003;480:117–123. doi: 10.1016/j.ejphar.2003.08.099. [DOI] [PubMed] [Google Scholar]
- Masiello P. Animal models of type 2 diabetes with reduced pancreatic beta-cell mass. Int J Biochem Cell Biol. 2006;38:873–893. doi: 10.1016/j.biocel.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Morrow TJ. Animal models of painful diabetic neuropathy: the STZ rat model. Curr Protoc Neurosci. 2004;Chapter 9(Unit 9):18. doi: 10.1002/0471142301.ns0918s29. [DOI] [PubMed] [Google Scholar]
- Murzi E, Contreras Q, Teneud L, Valecillos B, Parada MA, De Parada MP, Hernandez L. Diabetes decreases limbic extracellular dopamine in rats. Neurosci Lett. 1996;202:141–144. doi: 10.1016/0304-3940(95)12232-x. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Chen SA, Smith RT, Specio SE, Balster RL, Paterson NE, Markou A, Zorrilla EP, Koob GF. Extended access to nicotine self-administration leads to dependence: Circadian measures, withdrawal measures, and extinction behavior in rats. J Pharmacol Exp Ther. 2007;320:180–193. doi: 10.1124/jpet.106.105270. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Koob GF. `Nicotine deprivation effect' in rats with intermittent 23-hour access to intravenous nicotine self-administration. Pharmacol Biochem Behav. 2007;86:346–353. doi: 10.1016/j.pbb.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell LE, Parsons LH. Serotonin1B receptors in the ventral tegmental area modulate cocaine-induced increases in nucleus accumbens dopamine levels. J Pharmacol Exp Ther. 2004;311:711–719. doi: 10.1124/jpet.104.069278. [DOI] [PubMed] [Google Scholar]
- Ogawa A, Harris V, McCorkle SK, Unger RH, Luskey KL. Amylin secretion from the rat pancreas and its selective loss after streptozotocin treatment. J Clin Invest. 1990;85:973–976. doi: 10.1172/JCI114528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens WA, Sevak RJ, Galici R, Chang X, Javors MA, Galli A, France CP, Daws LC. Deficits in dopamine clearance and locomotion in hypoinsulinemic rats unmask novel modulation of dopamine transporters by amphetamine. J Neurochem. 2005;94:1402–1410. doi: 10.1111/j.1471-4159.2005.03289.x. [DOI] [PubMed] [Google Scholar]
- Owens WA, Williams JM, Saunders C, Avison MJ, Galli A, Daws LC. Rescue of dopamine transporter function in hypoinsulinemic rats by a D2 receptor-ERK-dependent mechanism. J Neurosci. 2012;32:2637–2647. doi: 10.1523/JNEUROSCI.3759-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. Academic Press; San Diego: 1998. [Google Scholar]
- Risner ME, Goldberg SR. A comparison of nicotine and cocaine self-administration in the dog: fixed-ratio and progressive-ratio schedules of intravenous drug infusion. J Pharmacol Exp Ther. 1983;224:319–326. [PubMed] [Google Scholar]
- Saitoh A, Morita K, Sodeyama M, Kamei J. Effects of the experimental diabetes on dopamine D1 receptor-mediated locomotor-enhancing activity in mice. Pharmacol Biochem Behav. 1998;60:161–166. doi: 10.1016/s0091-3057(97)00588-1. [DOI] [PubMed] [Google Scholar]
- Salković M, Sabolić I, Lacković Z. Striatal dopaminergic D1 and D2 receptors after intracerebroventricular application of alloxan and streptozocin in rat. J Neural Transm Gen Sect. 1995;100:137–145. doi: 10.1007/BF01271536. [DOI] [PubMed] [Google Scholar]
- Saller CF, Chiodo LA. Glucose suppresses basal firing and haloperidol-induced increases in the firing rate of central dopaminergic neurons. Science. 1980;210:1269–1271. doi: 10.1126/science.6254155. [DOI] [PubMed] [Google Scholar]
