Heart failure with preserved ejection fraction (HFpEF) is common and associated with high morbidity and mortality: HFpEF represents >50% of all heart failure (HF), and it is growing in prevalence (1); quality of life is generally poor, comparable to patients with end-stage renal disease (2); and 5-year survival is only 35% after HF hospitalization (3). In addition, care of patients with HFpEF can be frustrating: the diagnosis is often not straightforward; comorbidities are common and drive outcomes in these patients (4,5); and treatment of HFpEF remains an enigma, with disappointing results from several large randomized controlled trials (6). Thus, it is not surprising that many clinicians feel “therapeutic nihilism” towards HFpEF.
Why have prior HFpEF clinical trials failed? There are multiple possibilities (7), but for clinicians who care for HFpEF patients on a frequent basis, it is clear that the heterogeneity of HFpEF is one primary reason (8). HFpEF, like all forms of HF, is a syndrome and not a specific disease process. The overwhelming majority of patients with HFpEF have elevated left ventricular (LV) filling pressures, either at rest or with exertion. However, the severity of the left atrial pressure elevation, volume retention, and consequent pulmonary hypertension with right ventricular dysfunction is variable, as are the etiologic and pathophysiologic paths by which individual patients develop HFpEF. Thus, a “one size fits all” treatment strategy is unlikely to work for HFpEF and may underlie the failures of previous HFpEF clinical trials.
For future HFpEF clinical trials to be successful, better matching of therapies with the correct type of HFpEF patient, and endpoints tested, is necessary. Sometimes only in retrospect is it clear that the type of therapy tested in a clinical trial is not the right match for the types of patients enrolled (or the outcomes tested). Recent examples of this phenomenon include the Aldosterone Receptor Blockade in Diastolic Heart Failure (Aldo-DHF), which enrolled patients with early-stage HFpEF and not overt volume overload (9), and Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Heart Failure with Preserved Ejection Fraction (RELAX) (10), which enrolled symptomatic patients with volume overload but not necessarily those with overt pulmonary hypertension and right ventricular dysfunction. However, in other studies, the mechanism of the experimental drug is well matched to the type of patients enrolled and the endpoints tested.
In this issue of the Journal, Kosmala, Marwick, and colleagues report their results from just such a study: a short-term, randomized controlled trial of the effects of ivabradine vs. placebo on exercise capacity and hemodynamics in HFpEF (11). In this small, double-blind clinical trial of 61 patients with early-stage HFpEF and New York Heart Association (NYHA) class II-III symptoms, the investigators randomized study participants to 7 days of ivabradine 5 mg twice daily (N=30) or placebo (N=31). All study participants underwent cardiopulmonary exercise testing and diastolic stress echocardiography at baseline and on day 7. The co-primary endpoints were peak VO2 and peak exercise E/e’ (a non-invasive surrogate for LV filling pressure). The results of the trial were impressive: patients randomized to ivabradine had improved exercise capacity, increased peak VO2, and reduced exercise-induced rises in E/e’. Although the trial was only a short-term 7-day study, the safety and tolerability of ivabradine was also remarkable with no associated adverse effects and no need for dose reductions or study drug cessation due to bradycardia.
