Abstract
During brain injury, extracellular adenosine and glutamate levels increase rapidly and dramatically. We hypothesized that local glutamate levels in the brain dictates the adenosine–adenosine A2A receptor (A2AR) effects on neuroinflammation and brain damage outcome. Here, we showed that, in the presence of low concentrations of glutamate, the A2AR agonist 3-[4-[2-[[6-amino-9-[(2R,3R,4S,5S)-5-(ethylcarbamoyl)-3,4-dihydroxy-oxolan-2-yl]purin-2-yl]amino]ethyl]phenyl]propanoic acid (CGS21680) inhibited lipopolysaccharide (LPS)-induced nitric oxide synthase (NOS) activity of cultured microglial cells, an effect that was dependent on the protein kinase A (PKA) pathway. However, in high concentrations of glutamate, CGS21680 increased LPS-induced NOS activity in a protein kinase C (PKC)-dependent manner. Thus, increasing the local level of glutamate redirects A2AR signaling from the PKA to the PKC pathway, resulting in a switch in A2AR effects from antiinflammatory to proinflammatory. In a cortical impact model of traumatic brain injury (TBI) in mice, brain water contents, behavioral deficits, and expression of tumor necrosis factor-α, interleukin-1 mRNAs, and inducible NOS were attenuated by administering CGS21680 at post-TBI time when brain glutamate levels were low, or by administering the A2AR antagonist ZM241385 [4-(2-{[5-amino-2-(2-furyl)[1,2,4]triazolo[1,5-a][1,3,5]triazin-7-yl]amino}ethyl)phenol] at post-TBI time when brain glutamate levels were elevated. Furthermore, pre-TBI treatment with the glutamate release inhibitor (S)-4C3HPG [(S)-4-carboxy-3-hydroxyphenylglycine] converted the debilitating effect of CGS21680 administered at post-TBI time with high glutamate level to a neuroprotective effect. This further indicates that the switch in the effect of A2AR activation in intact animals from antiinflammatory to proinflammatory is dependent on glutamate concentration. These findings identify a novel role for glutamate in modulation of neuroinflammation and brain injury via the adenosine–A2AR system.
Introduction
Adenosine levels rise rapidly and markedly in response to ischemia, hypoxia, excitotoxicity, inflammation, and other brain insults (Pedata et al., 2001). Increased extracellular adenosine acting at the A2A receptor (A2AR) may exert a complex and probably “fine-tuning” modulation of brain injury (de Mendonça et al., 2000; Chen et al., 2007). On the one hand, A2AR inactivation can protect the brain against various insults including ischemia, excitotoxicity, and mitochondrial toxicity (Cunha, 2005). For example, genetic and pharmacological inactivation of A2ARs downregulates neuroinflammation and protects brain tissue against ischemia (Phillis, 1995; Chen et al., 1999), the dopaminergic neurotoxin MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) and 6-OHDA (6-hydroxydopamine) (Chen et al., 2001; Ikeda et al., 2002; Yu et al., 2008), excitotoxicity (Jones et al., 1998a,b; Popoli et al., 2002), β-amyloid aggregation (Dall'Igna et al., 2007; Canas et al., 2009), and traumatic brain injury (TBI) (Li et al., 2009). The protective effect of A2AR inactivation in the brain has been attributed to inhibition of glutamate release and suppression of proinflammatory cytokines (Popoli et al., 1995, 2003; Yu et al., 2004; Cunha, 2005; Chen et al., 2007; Stone and Behan, 2007). On the other hand, A2AR activation has also been shown to attenuate brain damage induced by intracerebral hemorrhage (Mayne et al., 2001), hippocampal kainate-induced excitotoxicity (Jones et al., 1998a), striatal lesion (Chou et al., 2005), and spinal cord injury (Li et al., 2006; Genovese et al., 2009). The neuroprotective effect of A2AR activation in these injury models has been primarily attributed to inhibition of inflammatory processes and vascular effects. Furthermore, the neuroprotective effects of A2AR agonists and antagonists are dependent on the stages of pathological processes (Li et al., 2006; Popoli et al., 2008), extents of brain injury (Blum et al., 2003), or routes of drug delivery (Jones et al., 1998a).
The apparent paradoxical effects of A2AR ligands on neuroinflammation and brain injury may in part reflect complex interactions between A2ARs and other neurotransmitters in the brain. In addition to adenosine, extracellular levels of glutamate increase rapidly (within minutes) and dramatically (up to 100-fold) in response to various brain injuries by presynaptic release from neurons and by exocytosis and possible reversal of glutamate uptake from astrocytes (Obrenovitch and Urenjak, 1997; Haydon and Carmignoto, 2006). According to the well documented role of extracellular glutamate in excitotoxicity, we reasoned that the interplay of extrasynaptic glutamate and adenosine in CNS inflammatory cells (e.g., microglial cells) may be critical to how A2AR activation impacts neuroinflammation and brain damage. We hypothesized that local increase of glutamate level switches the effect of A2AR activation from antiinflammatory effect and neuroprotection to proinflammatory effect and cytotoxicity.
To test this hypothesis, we investigated the effects of A2AR activation on neuroinflammation in the presence of varying concentrations of glutamate in primary cultures of microglial cells after treatment with lipopolysaccharide (LPS) and in intact animals after a cortical impact model of traumatic brain injury. The data we present here provide direct evidence that extrasynaptic glutamate levels dictate the switch from the A2AR activation-mediated antiinflammatory and neuroprotective to proinflammatory and cytotoxic role in vivo and in vitro. These findings identify a novel role for glutamate in modulation of neuroinflammation and brain injury via the adenosine–A2AR system.
Materials and Methods
Wild-type and A2AR knock-out mice
A2AR knock-out (KO) mice (A2AR−/−) and their wild-type (WT) controls (A2AR+/+) were bred in a C57BL/6 × 129SvEvSteel background. A2AR−/− mice were generated by gene targeting as previously described (Chen et al., 1999). The mice were maintained in a pathogen-free environment at the Animal Care Center of the Research Institute of Surgery and Daping Hospital (Third Military Medical University, Chongqing, China), with ad libitum access to regular mouse chow and drinking water. All procedures used in this study were reviewed and approved by the Institutional Animal Care and Use Committee of Third Military Medical University and performed under the supervision of the facility veterinary staff.
In vitro experiments
Primary culture of microglial cells.
Mouse microglial cell cultures were prepared as described previously (Si et al., 1996). In brief, cerebral cortices from neonatal mice (1–2 d of age) were dissected, carefully stripped of their meninges, and digested with 0.25% trypsin for 30 min at 37°C. Trypsinization was stopped by adding an equal volume of DMEM/F12 nutrient mixture containing 10% FBS. The suspension was pelleted and resuspended in culture medium and brought to a single-cell suspension by repeated pipetting, followed by passage through a 105 μm pore mesh. Cells were seeded at 1 × 106 cells/ml and cultured at 37°C in humidified 5% CO2/95% air until the mixed glial cultures were confluent (∼10 d). Floating and weakly attached cells on the mixed primary culture cell layer were obtained by gentle shaking for 10–15 min. The resulting cell suspension was seeded in a 24-well plate and allowed to adhere for 30 min at 37°C. Unattached cells were removed, leaving strongly adherent cells, which are primarily microglia. The purity of the microglial cultures was >95%, which was confirmed by immunofluorescence with microglial marker MAC-1 monoclonal antibody (see supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Purified microglial cultures were used for experiments within 2–3 d of isolation.
Pharmacological treatments.
