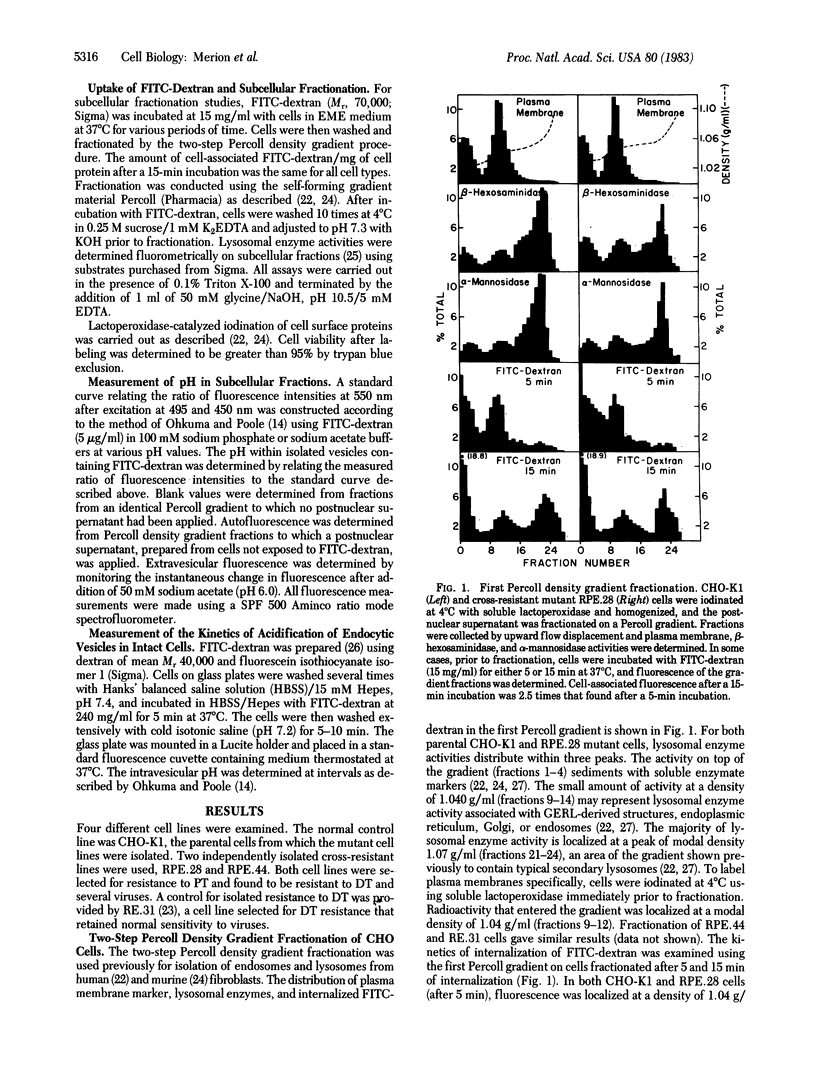
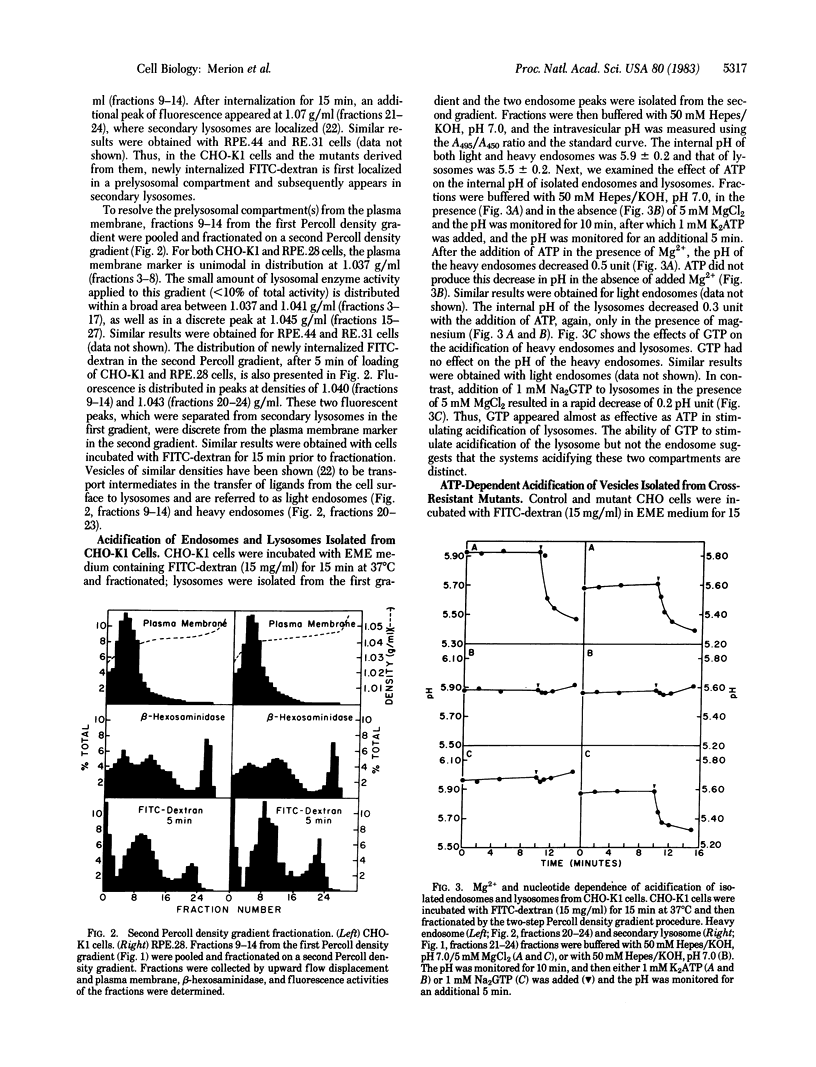
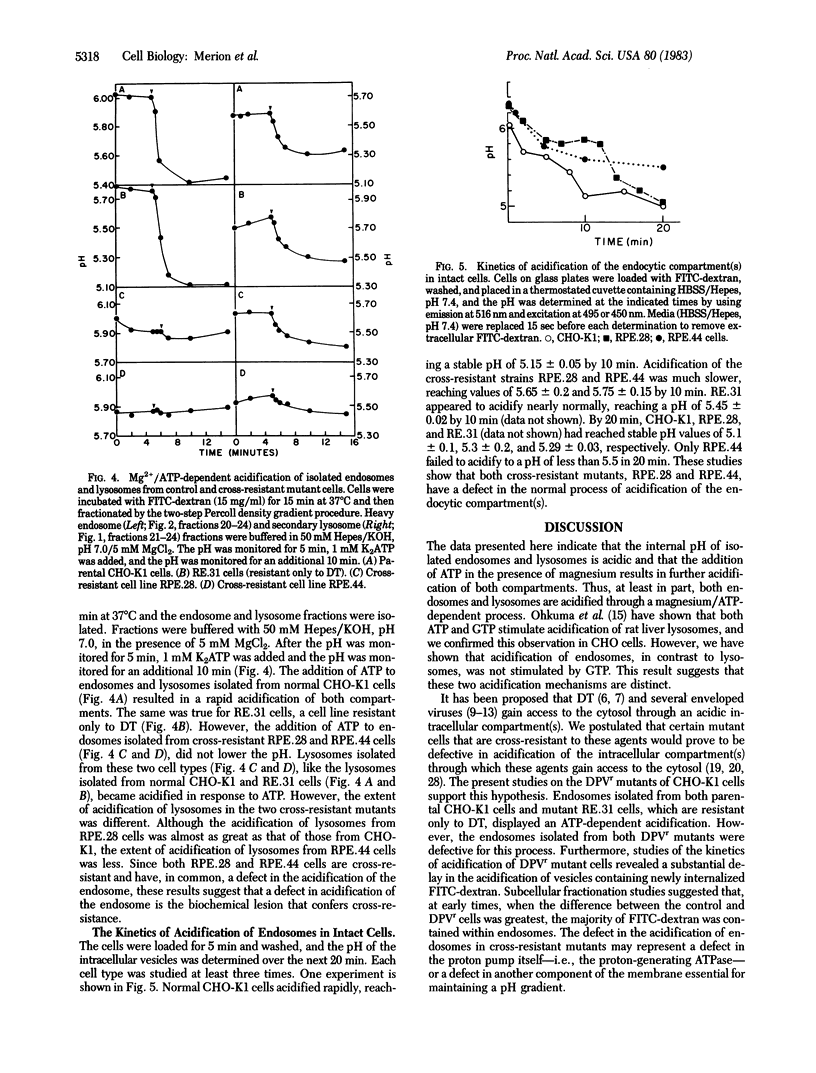
Abstract
Like many physiological ligands, several viruses and toxins enter mammalian cells through receptor-mediated endocytosis. Once internalized, the nucleic acids of several viruses and the toxic subunit of diphtheria toxin gain access to the cytosol of the host cell through an acidic intracellular compartment. In this report, we present evidence that one class of mutants of Chinese hamster ovary (CHO)-K1 cells, which is "cross-resistant" to Pseudomonas exotoxin A, diphtheria toxin, and several animal viruses, has a defect in acidification of the endosome. Cells were allowed to internalize fluorescein isothiocyanate-conjugated dextran before subcellular fractionation. Fluorescence measurements on subcellular fractions permitted measurement of the internal pH of the isolated endosomes and lysosomes. Our results show that (i) endosomes and lysosomes from CHO-K1 cells maintain an acidic pH, (ii) acidification of both endosomes and lysosomes is mediated by a Mg2+/ATP-dependent process, (iii) GTP can satisfy the ATP requirement for acidification of lysosomes but not of endosomes, and (iv) at least one class of mutants that is cross-resistant to toxins and animal viruses has a defect in the ATP-dependent acidification of their endosomes. These studies provide biochemical and genetic evidence that the mechanisms of acidification of endosomes and lysosomes are distinct and that a defect in acidification of endosomes is one biochemical basis for cross-resistance to toxins and viruses.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown M. S., Anderson R. G., Goldstein J. L. Recycling receptors: the round-trip itinerary of migrant membrane proteins. Cell. 1983 Mar;32(3):663–667. doi: 10.1016/0092-8674(83)90052-1. [DOI] [PubMed] [Google Scholar]