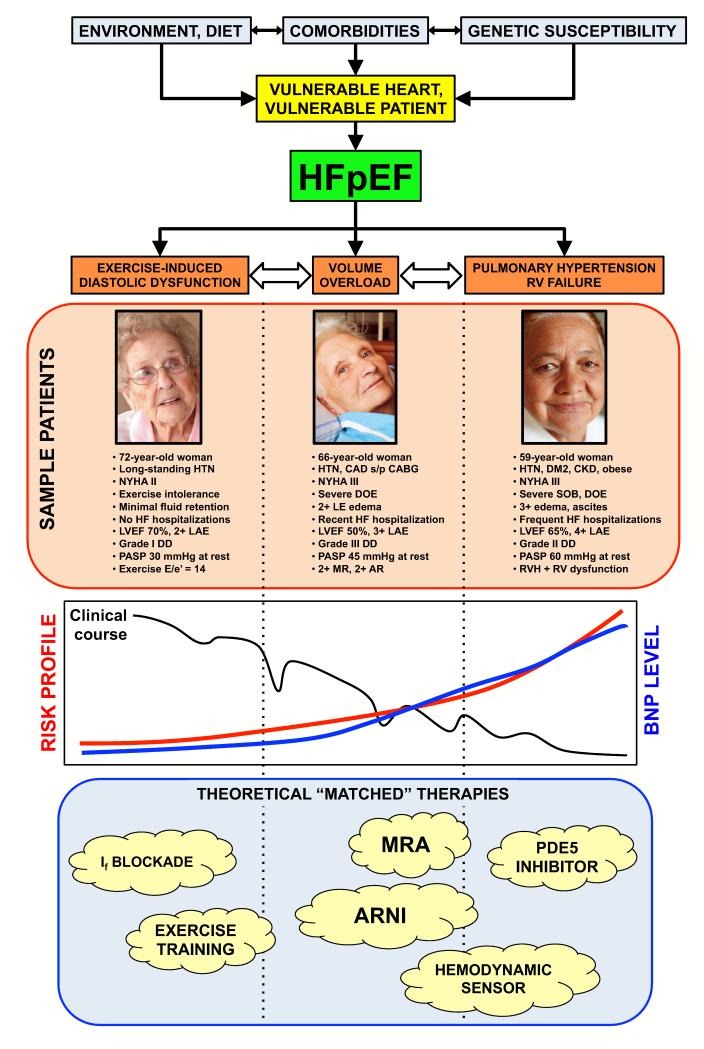
Why was the trial by Kosmala et al. successful? The primary reason may be the drug tested (ivabradine) and its beneficial effects in HFpEF. However, matching the drug and its proposed mechanism of benefit to the right type of HFpEF patient cannot be overemphasized, as shown by analyzing several recent HFpEF clinical trials (Table) (2,9-17). Figure 1 displays a theoretical schema of 3 different HFpEF patient types: exercise-induced diastolic dysfunction (i.e., exercise-induced rise in LV filling pressure); chronic volume overload; and associated right heart failure / pulmonary hypertension. Each type of patient can be classified as “HFpEF”; however, the 3 types of HFpEF may represent different stages of the HFpEF syndrome or different endo-phenotypes based on the culmination of environment, diet, comorbidities, and genetic susceptibility.
Table. Summary of Selected Recent or Pending Heart Failure with Preserved Ejection Fraction Randomized Controlled Trials.
| Trial | Intervention | HFpEF patient type* | Primary endpoint | Trial result | Trial “Matched” for Rx? |
|---|---|---|---|---|---|
| Kosmala et al. | Ivabradine | Exercise-induced DD | Peak VO2, peak E/e’ | Positive | Yes |
| CHAMPION | CardioMEMS sensor | Volume overload | HF hospitalization | Positive | Yes |
| Guazzi et al. | Sildenafil | Right heart failure / PH | Pulmonary hemodynamics, RV performance, QoL |
Positive | Yes |
| Kitzman et al. | Exercise training | Exercise-induced DD | Peak VO2 | Positive | Yes |
| PARAMOUN T |
LCZ696 (ARNI) | Volume overload | ΔNT-proBNP | Positive | Yes |
| TOPCAT | Sprionolactone | Volume overload | CV death, aborted cardiac arrest, or HF hospitalization |
Pending | Yes‡ |
| ALDO-DHF | Spironolactone | Exercise-induced DD | Peak VO2, ΔE/e’ | Negative† | No‡ |
| ELANDD | Nebivolol | Exercise-induced DD | 6-minute walk test | Negative | No§ |
| J-DHF | Carvedilol (low-dose) | Exercise-induced DD / volume overload |
Death or HF hospitalization | Negative | No§ |
| RAAM-PEF | Eplerenone | Volume overload | 6-minute walk test | Negative | No‡ |
| RELAX | Sildenafil | Volume overload | Peak VO2 | Negative | No |
HFpEF = heart failure with preserved ejection fraction; Rx = treatment; ARNI = angiotensin receptor-neprilysin inhibitor; DD = diastolic dysfunction; PH= pulmonary hypertension; HF = heart failure; RV = right ventricle; QoL = quality of life; NT-proBNP = change in N-terminal pro-B-type natriuretic peptide; CV = cardiovascular
HFpEF patient types include exercise-induced diastolic dysfunction (ambulatory patients with NYHA class II-III symptoms, grade I diastolic dysfunction, and normal or near-normal BNP levels); chronic volume overload (NYHA class II-IV symptoms with history of heart failure hospitalization, elevated BNP, and/or left atrial enlargement); and associated right heart failure / pulmonary hypertension (NYHA class III-IV symptoms with evidence of pulmonary vascular disease and/or right ventricular dysfunction). See also Figure for examples of each patient type.
ALDO-DHF had co-primary end-points and was negative for the peak VO2 endpoint but positive for the ΔE/e’ endpoint.
Prior HF trials of mineralocorticoid receptor antagonists have shown that these drugs reduce volume overload and improve symptoms, but they do not improve exercise capacity or functional class.
Given the vasodilating effects of nebivolol and carvedilol, ELANDD and J-DHF may have been better suited with chronic volume overload type of patients with HF hospitalization as an endpoint; J-DHF may have proven to be positive if higher doses of carvedilol were used in the study.
Figure 1.