Microglial cells derived from A2AR+/+ and A2AR−/− mice were pretreated with 0, 0.1, 0.5, or 5.0 mm glutamate, followed by a combination of LPS (1000 ng/ml) and the A2AR agonist 3-[4-[2-[[6-amino-9-[(2R,3R,4S,5S)-5-(ethylcarbamoyl)-3,4-dihydroxy-oxolan-2-yl]purin-2-yl]amino]ethyl]phenyl]propanoic acid (CGS21680) (100 nm) (Saura et al., 2005). For LPS and CGS21680 cotreatment, CGS21680 was added 10 min before LPS treatment. To elucidate the signaling pathway associated with A2AR action, 10 μm N-[2-[[3-(4-bromophenyl)-2-propenyl]amino]ethyl]-5-isoquinolinesulfonamide dihydrate dihydrochloride (H-89) or 5 μm 2-[1-(3-dimethylaminopropyl)-1H-indol-3-yl]-3-(1H-indol-3-yl)maleimide (GF109203X) was added to microglial cultures 30 min before glutamate was added. At these doses, H-89 (IC50, 5 μm) (Bracken et al., 2006) and GF109203X (IC50, 1–2 μm) (Cobine et al., 2007) can exert the inhibitory effect on protein kinase A (PKA) or protein kinase C (PKC) substrate phosphorylation and related cellular functions, respectively. All reported values were presented as means ± SEM of three independent experiments conducted in triplicate.
Measurement of NO levels.
Twelve hours after agents were added, the supernatants were collected, and the NO level was assayed according to the instructions in the NO detection kit (Cell Technology) based on the Griess reaction as an indirect indicator of nitric oxide synthase (NOS) activity.
cAMP content in microglial cells.
The cAMP content in the cell lysates was determined using the overnight acetylation protocol of a 125I-cAMP SPA kit (GE Healthcare), which is based on a 125I competitive binding assay.
In vivo experiments
Cortical impact model of TBI.
A moderate cortical impact was performed by the weight-dropping method as previously described (Li et al., 2008, 2009). Briefly, to minimize discomfort and pain, mice were anesthetized with intraperitoneal injection of 50 mg/kg pentobarbital sodium and then placed in a stereotaxic frame and subjected to 2-mm-diameter craniotomy over the left parietal cortex, with the center between the bregma and lambdoid suture. After TBI, the bone flap was repositioned, and the skin was closed with continuous sutures. Approximately 3–4 h after surgery, mice regained consciousness. Anesthesia (pentobarbital sodium, 50 mg/kg) was also used when mice were killed for analysis.
Determination of glutamate concentrations in the CSF.
At five different time points after TBI (15 min, 3, 6, 12, and 24 h), glutamate levels in CSF were assayed by HPLC as previously reported (Begley et al., 1994).
Pharmacological treatments.
Based on the time course of glutamate level in the CSF, the mice subjected to TBI were injected intraperitoneally with the A2AR agonist CGS21680 (0.1 mg/kg) or the A2AR antagonist 4-(2-{[5-amino-2-(2-furyl)[1,2,4]triazolo[1,5-a][1,3,5]triazin-7-yl]amino}ethyl)phenol (ZM241385) (1 mg/kg) at 15 min, 3, 6, or 12 h after TBI. In a separate experiment, the mice were treated with glutamate release inhibitor (S)-4-carboxy-3-hydroxyphenylglycine [(S)-4C3HPG] (Lorenc-Koci et al., 2001) at dose of 1, 5, or 10 mg/kg (intraperitoneally) 30 min before TBI, and the dose of (S)-4C3HPG that reduced the glutamate level in CSF at 15 min after TBI was selected to combine with CGS21680 treatment at 15 min after TBI. The effects of these agents on brain damage were evaluated at 24 h after TBI (Li et al., 2009).
Neurological deficits, brain water content, and the mRNA level of inflammatory cytokine in cortex and cultured microglial cells were determined as we descried previously (Li et al., 2008, 2009).
Detection of inducible NOS density.
Inducible NOS (iNOS) density in mouse brain after TBI was determined with standard immunofluorescence immunohistochemistry. Briefly, frozen sections of brain tissue (24 h after TBI) were acetone-fixed for 10 min, followed by incubation for 30 min at 37°C with rabbit anti-mouse iNOS antibody (Santa Cruz; 1:100). After washing three times with PBS and incubation with FITC-conjugated goat anti-rabbit secondary antibody for 30 min at 37°C, the sections were washed in a similar manner and examined by fluorescence microscopy.
Statistical analysis
Neurological deficit scores were analyzed by nonparametric Mann–Whitney U test. Statistical comparisons of more than two groups were performed by factorial ANOVA followed by Bonferroni's post hoc test. Graphic data represent the mean ± SEM. A value of p < 0.01 was considered statistically significant.
Results
The effect of A2AR activation on LPS-induced NOS activity in microglial cells in vitro is dependent on extracellular glutamate concentration
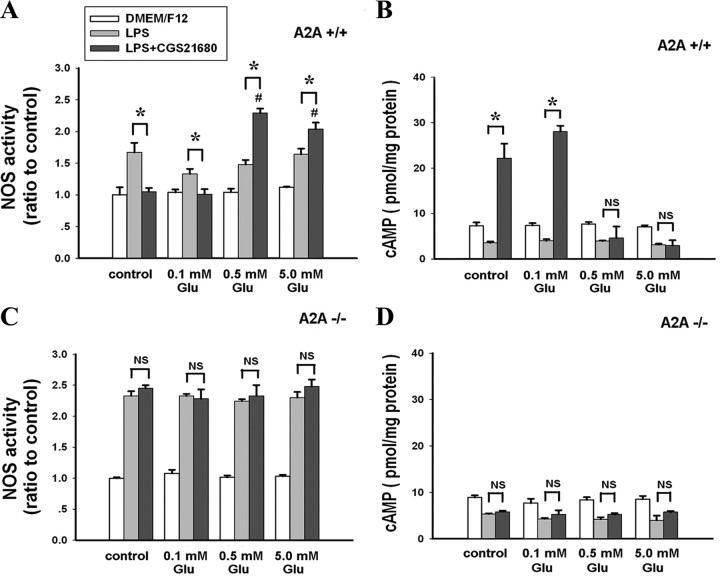
Microglial cells are critically involved in neuroinflammation. We measured LPS-induced NOS and cAMP production by microglial cells. Twelve hours after drug addition, CGS21680 inhibited LPS-induced NOS activity and significantly increased cAMP production in control medium and in the presence of 0.1 mm glutamate in microglial cells derived from A2AR+/+. By contrast, in the presence of 0.5 and 5.0 mm glutamate, CGS21680 promoted LPS-induced NOS activity and had no effect on LPS-induced cAMP production (Fig. 1 A,B). Once again, in microglial cells derived from A2AR−/−, CGS21680 had no significant effect on LPS-induced NOS activity at any concentration of glutamate, confirming that the bidirectional effects of CGS21680 on NOS activity in microglial cells was mediated by the A2AR (Fig. 1 C,D). A similar modulation of glutamate concentration on the effect of CGS21680 on NOS activity was observed in microglial cells 24 h after drug treatment, although at this time point, glutamate treatment alone caused some change in NOS activity compared with the control (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). Thus, glutamate concentration determines whether A2AR activation inhibits or stimulates NOS activity in microglial cells. In addition, we observed a similar glutamate-dependent switch in the effect of CGS21680 on LPS-induced tumor necrosis factor-α (TNF-α) expression in microglia cells in a separate experiment (supplemental Fig. 3, available at www.jneurosci.org as supplemental material).
Figure 1.
Activation of A2AR potentiates LPS-induced cAMP-independent NOS activity by microglial cells in the presence of high concentrations of glutamate. Primary cultures of mouse microglial cells were treated with CGS21680 and LPS in the presence of increasing concentrations of glutamate. Twelve hours later, NOS activity was normalized to the NOS activity in the control cultures. Data are reported as the mean ± SEM of three independent experiments. A, NOS activity in A2AR+/+ microglial cells. B, cAMP levels in A2AR+/+ microglial cells. C, NOS activity in A2AR−/− microglial cells. D, cAMP levels in A2AR−/− microglial cells. *p < 0.01 between the two groups; # p < 0.01 compared with the LPS+CGS21680 control group. NS, Not significant.