- Dorland R. B., Middlebrook J. L., Leppla S. H. Receptor-mediated internalization and degradation of diphtheria toxin by monkey kidney cells. J Biol Chem. 1979 Nov 25;254(22):11337–11342. [PubMed] [Google Scholar]
- Draper R. K., Simon M. I. The entry of diphtheria toxin into the mammalian cell cytoplasm: evidence for lysosomal involvement. J Cell Biol. 1980 Dec;87(3 Pt 1):849–854. doi: 10.1083/jcb.87.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser J. H., Sly W. S. Beta-glucuronidase deficiency mucopolysaccharidosis: methods for enzymatic diagnosis. J Lab Clin Med. 1973 Dec;82(6):969–977. [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Helenius A., Kartenbeck J., Simons K., Fries E. On the entry of Semliki forest virus into BHK-21 cells. J Cell Biol. 1980 Feb;84(2):404–420. doi: 10.1083/jcb.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Marsh M., White J. Inhibition of Semliki forest virus penetration by lysosomotropic weak bases. J Gen Virol. 1982 Jan;58(Pt 1):47–61. doi: 10.1099/0022-1317-58-1-47. [DOI] [PubMed] [Google Scholar]
- Lenard J., Miller D. K. pH-dependent hemolysis by influenza, Semliki, Forest virus, and Sendai virus. Virology. 1981 Apr 30;110(2):479–482. doi: 10.1016/0042-6822(81)90079-9. [DOI] [PubMed] [Google Scholar]
- Marnell M. H., Stookey M., Draper R. K. Monensin blocks the transport of diphtheria toxin to the cell cytoplasm. J Cell Biol. 1982 Apr;93(1):57–62. doi: 10.1083/jcb.93.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Bolzau E., Helenius A. Penetration of Semliki Forest virus from acidic prelysosomal vacuoles. Cell. 1983 Mar;32(3):931–940. doi: 10.1016/0092-8674(83)90078-8. [DOI] [PubMed] [Google Scholar]