Teoretical Schema of Heart Failure with Preserved Ejection Fraction Patient Types with Sample Patients, Risk Profiles, and Matched Therapies
HTN = hypertension; NYHA = New York Heart Association functional class; HF = heart failure; LVEF = left ventricular ejection fraction; LAE = left atrial enlargement; DD = diastolic dysfunction; PASP = pulmonary artery systolic pressure; E/e’ = ratio of early mitral inflow to early mitral annular diastolic tissue velocity; CAD s/p CABG = coronary artery disease status-post coronary artery bypass grafting; DOE = dyspnea on exertion; MR = mitral regurgitation; AR = aortic regurgitation; DM2 = type 2 diabetes mellitus; CKD = chronic kidney disease; SOB = shortness of breath; RVH = right ventricular hypertrophy; RV = right ventricular; BNP = B-type natriuretic peptide; If = inward “funny” channel; MRA = mineralocorticoid receptor antagonist; ARNI = angiotensin receptor / neprilysin inhibitor; PDE5 = phosphodiesterase-5
The trial by Kosmala et al. specifically tested the first group of HFpEF, which is primarily characterized by exercise-induced elevations in LV filling pressures. These patients often do not have significant signs of fluid overload at rest, and typically have NYHA functional class II symptoms, normal or near-normal natriuretic peptide levels, and grade I diastolic dysfunction on resting echocardiography. Many of these patients do not even require maintenance diuretic therapy. However, with exercise, their LV filling pressures (and left atrial pressures) rise significantly, resulting in exercise intolerance and dyspnea. These patients are not likely to benefit from either spironolactone or phosphodiesterase-5 inhibition, as shown in recent clinical trials (9,10). Instead, a drug with heart-rate lowering and lusitropic effects may be more desirable. Ivabradine is just such as drug.
Ivabradine is a highly selective blocker of inward “funny” (If) channels, which are central regulators of spontaneous depolarization in pacemaker cells (18). Thus, ivabradine selectively decreases heart rate without having negative inotropic or lusitropic effects, as can occur with beta-blockers. Furthermore, animal and human studies have shown that ivabradine can decrease heart rate while simultaneously improving stroke volume and cardiac output. An elegant study, which used a novel HFpEF animal model, the db/db (leptin-receptor deficient) mouse, found that heart rate lowering with ivabradine had several beneficial effects, including reduced effective arterial elastance (Ea), increased aortic distensibility, and decreased LV end-systolic elastance (Ees) (19). In addition, ivabradine accelerated myocardial relaxation by increased phosphorylation of phospholamban, reversing the SERCA2a inhibition that was present in the db/db mouse. Improving the activity of SERCA2a has several beneficial downstream effects including reduction of titin N2B isoform expression and lowering myocardial collagen content (19). Thus, ivabradine may be useful in the short-term with its lusitropic and hemodynamic effects, thereby improving symptoms and exercise capacity, and it may also be useful in the long-term, decreasing myocardial stiffness and thereby preventing development of worsening heart failure (volume overload).
The study by Kosmala and colleagues has several strengths, including its use of detailed exercise and echocardiographic testing, specific enrollment criteria (signs and symptoms of HFpEF; evidence of diastolic dysfunction; exercise capacity < 80% of age- and sex- predicted; and E/e’ > 13 at peak stress). The 7-day duration of the trial could be viewed as a positive aspect of the study because it allowed for rapid determination of the drug’s efficacy in improving exercise tolerance. Finally, as stated above, perhaps the biggest strength of the study was the accurate “matchmaking” between experimental therapy (ivabradine) and patient type (early-stage HFpEF with primary symptoms of exercise intolerance due to exercise-induced elevations in LV filling pressure).
Several limitations of the study should also be considered. First, the study was small, and included only white participants. Thus, the study results may not be generalizable to other HFpEF patient types and populations, and a larger ivabradine trial must be performed in HFpEF before its use can be advocated in clinical practice. Second, the strict inclusion/exclusion criteria benefitted the trial by enrolling only carefully selected patients; however, future larger-scale clinical trials of ivabradine in HFpEF must enroll patients using a different strategy than the typical large multi-center HFpEF trials (2), which often use elevated natriuretic peptides and/or prior HF hospitalization as key inclusion criteria. Third, in HFpEF, heart rate lowering can be problematic in (a) patients who have advanced diastolic dysfunction and a stiff LV (and a relatively fixed stroke volume), because of the dependence on heart rate to augment cardiac output in these cases; and (b) in patients who have chronotropic incompetence in whom heart rate lowering could also exacerbate symptoms (20). Finally, because of the small sample size of the trial, the subgroup analyses presented by Kosmala et al. are limited and thus may have missed adverse effects in problematic patient populations, such as those with lower baseline heart rates or those with undiagnosed chronotropic incompetence.