The inhibitory and facilitative effects of A2AR activation on NOS activity in microglial cells at different concentrations of glutamate require distinct signaling pathways
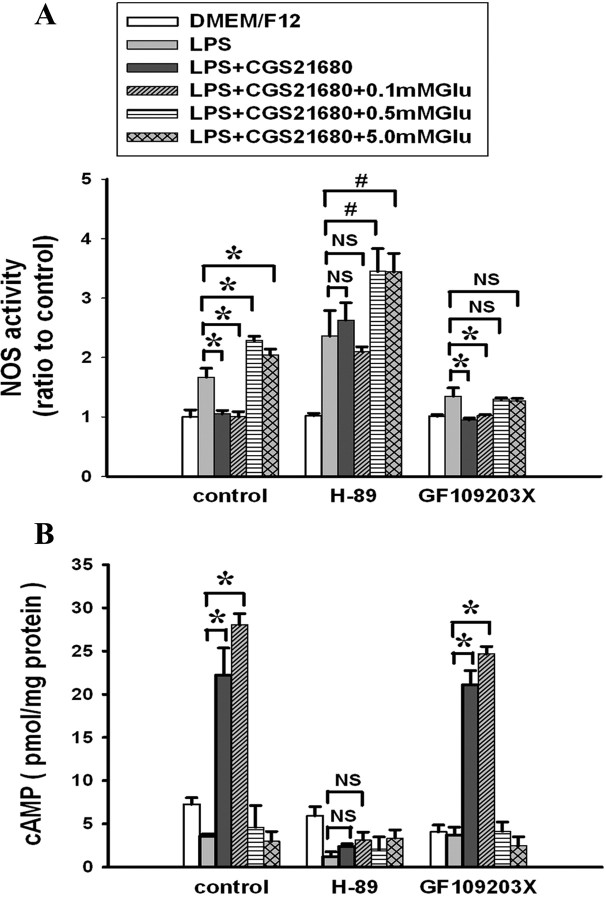
We next investigated whether the glutamate-induced switch in the effect of A2AR on LPS-induced NOS activity in microglial cells indicates a change the predominant signaling cascades activated by A2AR. It is well documented that A2AR activation can signal through either PKA or PKC pathways (Fredholm et al., 2007) and that the PKA pathway underlies the suppression of inflammation by A2AR activation in many tissues (Sitkovsky et al., 2004). We therefore hypothesized that increasing glutamate concentrations suppress PKA signaling from the A2AR, leaving PKC as the predominant signaling pathway. To explore this hypothesis, cultured microglial cells were treated with the PKA inhibitor H-89 or the PKC inhibitor GF109203X before treatment with CGS21680 and glutamate followed by LPS treatment. In the control medium or in the presence of 0.1 mm glutamate, H-89 blocked CGS21680-induced inhibition of LPS-induced NOS activity, whereas GF109203X had no effect on the CGS2180 inhibition (Fig. 2 A). By contrast, in the presence of 0.5 and 5.0 mm glutamate, GF109203X blocked CGS21680-induced potentiation of LPS-induced NOS activity, whereas H-89 had no effect (Fig. 2 A). As expected, H-89 blocked the LPS-induced increase in cAMP production, whereas GF109203X had no effect (Fig. 2 B). These results indicate that the CGS21680-induced inhibition of NOS activity in low concentrations of glutamate depends on the cAMP–PKA signaling cascade. By contrast, the CGS21680-induced potentiation of NOS activity in the presence of high concentrations of glutamate requires PKC activity.
Figure 2.
Effect of kinase inhibitors on modulation of NOS activity by A2AR activation at varying concentrations of glutamate. The experiments were as described in Figure 1 except that the PKA inhibitor H-89 or the PKC inhibitor GF109203X was added 30 min before glutamate was added to cultured A2AR+/+ microglial cells. NOS activity and cAMP levels were assayed as described in Materials and Methods. NOS activity was normalized to the activities in untreated control cultures. Graphs show mean ± SEM of three independent experiments. A, NOS activity in A2AR+/+ microglial cells. B, cAMP levels in WT microglial cells. *p < 0.01 between the two groups; # p < 0.05 between the two groups. NS, Not significant.
Modulation of brain injury by A2AR agonists and antagonists is dependent on the time of drug administration after TBI and is associated with the time course of changes in brain glutamate concentration
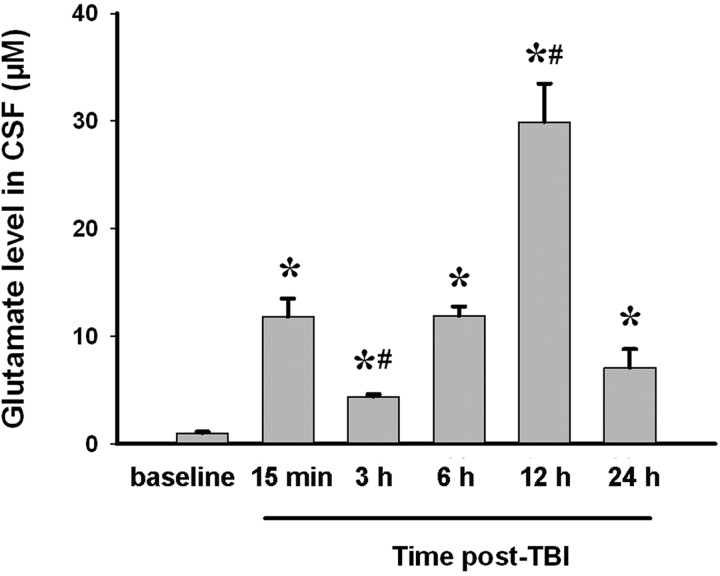
Next, we investigated whether local glutamate levels control the effect of A2AR activation on neuroinflammation and brain injury in intact animals using a mouse cortical impact model of TBI. We first determined glutamate levels in the CSF of mice at various times after TBI (Fig. 3). The glutamate level was lowest at 3 h after TBI and peaked at 12 h after TBI, declining thereafter but remaining elevated above the baseline at 24 h after TBI.
Figure 3.
Changes in glutamate level in the CSF of mice over 24 h after TBI. WT mice were subjected to TBI. Before TBI and at 15 min, 3, 6, 12, and 24 h after TBI, levels of glutamate in CSF collected from the cisterna magna were determined by HPLC. Data are expressed as mean ± SEM. *p < 0.01 compared with baseline; # p < 0.01 compared with 15 min after TBI. n = 10 mice per time point. A different set of animals was used for each time point.
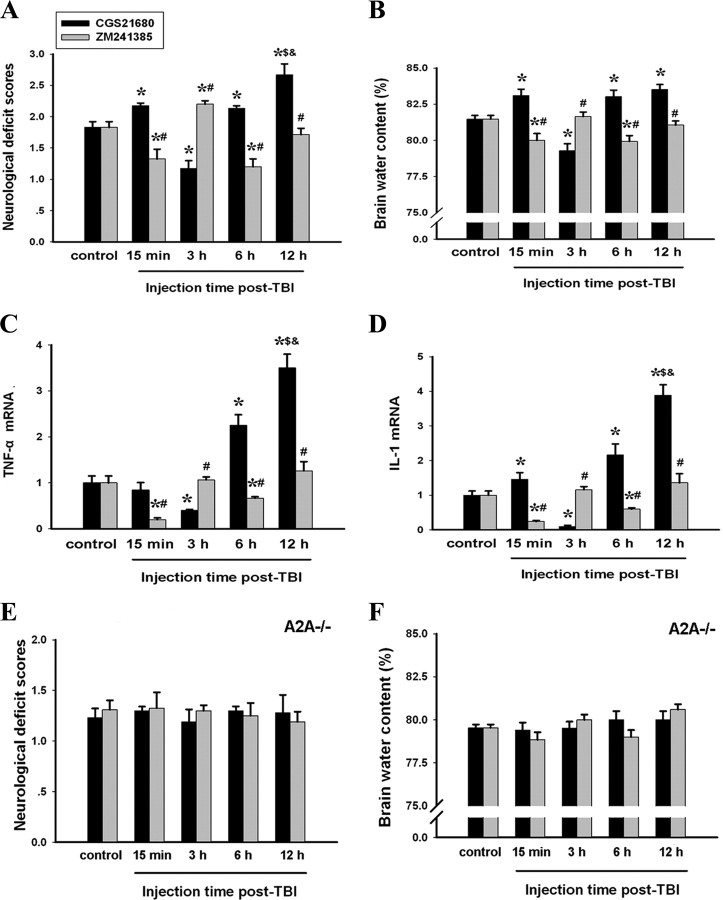
Based on this time course of changes in glutamate concentration, we treated the animals with CGS21680 or the A2AR antagonist ZM241385 at 15 min, 3, 6, and 12 h after TBI and assessed behavioral deficits and brain water content at 24 h after TBI. CGS21680 administered at 15 min, 6, or 12 h after TBI, when glutamate levels were higher than the control, increased the neurological deficit scores compared with mice treated with vehicle (note: CGS21680 caused death in ∼50% of mice when administered at 12 h after TBI and no death when administered at other time points after TBI). Conversely, the A2AR antagonist ZM241385 administered at these time points reduced the behavioral deficit scores compared with mice treated with vehicle. However, when administered at 3 h after TBI, when glutamate concentration is at the lowest post-TBI level, CGS21680 attenuated the neurological deficits seen at 24 h, whereas ZM241385 aggravated the deficits (Fig. 4 A). Consistent with these behavioral results, CGS21680 reduced, and ZM241385 increased, brain water content when administered 3 h after TBI, but both drugs had the opposite effect when administered at 15 min, 6, or 12 h after TBI (Fig. 4 B). The effects of CGS21680 and ZM241385 administered at different post-TBI time points on TBI-induced TNF-α and IL-1 mRNA expression paralleled the changes in behavioral and brain water contents (Figs. 4 C,D). Last, CGS21680 and ZM241385 had no effect on behavioral deficit score and brain water content after TBI in A2AR KO mice, confirming that the effects of both drugs were mediated by the A2AR (Fig. 4 E,F).