- Saller CF. Dopaminergic activity is reduced in diabetic rats. Neurosci Lett. 1984;49:301–306. doi: 10.1016/0304-3940(84)90306-9. [DOI] [PubMed] [Google Scholar]
- Scemama O, Hamo-Tchatchouang E, Le Faou AL, Altman JJ. Difficulties of smoking cessation in diabetic inpatients benefiting from a systematic consultation to help them to give up smoking. Diabetes Metab. 2006;32:435–441. doi: 10.1016/s1262-3636(07)70301-4. [DOI] [PubMed] [Google Scholar]
- Sevak RJ, Koek W, France CP. Streptozotocin-induced diabetes differentially modifies haloperidol- and gamma-hydroxybutyric acid (GHB)-induced catalepsy. Eur J Pharmacol. 2005;517:64–67. doi: 10.1016/j.ejphar.2005.05.043. [DOI] [PubMed] [Google Scholar]
- Sevak RJ, Owens WA, Koek W, Galli A, Daws LC, France CP. Evidence for D2 receptor mediation of amphetamine-induced normalization of locomotion and dopamine transporter function in hypoinsulinemic rats. J Neurochem. 2007;101:151–159. doi: 10.1111/j.1471-4159.2006.04358.x. [DOI] [PubMed] [Google Scholar]
- Sevak RJ, Koek W, Daws LC, Owens WA, Galli A, France CP. Behavioral effects of amphetamine in streptozotocin-treated rats. Eur J Pharmacol. 2008;581:105–112. doi: 10.1016/j.ejphar.2007.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton PM, Paxinos G, Kenney MA, Wookey PJ, Beaumont K. In vitro autoradiographic localization of amylin binding sites in rat brain. Neuroscience. 1994;62:553–567. doi: 10.1016/0306-4522(94)90388-3. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Ichikawa J, Meltzer HY. The effect of streptozotocin-induced diabetes on dopamine2, serotonin1A and serotonin2A receptors in the rat brain. Neuropsychopharmacology. 1997;16:183–190. doi: 10.1016/S0893-133X(96)00185-6. [DOI] [PubMed] [Google Scholar]
- Tonstad S. Cigarette smoking, smoking cessation, and diabetes. Diabetes Res Clin Pract. 2009;85:4–13. doi: 10.1016/j.diabres.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Valentine JD, Hokanson JS, Matta SG, Sharp BM. Self-administration in rats allowed unlimited access to nicotine. Psychopharmacology (Berl) 1997;133:300–304. doi: 10.1007/s002130050405. [DOI] [PubMed] [Google Scholar]
- van Rossum D, Ménard DP, Fournier A, St-Pierre S, Quirion R. Autoradiographic distribution and receptor binding profile of [125I]Bolton Hunter-rat amylin binding sites in the rat brain. J Pharmacol Exp Ther. 1994;270:779–787. [PubMed] [Google Scholar]
- Wei M, Ong L, Smith MT, Ross FB, Schmid K, Hoey AJ, Burstow D, Brown L. The streptozotocin-diabetic rat as a model of the chronic complications of human diabetes. Heart Lung Circ. 2003;12:44–50. doi: 10.1046/j.1444-2892.2003.00160.x. [DOI] [PubMed] [Google Scholar]
- Williams JM, Owens WA, Turner GH, Saunders C, Dipace C, Blakely RD, France CP, Gore JC, Daws LC, Avison MJ, Galli A. Hypoinsulinemia regulates amphetamine-induced reverse transport of dopamine. PLoS Biol. 2007;5:e274. doi: 10.1371/journal.pbio.0050274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky TD, Abrahamsen GC, Carr KD. Diabetes alters mu and kappa opioid binding in rat brain regions: comparison with effects of food restriction. Brain Res. 1996;738:167–171. doi: 10.1016/0006-8993(96)00994-8. [DOI] [PubMed] [Google Scholar]
- Yokel RA, Wise RA. Attenuation of intravenous amphetamine reinforcement by central dopamine blockade in rats. Psychopharmacology (Berl) 1976;48:311–318. doi: 10.1007/BF00496868. [DOI] [PubMed] [Google Scholar]