What are the next steps for ivabradine in HFpEF? Based on the data shown in the study by Kosmala et al., a large-scale, longer-duration clinical trial of ivabradine should be conducted in patients with HFpEF; however, as noted above, the inclusion/exclusion criteria should focus on early-stage HFpEF patients in whom exercise intolerance is the key symptom, and in whom there is objective evidence of exercise-induced rise in LV filling pressure. Elevated natriuretic peptide levels and/or prior HF hospitalization should not be used as entry criteria, because they may result in the selection of more advanced HFpEF patients who are unlikely to benefit from ivabradine. Finally, the primary endpoints for a large-scale clinical trial of ivabradine in HFpEF should be exercise capacity and quality of life, with prevention of worsening HF (i.e., HF hospitalization) as a secondary, exploratory endpoint.
In conclusion, Kosmala, Marwick, and colleagues should be congratulated for carrying out a carefully conducted and detailed exercise hemodynamic study in HFpEF patients. By taking ivabradine, a blocker of the inward “funny” current, and matching it with the right type of HFpEF patient, coupled with appropriate endpoints (peak VO2 and exercise E/e’), the authors were successful matchmakers and may have found a novel therapy for an otherwise difficult-to-manage patient population.
Acknowledgments
Funding Sources: National Institutes of Health (R01 HL107557) and the American Heart Association (0835488N) (both to S.J.S.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: S.J.S. reports receiving grant funding from the National Institutes of Health and the American Heart Association; and honoraria from the Pulmonary Hypertension Association, Novartis, and Bayer Schering Pharma.
REFERENCES
- 1.Steinberg BA, Zhao X, Heidenreich PA, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126:65–75. doi: 10.1161/CIRCULATIONAHA.111.080770. [DOI] [PubMed] [Google Scholar]
- 2.Shah SJ, Heitner JF, Sweitzer NK, et al. Baseline characteristics of patients in the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial. Circ Heart Fail. 2013;6:184–92. doi: 10.1161/CIRCHEARTFAILURE.112.972794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 4.Ather S, Chan W, Bozkurt B, et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol. 2012;59:998–1005. doi: 10.1016/j.jacc.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah SJ, Gheorghiade M. Heart failure with preserved ejection fraction: treat now by treating comorbidities. JAMA. 2008;300:431–3. doi: 10.1001/jama.300.4.431. [DOI] [PubMed] [Google Scholar]
- 6.Borlaug BA, Redfield MM. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation. 2011;123:2006–13. doi: 10.1161/CIRCULATIONAHA.110.954388. discussion 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gheorghiade M, Vaduganathan M, Shah SJ. Evaluative framework for Phase II studies in patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2013;1:123–126. doi: 10.1016/j.jchf.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Shah AM, Pfeffer MA. The many faces of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2012;9:555–6. doi: 10.1038/nrcardio.2012.123. [DOI] [PubMed] [Google Scholar]
- 9.Edelmann F, Wachter R, Schmidt AG, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309:781–91. doi: 10.1001/jama.2013.905. [DOI] [PubMed] [Google Scholar]
- 10.Redfield MM, Chen HH, Borlaug BA, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–77. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kosmala W, Holland DJ, Rojek A, Wright L, Przewlocka-Kosmala M, Marwick TH. Effect of If-channel inhibition on hemodynamics and exericse tolerance in heart failure with preserved ejection fraction: A randomized trial. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 12.Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–66. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 13.Conraads VM, Metra M, Kamp O, et al. Effects of the long-term administration of nebivolol on the clinical symptoms, exercise capacity, and left ventricular function of patients with diastolic dysfunction: results of the ELANDD study. Eur J Heart Fail. 2012;14:219–25. doi: 10.1093/eurjhf/hfr161. [DOI] [PubMed] [Google Scholar]
- 14.Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation. 2011;124:164–74. doi: 10.1161/CIRCULATIONAHA.110.983866. [DOI] [PubMed] [Google Scholar]
- 15.Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail. 2010;3:659–67. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solomon SD, Zile M, Pieske B, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380:1387–95. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto K, Origasa H, Hori M. Effects of carvedilol on heart failure with preserved ejection fraction: the Japanese Diastolic Heart Failure Study (J-DHF) Eur J Heart Fail. 2013;15:110–8. doi: 10.1093/eurjhf/hfs141. [DOI] [PubMed] [Google Scholar]
- 18.Reil JC, Reil GH, Bohm M. Heart rate reduction by I(f)-channel inhibition and its potential role in heart failure with reduced and preserved ejection fraction. Trends Cardiovasc Med. 2009;19:152–7. doi: 10.1016/j.tcm.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Reil JC, Hohl M, Reil GH, et al. Heart rate reduction by If-inhibition improves vascular stiffness and left ventricular systolic and diastolic function in a mouse model of heart failure with preserved ejection fraction. Eur Heart J. 2012 doi: 10.1093/eurheartj/ehs218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borlaug BA, Melenovsky V, Russell SD, et al. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–47. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]