Figure 4.
Activation of A2AR at different times after TBI exerts different effects on inflammation and outcome at 24 h after TBI. A–F, At 15 min, 3, 6, and 12 h after TBI, injured WT (A–D) and A2AR KO (E, F) mice were injected with the A2AR agonist CGS21680 or the antagonist ZM241385. At 24 h after TBI, neurological deficits, brain water content, and TNF-α and IL-1 mRNA expression in the injured brain tissue were evaluated. A, E, Neurological deficit scores. B, F, Brain water content (the percentage brain water content of TBI brains was assayed using the wet–dry method and calculated as the difference between wet and dry weights divided by the wet weight of the brain). C, Relative expression of TNF-α mRNA. D, Relative expression of IL-1 mRNA. All the mRNA expression levels were normalized to GAPDH mRNA levels, and the data are expressed as the ratios relative to the control. *p < 0.01 compared with control; # p < 0.01 compared with the group treated with CGS21680 at the same time point; $ p < 0.01 compared with the group treated with CGS21680 at 15 min after TBI; & p < 0.01 compared with the group treated with CGS21680 at 6 h after TBI; n = 10 mice per group. Error bars indicate SEM.
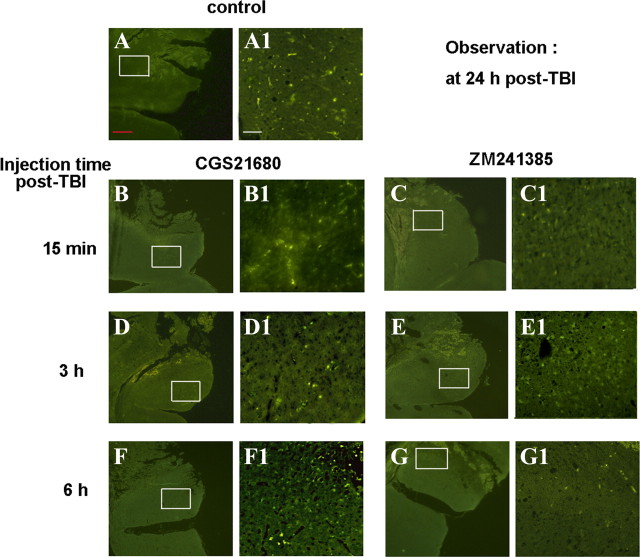
In addition, at 24 h after TBI, more iNOS-immunoreactive (iNOS-IR) cells were observed in brain sections from mice treated with CGS21680 at 15 min or 6 h after TBI than in sections from the vehicle-treated mice. Conversely, treatment with ZM241385 at 15 min or 6 h after TBI decreased iNOS-IR cells compared with the vehicle. At an intermediate time point, 3 h after TBI, the effect of the A2AR ligands was reversed: CGS21680 decreased the number of iNOS-IR neurons, whereas ZM241385 increased iNOS-IR neurons compared with the vehicle group (Fig. 5).
Figure 5.
Activation of A2AR at different times after TBI exerts different effects on iNOS density at 24 h after TBI. At 15 min, 3, and 6 h after TBI, injured WT mice were injected with the A2AR agonist CGS21680 or the antagonist ZM241385. At 24 h after TBI, iNOS density in the injured brain tissue was evaluated by immunoflourescence with anti-iNOS antibody. A, A1, WT mice with TBI as control. B, B1, Injured WT mice injected with CGS21680 at 15 min post-TBI. C, C1, Injured WT mice injected with ZM241385 at 15 min post-TBI. D, D1, Injured WT mice injected with CGS21680 at 3 h post-TBI. E, E1, Injured WT mice injected with ZM241385 at 3 h post-TBI. F, F1, Injured WT mice injected with CGS21680 at 6 h post-TBI. G, G1, Injured WT mice injected with ZM241385 at 6 h post-TBI. Magnification: A–G, 50×; A1–G1, 200×. Scale bars: red, 200 μm; white, 50 μm.
These results suggested that the direction of the effect of A2AR ligands on inflammation and brain injury after TBI in mice were dependent on the extracellular glutamate concentration in the local environment at the time of ligand administration.
Treatment with the glutamate release inhibitor (S)-4C3HPG converts the effect of CGS21680 on post-TBI brain injury from exacerbation to attenuation
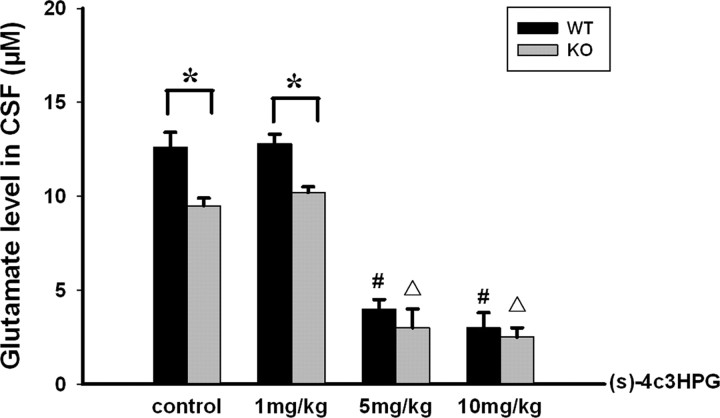
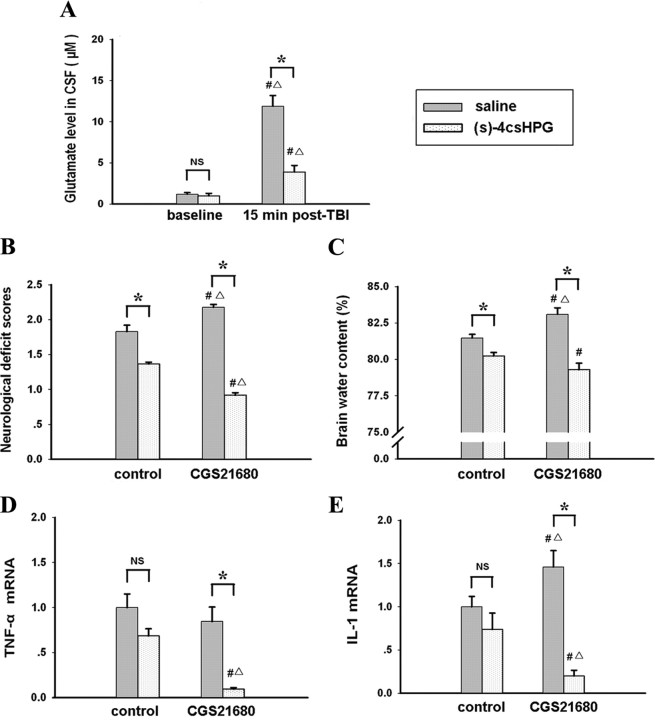
To substantiate the hypothesis that high concentrations of glutamate prompted the change in the effect of A2AR activation on inflammation and brain injury, we reduced endogenous glutamate release by administering (S)-4C3HPG before TBI. As expected, (S)-4C3HPG dose-dependently decreased the glutamate level in mouse CSF at 15 min after TBI in WT as well as A2AR KO mice (Fig. 6). As shown in Fig. 7 A, (S)-4C3HPG at 5 mg/kg reduced the glutamate level from 12.5 to 3.9 μm at 15 min after TBI. Thus, we selected 5 mg/kg (S)-4C3HPG for the combined treatment with CGS21680 since (S)-4C3HPG at 10 mg/kg resulted in some mortalities of mice after TBI. When we administered CGS21680 at 15 min after TBI and assessed neurological deficits and neuroinflammation markers at 24 h after TBI, we again found that CGS21580 exacerbated the neurological deficit and increased neuroinflammation markers induced by TBI but also found that (S)-4C3HPG pretreatment altered this effect significantly, attenuating the neurological deficits and reducing brain water content and expression of TNF-α mRNA and IL-1 mRNA (Fig. 7 B–E). Thus, by blocking the TBI-induced increase in local glutamate level, we converted the direction of the CGS21680 effect on brain injury and neuroinflammation in vivo from exacerbation to attenuation.