- Matlin K. S., Reggio H., Helenius A., Simons K. Pathway of vesicular stomatitis virus entry leading to infection. J Mol Biol. 1982 Apr 15;156(3):609–631. doi: 10.1016/0022-2836(82)90269-8. [DOI] [PubMed] [Google Scholar]
- Merion M., Poretz R. D. The resolution of two populations of lysosomal organelles containing endocytosed Wistaria floribunda agglutinin from murine fibroblasts. J Supramol Struct Cell Biochem. 1981;17(4):337–346. doi: 10.1002/jsscb.380170405. [DOI] [PubMed] [Google Scholar]
- Merion M., Sly W. S. The role of intermediate vesicles in the adsorptive endocytosis and transport of ligand to lysosomes by human fibroblasts. J Cell Biol. 1983 Mar;96(3):644–650. doi: 10.1083/jcb.96.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. K., Lenard J. Antihistaminics, local anesthetics, and other amines as antiviral agents. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3605–3609. doi: 10.1073/pnas.78.6.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehring J. M., Moehring T. J. Characterization of the diphtheria toxin-resistance system in Chinese hamster ovary cells. Somatic Cell Genet. 1979 Jul;5(4):453–468. doi: 10.1007/BF01538880. [DOI] [PubMed] [Google Scholar]
- Moehring T. J., Moehring J. M. Response of cultured mammalian cells to diphtheria toxin. IV. Isolation of KB cells resistant to diphtheria toxin. Infect Immun. 1972 Oct;6(4):487–492. doi: 10.1128/iai.6.4.487-492.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma S., Moriyama Y., Takano T. Identification and characterization of a proton pump on lysosomes by fluorescein-isothiocyanate-dextran fluorescence. Proc Natl Acad Sci U S A. 1982 May;79(9):2758–2762. doi: 10.1073/pnas.79.9.