Figure 6.
Dose-dependent attenuation of glutamate levels in CSF after TBI by pretreatment with (S)-4C3HPG. Mice were injected intraperitoneally with (S)-4C3HPG 30 min before TBI. Fifteen minutes after TBI, CSF was collected from injured mice and glutamate levels were assayed by HPLC. *p < 0.01 between the two groups; # p < 0.01 compared with WT control; ▵ p < 0.01 compared with A2AR KO control. (S)-4C3HPG treatment dose-dependently reduced the glutamate level after TBI in both in WT and A2AR KO mice.
Figure 7.
Inhibition of glutamate release with (S)-4C3HPG restores the antiinflammatory and protective effect of A2AR activity. Mice were treated with the glutamate release inhibitor (S)-4C3HPG 30 min before TBI. A, The glutamate concentration in injured mice was assayed at 15 min after TBI to confirm inhibition of glutamate release. (S)-4C3HPG-treated mice were treated with CGS21680 at 15 min after TBI. B–E, The effects of combination treatment were evaluated at 24 h after TBI, including evaluating neurological deficit (B), brain water content (C), and inflammatory cytokine expression (D, E). All mRNA expressions levels were normalized to GAPDH mRNA levels, and the data are expressed as the ratios relative to the control. *p < 0.01 between the two groups; # p < 0.01 compared with the baseline of intact mice treated with saline (A) or the TBI group treated with saline in control (B–E); ▵ p < 0.01 compared with the baseline of intact mice treated with (S)-4C3HPG (A) or the TBI group treated with (S)-4C3HPG in control (B–E). NS, Not significant; n = 10 mice per group. Error bars indicate SEM.
Discussion
The local level of glutamate determines whether A2AR activation enhances or inhibits the neuroinflammatory responses of microglia cells
Our in vitro experiments were designed to test directly the hypothesis that the local concentration of glutamate controls the effect of A2AR activity on neuroinflammation. We found that, in the presence of low levels of glutamate, A2AR activation inhibited the LPS-induced inflammatory response in cultured microglial cells. However, in the presence of high concentrations of glutamate, A2AR activation augmented the inflammatory response in microglial cells. Thus, the local glutamate concentrations dictate the direction of the effect of A2AR activation: it is antiinflammatory at low glutamate concentrations and proinflammatory at high glutamate concentrations. The potentiation of LPS-induced NOS activity by A2AR activation in microglial cells is consistent with a previous report showing that the A2AR receptor agonist CGS21680 potentiated LPS-induced NO release and NOS-II expression in mixed glial cultures (75% astrocytes, 25% microglia) (Saura et al., 2005). In our study, the enhanced effect of CGS21680 occurred only at high concentrations of glutamate; the concentration of glutamate in the culture medium used by Saura et al. is not indicated. Extracellular glutamate concentration had a similar effect on the modulation of LPS-induced TNF-α expression by A2AR activation in microglia cells. In the CNS, glutamate can be released from presynaptic glutamatergic terminals such as corticostriatal terminals (Obrenovitch and Urenjak, 1997). The A2AR at these terminals have been shown to modify the amount and frequency of glutamate release (Marchi et al., 2002; Popoli et al., 2003) and extracellular glutamate outflow in various brain regions (Popoli et al., 1995; Corsi et al., 1999). Another important source of extracellular glutamate in the brain likely come from glial cells, as suggested by studies showing that astrocytes release large amounts of glutamate by physiological astrocytic calcium levels and by inversion of glutamate transporter activity during hypoxia (Haydon and Carmignoto, 2006). With both neurons and astrocytes releasing glutamate after injury, the local CNS environment is likely to be glutamate-enriched and A2AR activation would be expected to exert a proinflammatory effect.
Glutamate-dependent switch from antiinflammatory to proinflammatory of A2AR activation is associated with signaling from the PKA to PKC pathway
Our results with kinase inhibitors in cultured microglial cells indicate that the antiinflammatory effect of A2AR activation at low glutamate concentrations is PKA dependent, whereas the proinflammatory effect of A2AR activation at higher glutamate concentrations is PKC dependent. The cAMP–PKA pathway has been identified as the main signaling cascade responsible for A2AR-mediated inhibition of acute inflammation (Cronstein, 1994; Haskó and Cronstein, 2004; Sitkovsky et al., 2004; Fredholm et al., 2007). However, A2AR activation can signal through cAMP–PKA independent pathways as well (Fredholm et al., 2007), including PKC (Lai et al., 1997; Cunha and Ribeiro, 2000; Pinto-Duarte et al., 2005), MAPK (mitogen-activated protein kinase) (Cheng et al., 2002; Schulte and Fredholm, 2003; Gianfriddo et al., 2004; Melani et al., 2006), β-arrestin (Khoa et al., 2006), and even Src–TrkA pathway (Malek et al., 1999). In addition, recent studies show that the C terminus of the A2AR binds to several interacting proteins [actinin, ARNO (ARF nucleotide binding site opener), Usp4, and TRAX (Translin-associated factor X)] and might mediate the G-protein-independent function of A2ARs (for review, see Fredholm et al., 2007). These cAMP-independent signal pathways may be responsible for A2AR-mediated enhancement of inflammatory response in the presence of high concentrations of glutamate. The PKC pathway is particularly relevant since activation of PKC by A2ARs via a pertussis toxin-sensitive G-protein (Gαx) can phosphorylate adenylyl cyclase, leading to desensitization of A2AR–cAMP signal pathway (Lai et al., 1997). In the present study, we demonstrated that, in the presence of high concentrations of glutamate, the PKC inhibitor GF109203X but not the PKA inhibitor H-89 blocked the proinflammatory activity of A2AR, indicating the involvement of the PKC signaling pathway in A2AR proinflammatory responses. This raises the possibility that the proinflammatory effects of A2AR activation at high concentrations of glutamate are attributable to a switch in A2AR signal transduction pathways from cAMP–PKA to PKC. However, our study cannot distinguish the possible direct effect of PKC inhibitor on glutamate-mediated (such as mGluR5) PKC signaling from A2AR-mediated PKC signaling, and additional studies are required to confirm this hypothesized switch in A2AR signaling pathway.
It is not clear how high glutamate concentrations might trigger this switch. Heteromeric complexes contain both A2ARs and mGlu5 receptors and functional synergism between the two receptors has been demonstrated in striatal neurons (Ferré et al., 2002; Coccurello et al., 2004; Kachroo et al., 2005; Tebano et al., 2005). Since both mGluR and A2ARs are expressed on inflammatory cells (polymorphonuclear neutrophils and microglial cells) (Gill and Pulido, 2001), this cross talk between the A2ARs and mGluR5 receptors is one possible molecular basis for the glutamate concentration-mediated regulation of A2AR function. Although our in vitro experiments demonstrated a critical role of A2AR on microglia cells in this molecular switch, the A2AR effects on neuroinflammation and brain injury in intact animals after TBI likely involve the intricate interplay between A2AR and glutamate signaling not only in microglia cells but also in neurons, astrocytes, and infiltrated white blood cells. Moreover, A2AR activation facilitates glutamate release, whereas inactivation of the A2AR attenuates glutamate release in response to ischemic and excitotoxic injury (Cunha, 2005; Chen et al., 2007). Thus, we speculate that enhanced glutamate release switches the effect of A2AR activation from antiinflammatory effect to proinflammatory, leading to a possible feedforward loop and ultimately brain injury.
Local glutamate levels in the brain after traumatic brain injury dictate whether A2AR agonists or antagonists have a neuroprotective effect
Neuroinflammation is common pathological response to a variety of brain insults. Thus, we reasoned that the glutamate concentration dependence of the effect of A2AR activity on neuroinflammatory processes in microglial cells may explain the variable effects of A2AR agonists and antagonists on brain injury in intact animals. Using a mouse model of TBI, we evaluated the effect of CNS glutamate concentrations on A2AR modulation of brain injury and found the following: (1) After TBI, glutamate levels in CSF undergo dynamic changes. (2) When administered at a time of low glutamate concentrations, A2AR activation attenuated brain damage by inhibiting inflammatory cytokine expression and iNOS activity. (3) By contrast, when administered at a time when concentrations of glutamate were high, A2AR activation aggravated brain damage by potentiating inflammatory cytokine expression and iNOS activity. (4) Importantly, when the high concentration of glutamate at 15 min after TBI was pharmacologically reduced by pretreatment with an inhibitor of glutamate release, the debilitating effect of A2AR activation was converted to a protective effect. (5) Last, the specificity of the effect of A2AR agonists and antagonists in WT mice was validated by the disappearance of these pharmacological effects in A2AR KO mice. These results complement our in vitro results, demonstrating that local glutamate level dictates brain injury outcome by determining the effect of A2AR on neuroinflammation. Furthermore, the combined effect of mGluR antagonism and A2AR antagonism has been explored as the possible treatment to improve motor activity in animal model of Parkinson's disease (Coccurello et al., 2004; Kachroo et al., 2005). Additional studies are needed to investigate the combined effect of A2ARs and mGluR5 antagonism on brain injury and to better characterize the mechanism by which (S)-4C3HPG reduces glutamate level.
Our results clearly demonstrated the A2AR effects on brain injury is context dependent, critically depending on local glutamate levels, and thus provide at least partial explanation for the paradoxical results reported with A2AR agonists and antagonists in some CNS injury models and. For instance, Blum et al. (2003) reported that, when a high dose of 3-nitropropionic acid was administered to induce high glutamate outflow in the striatum, A2AR activation exerted a deleterious effect; however, at a low dose of 3-nitropropionic acid and therefore lower glutamate outflow, A2AR activation exerted a protective effect. In a spinal cord injury model, administration of A2AR agonists was neuroprotective in early stages postinjury, whereas A2AR inhibition was neuroprotective at later stages (Li et al., 2006). This time-dependent effect for A2AR agonists and antagonists may well be attributable to changes in the local glutamate concentration in the spinal cord at different stages of the injury response, as we demonstrated here for brain glutamate concentrations after TBI. Similarly, Jones et al. (1998a,b) found that peripheral administration of the A2AR agonist CGS21680 or central injection of antagonist ZM241385 into the hippocampus protects the hippocampus against kainate-induced excitotoxicity. It is tempting to speculate that different local glutamate levels in hippocampus (i.e., high glutamate) and in peripheral blood (relatively low level of glutamate) may contribute to this injection site-dependent difference in effects of A2AR ligands.
After brain injury, the extracellular level of adenosine increases rapidly and significantly, with preferential activation of A1R at relatively low levels of adenosine and preferential activation of the A2AR at higher levels of adenosine (Cunha, 2005). After ischemic brain injury, extracellular adenosine levels peak ∼3 h (Latini and Pedata, 2001), which is coincident with our finding of lower level of glutamate in the brain at 3–4 h after TBI. At this specific time window, activation of A2ARs by extracellular adenosine is protective, a finding consistent with previously reported motor functional improvement by the nonselective A1/A2A agonist 2-chloroadenosine after TBI (Varma et al., 2002). The finding also suggests that the documented general protective effect of extracellular adenosine may be mediated not only by effects at the A1 receptor (de Mendonça et al., 2000) but also possibly by the effects at the A2AR at specific time point. However, we also found that, at 6 h after TBI, which is considered the optimal time to treat TBI, CSF glutamate levels had returned to a relatively high level and A2AR agonists exacerbated injury. This narrow therapeutic window for A2AR agonists is consistent with the fact that most studies found that inactivation of A2ARs is protective after brain injury (Chen et al., 2007) and supports the broad spectrum of neuroprotection by A2A receptor antagonists, whereas A2AR agonists may be considered under some specific pathological conditions when brain glutamate level is relatively low.
A novel role for glutamate in neuroinflammation and brain injury
In summary, in addition to the well documented excitotoxic effect of glutamate, our findings provide critical evidence for glutamate regulation of neuroinflammation and brain injury via the adenosine–A2AR system. Furthermore, this novel control mechanism provides a plausible explanation for some reported variability in the effect of A2AR activation on CNS injuries and provides compelling evidence that the functional outcome of A2AR activation during brain injury is context dependent, critically depending on local glutamate level. These findings offer insight into the complexity of A2AR–glutamate interactions and suggest a novel strategy for controlling inflammatory processes and limiting brain injury by regulating A2AR activity according to local glutamate levels.
Footnotes
This work was supported by National Natural Science Foundation of China Grants 30328015 and 30671918, and U.S. Public Health Service Grants NS41083 and NS48995. We gratefully acknowledge the technical and equipment support provided by Drs. Minghui Xu and Guansong Wang. We thank Chun Hu and Hao Wang for handling of the animals and Dr. Susan E. Lewis for editing this manuscript.