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. R., Peng S. S., Marshall J. L. Mutant Chinese hamster ovary cells pleiotropically defective in receptor-mediated endocytosis. J Cell Biol. 1983 Apr;96(4):1064–1071. doi: 10.1083/jcb.96.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome L. H., Garvin A. J., Allietta M. M., Neufeld E. F. Two species of lysosomal organelles in cultured human fibroblasts. Cell. 1979 May;17(1):143–153. doi: 10.1016/0092-8674(79)90302-7. [DOI] [PubMed] [Google Scholar]
- Rothenberg P., Glaser L., Schlesinger P., Cassel D. Epidermal growth factor stimulates amiloride-sensitive 22Na+ uptake in A431 cells. Evidence for Na+/H+ exchange. J Biol Chem. 1983 Apr 25;258(8):4883–4889. [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Diphtheria toxin entry into cells is facilitated by low pH. J Cell Biol. 1980 Dec;87(3 Pt 1):828–832. doi: 10.1083/jcb.87.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Entry of the toxic proteins abrin, modeccin, ricin, and diphtheria toxin into cells. II. Effect of pH, metabolic inhibitors, and ionophores and evidence for toxin penetration from endocytotic vesicles. J Biol Chem. 1982 Jul 10;257(13):7504–7513. [PubMed] [Google Scholar]
- Tycko B., Maxfield F. R. Rapid acidification of endocytic vesicles containing alpha 2-macroglobulin. Cell. 1982 Mar;28(3):643–651. doi: 10.1016/0092-8674(82)90219-7. [DOI] [PubMed] [Google Scholar]
- White J., Helenius A. pH-dependent fusion between the Semliki Forest virus membrane and liposomes. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3273–3277. doi: 10.1073/pnas.77.6.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Matlin K., Helenius A. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J Cell Biol. 1981 Jun;89(3):674–679. doi: 10.1083/jcb.89.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]