References
- Begley DJ, Reichel A, Ermisch A. Simple high-performance liquid chromatographic analysis of free primary amino acid concentrations in rat plasma and cisternal cerebrospinal fluid. J Chromatogr B Biomed Appl. 1994;657:185–191. doi: 10.1016/0378-4347(94)80085-5. [DOI] [PubMed] [Google Scholar]
- Blum D, Galas MC, Pintor A, Brouillet E, Ledent C, Muller CE, Bantubungi K, Galluzzo M, Gall D, Cuvelier L, Rolland AS, Popoli P, Schiffmann SN. A dual role of adenosine A2A receptors in 3-nitropropionic acid-induced striatal lesions: implications for the neuroprotective potential of A2A antagonists. J Neurosci. 2003;23:5361–5369. doi: 10.1523/JNEUROSCI.23-12-05361.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracken N, Elkadri M, Hart G, Hussain M. The role of constitutive PKA-mediated phosphorylation in the regulation of basal I Ca in isolated rat cardiac myocytes. Br J Pharmacol. 2006;148:1108–1115. doi: 10.1038/sj.bjp.0706809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canas PM, Porciúncula LO, Cunha GM, Silva CG, Machado NJ, Oliveira JM, Oliveira CR, Cunha RA. Adenosine A2A receptor blockade prevents synaptotoxicity and memory dysfunction caused by β-amyloid peptides via p38 mitogen-activated protein kinase pathway. J Neurosci. 2009;29:14741–14751. doi: 10.1523/JNEUROSCI.3728-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, Moskowitz MA, Fink JS, Schwarzschild MA. A2A adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Xu K, Petzer JP, Staal R, Xu YH, Beilstein M, Sonsalla PK, Castagnoli K, Castagnoli N, Jr, Schwarzschild MA. Neuroprotection by caffeine and A2A adenosine receptor inactivation in a model of Parkinson's disease. J Neurosci. 2001;21 doi: 10.1523/JNEUROSCI.21-10-j0001.2001. RC143(1–6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Sonsalla PK, Pedata F, Melani A, Domenici MR, Popoli P, Geiger J, Lopes LV, de Mendonça A. Adenosine A2A receptors and brain injury: broad spectrum of neuroprotection, multifaceted actions and “fine tuning” modulation. Prog Neurobiol. 2007;83:310–331. doi: 10.1016/j.pneurobio.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Cheng HC, Shih HM, Chern Y. Essential role of cAMP-response element-binding protein activation by A2A adenosine receptors in rescuing the nerve growth factor-induced neurite outgrowth impaired by blockage of the MAPK cascade. J Biol Chem. 2002;277:33930–33942. doi: 10.1074/jbc.M201206200. [DOI] [PubMed] [Google Scholar]
- Chou SY, Lee YC, Chen HM, Chiang MC, Lai HL, Chang HH, Wu YC, Sun CN, Chien CL, Lin YS, Wang SC, Tung YY, Chang C, Chern Y. CGS21680 attenuates symptoms of Huntington's disease in a transgenic mouse model. J Neurochem. 2005;93:310–320. doi: 10.1111/j.1471-4159.2005.03029.x. [DOI] [PubMed] [Google Scholar]
- Cobine CA, Callaghan BP, Keef KD. Role of L-type calcium channels and PKC in active tone development in rabbit coronary artery. Am J Physiol Heart Circ Physiol. 2007;292:H3079–H3088. doi: 10.1152/ajpheart.01261.2006. [DOI] [PubMed] [Google Scholar]
- Coccurello R, Breysse N, Amalric M. Simultaneous blockade of adenosine A2A and metabotropic glutamate mGlu5 receptors increase their efficacy in reversing parkinsonian deficits in rats. Neuropsychopharmacology. 2004;29:1451–1461. doi: 10.1038/sj.npp.1300444. [DOI] [PubMed] [Google Scholar]
- Corsi C, Melani A, Bianchi L, Pepeu G, Pedata F. Striatal A2A adenosine receptors differentially regulate spontaneous and K+-evoked glutamate release in vivo in young and aged rats. Neuroreport. 1999;10:687–691. doi: 10.1097/00001756-199903170-00005. [DOI] [PubMed] [Google Scholar]
- Cronstein BN. Adenosine, an endogenous anti-inflammatory agent. J Appl Physiol. 1994;76:5–13. doi: 10.1152/jappl.1994.76.1.5. [DOI] [PubMed] [Google Scholar]
- Cunha RA. Neuroprotection by adenosine in the brain: from A1 receptor activation to A2A receptor blockade. Purinergic Signal. 2005;1:111–134. doi: 10.1007/s11302-005-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA, Ribeiro JA. Adenosine A2A receptor facilitation of synaptic transmission in the CA1 area of the rat hippocampus requires protein kinase C but not protein kinase A activation. Neurosci Lett. 2000;289:127–130. doi: 10.1016/s0304-3940(00)01295-7. [DOI] [PubMed] [Google Scholar]
- Dall'Igna OP, Fett P, Gomes MW, Souza DO, Cunha RA, Lara DR. Caffeine and adenosine A2A receptor antagonists prevent beta-amyloid (25–35)-induced cognitive deficits in mice. Exp Neurol. 2007;203:241–245. doi: 10.1016/j.expneurol.2006.08.008. [DOI] [PubMed] [Google Scholar]
- de Mendonça A, Sebastião AM, Ribeiro JA. Adenosine: does it have a neuroprotective role after all? Brain Res Brain Res Rev. 2000;33:258–274. doi: 10.1016/s0165-0173(00)00033-3. [DOI] [PubMed] [Google Scholar]
- Ferré S, Karcz-Kubicha M, Hope BT, Popoli P, Burgueño J, Gutiérrez MA, Casadó V, Fuxe K, Goldberg SR, Lluis C, Franco R, Ciruela F. Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: implications for striatal neuronal function. Proc Natl Acad Sci U S A. 2002;99:11940–11945. doi: 10.1073/pnas.172393799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Chern Y, Franco R, Sitkovsky M. Aspects of the general biology of adenosine A2A signaling. Prog Neurobiol. 2007;83:263–276. doi: 10.1016/j.pneurobio.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Genovese T, Melani A, Esposito E, Mazzon E, Di Paola R, Bramanti P, Pedata F, Cuzzocrea S. The selective adenosine A2A receptor agonist CGS 21680 reduces JNK MAPK activation in oligodendrocytes in injured spinal cord. Shock. 2009;32:578–585. doi: 10.1097/SHK.0b013e3181a20792. [DOI] [PubMed] [Google Scholar]
- Gianfriddo M, Melani A, Turchi D, Giovannini MG, Pedata F. Adenosine and glutamate extracellular concentrations and mitogen-activated protein kinases in the striatum of Huntington transgenic mice. Selective antagonism of adenosine A2A receptors reduces transmitter outflow. Neurobiol Dis. 2004;17:77–88. doi: 10.1016/j.nbd.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Gill SS, Pulido OM. Glutamate receptors in peripheral tissues: current knowledge, future research, and implications for toxicology. Toxicol Pathol. 2001;29:208–223. doi: 10.1080/019262301317052486. [DOI] [PubMed] [Google Scholar]
- Haskó G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Kurokawa M, Aoyama S, Kuwana Y. Neuroprotection by adenosine A2A receptor blockade in experimental models of Parkinson's disease. J Neurochem. 2002;80:262–270. doi: 10.1046/j.0022-3042.2001.00694.x. [DOI] [PubMed] [Google Scholar]
- Jones PA, Smith RA, Stone TW. Protection against kainate-induced excitotoxicity by adenosine A2A receptor agonists and antagonists. Neuroscience. 1998a;85:229–237. doi: 10.1016/s0306-4522(97)00613-1. [DOI] [PubMed] [Google Scholar]
- Jones PA, Smith RA, Stone TW. Protection against hippocampal kainate excitotoxicity by intracerebral administration of an adenosine A2A receptor antagonist. Brain Res. 1998b;800:328–335. doi: 10.1016/s0006-8993(98)00540-x. [DOI] [PubMed] [Google Scholar]
- Kachroo A, Orlando LR, Grandy DK, Chen JF, Young AB, Schwarzschild MA. Interactions between metabotropic glutamate 5 and adenosine A2A receptors in normal and parkinsonian mice. J Neurosci. 2005;25:10414–10419. doi: 10.1523/JNEUROSCI.3660-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoa ND, Postow M, Danielsson J, Cronstein BN. Tumor necrosis factor-α prevents desensitization of Gαs-coupled receptors by regulating GRK2 association with the plasma membrane. Mol Pharmacol. 2006;69:1311–1319. doi: 10.1124/mol.105.016857. [DOI] [PubMed] [Google Scholar]
- Lai HL, Yang TH, Messing RO, Ching YH, Lin SC, Chern Y. Protein kinase C inhibits adenylyl cyclase type VI activity during desensitization of the A2A-adenosine receptor-mediated cAMP response. J Biol Chem. 1997;272:4970–4977. doi: 10.1074/jbc.272.8.4970. [DOI] [PubMed] [Google Scholar]
- Latini S, Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem. 2001;79:463–484. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- Li W, Dai S, An J, Li P, Chen X, Xiong R, Liu P, Wang H, Zhao Y, Zhu M, Liu X, Zhu P, Chen JF, Zhou Y. Chronic but not acute treatment with caffeine attenuates traumatic brain injury in the mouse cortical impact model. Neuroscience. 2008;151:1198–1207. doi: 10.1016/j.neuroscience.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Li W, Dai S, An J, Xiong R, Li P, Chen X, Zhao Y, Liu P, Wang H, Zhu P, Chen J, Zhou Y. Genetic inactivation of adenosine A2A receptors attenuates acute traumatic brain injury in the mouse cortical impact model. Exp Neurol. 2009;215:69–76. doi: 10.1016/j.expneurol.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Li Y, Oskouian RJ, Day YJ, Rieger JM, Liu L, Kern JA, Linden J. Mouse spinal cord compression injury is reduced by either activation of the adenosine A2A receptor on bone marrow-derived cells or deletion of the A2A receptor on non-bone marrow-derived cells. Neuroscience. 2006;141:2029–2039. doi: 10.1016/j.neuroscience.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Lorenc-Koci E, Wardas J, Wolfarth S, Pilc A. (S)-4C3HPG, a mixed group I mGlu receptor antagonist and a group II agonist, administered intrastriatally, counteracts parkinsonian-like muscle rigidity in rats. Brain Res. 2001;903:177–184. doi: 10.1016/s0006-8993(01)02438-6. [DOI] [PubMed] [Google Scholar]
- Malek RL, Nie Z, Ramkumar V, Lee NH. Adenosine A2A receptor mRNA regulation by nerve growth factor is TrkA-, Src-, and Ras-dependent via extracellular regulated kinase and stress-activated protein kinase/c-Jun NH2-terminal kinase. J Biol Chem. 1999;274:35499–35504. doi: 10.1074/jbc.274.50.35499. [DOI] [PubMed] [Google Scholar]
- Marchi M, Raiteri L, Risso F, Vallarino A, Bonfanti A, Monopoli A, Ongini E, Raiteri M. Effects of adenosine A1 and A2A receptor activation on the evoked release of glutamate from rat cerebrocortical synaptosomes. Br J Pharmacol. 2002;136:434–440. doi: 10.1038/sj.bjp.0704712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayne M, Fotheringham J, Yan HJ, Power C, Del Bigio MR, Peeling J, Geiger JD. Adenosine A2A receptor activation reduces proinflammatory events and decreases cell death following intracerebral hemorrhage. Ann Neurol. 2001;49:727–735. doi: 10.1002/ana.1010. [DOI] [PubMed] [Google Scholar]
- Melani A, Gianfriddo M, Vannucchi MG, Cipriani S, Baraldi PG, Giovannini MG, Pedata F. The selective A2A receptor antagonist SCH 58261 protects from neurological deficit, brain damage and activation of p38 MAPK in rat focal cerebral ischemia. Brain Res. 2006;1073–1074:470–480. doi: 10.1016/j.brainres.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Obrenovitch TP, Urenjak J. Altered glutamatergic transmission in neurological disorders: from high extracellular glutamate to excessive synaptic efficacy. Prog Neurobiol. 1997;51:39–87. doi: 10.1016/s0301-0082(96)00049-4. [DOI] [PubMed] [Google Scholar]
- Pedata F, Corsi C, Melani A, Bordoni F, Latini S. Adenosine extracellular brain concentrations and role of A2A receptors in ischemia. Ann N Y Acad Sci. 2001;939:74–84. doi: 10.1111/j.1749-6632.2001.tb03614.x. [DOI] [PubMed] [Google Scholar]
- Phillis JW. The effects of selective A1 and A2A adenosine receptor antagonists on cerebral ischemic injury in the gerbil. Brain Res. 1995;705:79–84. doi: 10.1016/0006-8993(95)01153-6. [DOI] [PubMed] [Google Scholar]
- Pinto-Duarte A, Coelho JE, Cunha RA, Ribeiro JA, Sebastião AM. Adenosine A2A receptors control the extracellular levels of adenosine through modulation of nucleoside transporters activity in the rat hippocampus. J Neurochem. 2005;93:595–604. doi: 10.1111/j.1471-4159.2005.03071.x. [DOI] [PubMed] [Google Scholar]
- Popoli P, Betto P, Reggio R, Ricciarello G. Adenosine A2A receptor stimulation enhances striatal extracellular glutamate levels in rats. Eur J Pharmacol. 1995;287:215–217. doi: 10.1016/0014-2999(95)00679-6. [DOI] [PubMed] [Google Scholar]
- Popoli P, Pintor A, Domenici MR, Frank C, Tebano MT, Pèzzola A, Scarchilli L, Quarta D, Reggio R, Malchiodi-Albedi F, Falchi M, Massotti M. Blockade of striatal adenosine A2A receptor reduces, through a presynaptic mechanism, quinolinic acid-induced excitotoxicity: possible relevance to neuroprotective interventions in neurodegenerative diseases of the striatum. J Neurosci. 2002;22:1967–1975. doi: 10.1523/JNEUROSCI.22-05-01967.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli P, Frank C, Tebano MT, Potenza RL, Pintor A, Domenici MR, Nazzicone V, Pèzzola A, Reggio R. Modulation of glutamate release and excitotoxicity by adenosine A2A receptors. Neurology. 2003;61:S69–S71. doi: 10.1212/01.wnl.0000095216.89483.a2. [DOI] [PubMed] [Google Scholar]
- Popoli P, Blum D, Domenici MR, Burnouf S, Chern Y. A critical evaluation of adenosine A2A receptors as potentially “druggable” targets in Huntington's disease. Curr Pharm Des. 2008;14:1500–1511. doi: 10.2174/138161208784480117. [DOI] [PubMed] [Google Scholar]
- Saura J, Angulo E, Ejarque A, Casadó V, Tusell JM, Moratalla R, Chen JF, Schwarzschild MA, Lluis C, Franco R, Serratosa J. Adenosine A2A receptor stimulation potentiates nitric oxide release by activated microglia. J Neurochem. 2005;95:919–929. doi: 10.1111/j.1471-4159.2005.03395.x. [DOI] [PubMed] [Google Scholar]
- Schulte G, Fredholm BB. Signalling from adenosine receptors to mitogen-activated protein kinases. Cell Signal. 2003;15:813–827. doi: 10.1016/s0898-6568(03)00058-5. [DOI] [PubMed] [Google Scholar]
- Si QS, Nakamura Y, Schubert P, Rudolphi K, Kataoka K. Adenosine and propentofylline inhibit the proliferation of cultured microglial cells. Exp Neurol. 1996;137:345–349. doi: 10.1006/exnr.1996.0035. [DOI] [PubMed] [Google Scholar]
- Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- Stone TW, Behan WM. Interleukin-1beta but not tumor necrosis factor-alpha potentiates neuronal damage by quinolinic acid: protection by an adenosine A2A receptor antagonist. J Neurosci Res. 2007;85:1077–1085. doi: 10.1002/jnr.21212. [DOI] [PubMed] [Google Scholar]
- Tebano MT, Martire A, Rebola N, Pepponi R, Domenici MR, Grò MC, Schwarzschild MA, Chen JF, Cunha RA, Popoli P. Adenosine A2A receptors and metabotropic glutamate 5 receptors are co-localized and functionally interact in the hippocampus: a possible key mechanism in the modulation of N-methyl-d-aspartate effects. J Neurochem. 2005;95:1188–1200. doi: 10.1111/j.1471-4159.2005.03455.x. [DOI] [PubMed] [Google Scholar]
- Varma MR, Dixon CE, Jackson EK, Peters GW, Melick JA, Griffith RP, Vagni VA, Clark RS, Jenkins LW, Kochanek PM. Administration of adenosine receptor agonists or antagonists after controlled cortical impact in mice: effects on function and histopathology. Brain Res. 2002;951:191–201. doi: 10.1016/s0006-8993(02)03161-x. [DOI] [PubMed] [Google Scholar]
- Yu L, Huang Z, Mariani J, Wang Y, Moskowitz M, Chen JF. Selective inactivation or reconstitution of adenosine A2A receptors in bone marrow cells reveals their significant contribution to the development of ischemic brain injury. Nat Med. 2004;10:1081–1087. doi: 10.1038/nm1103. [DOI] [PubMed] [Google Scholar]
- Yu L, Shen HY, Coelho JE, Araújo IM, Huang QY, Day YJ, Rebola N, Canas PM, Rapp EK, Ferrara J, Taylor D, Müller CE, Linden J, Cunha RA, Chen JF. Adenosine A2A receptor antagonists exert motor and neuroprotective effects by distinct cellular mechanisms. Ann Neurol. 2008;63:338–346. doi: 10.1002/ana.21313. [DOI] [PubMed] [Google Scholar]