Abstract
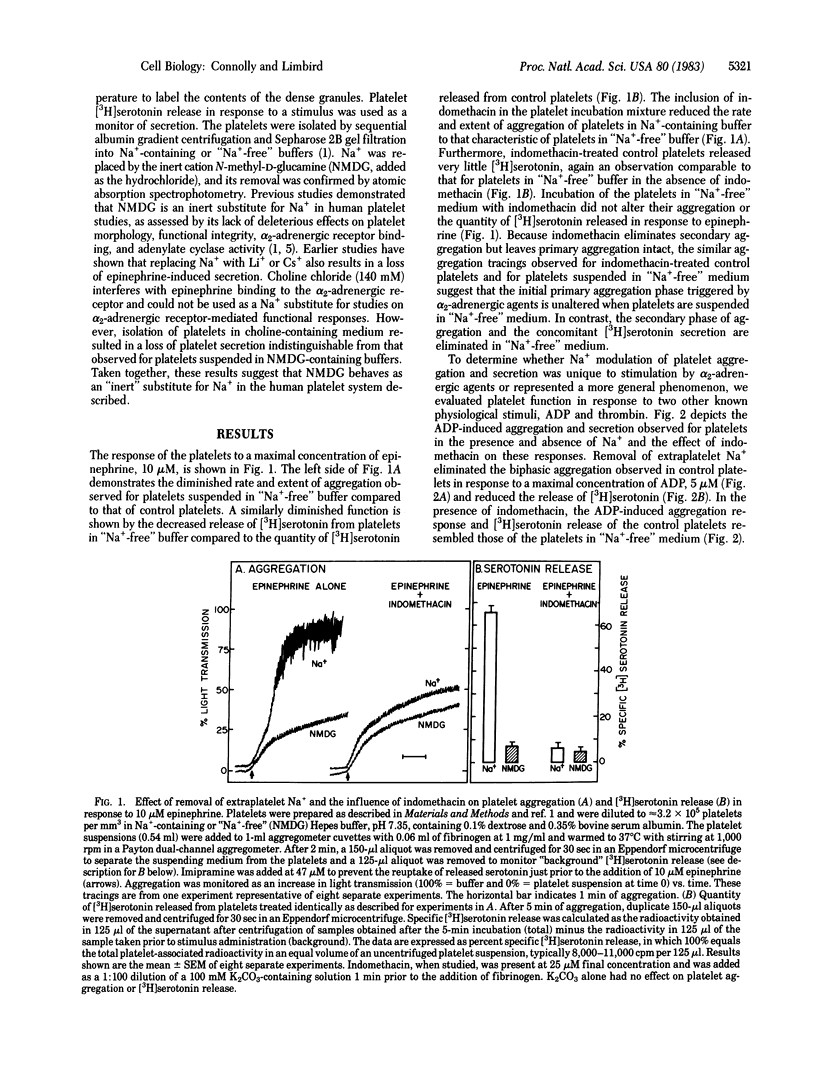
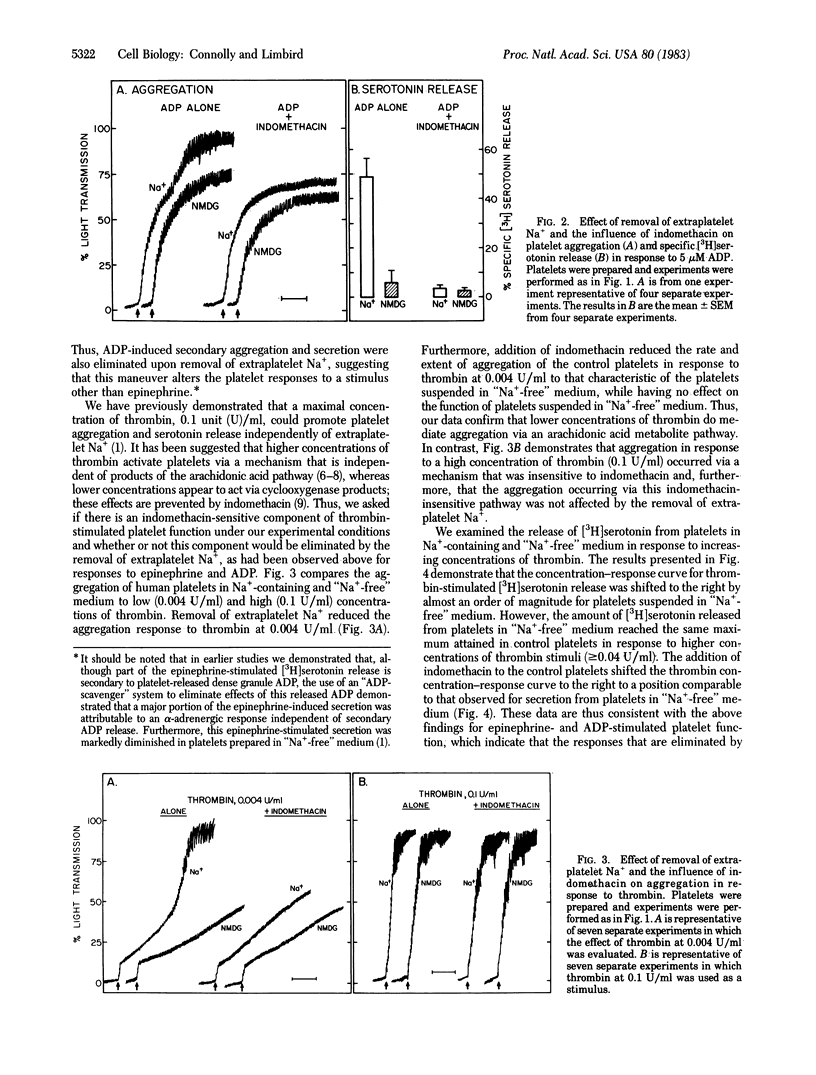
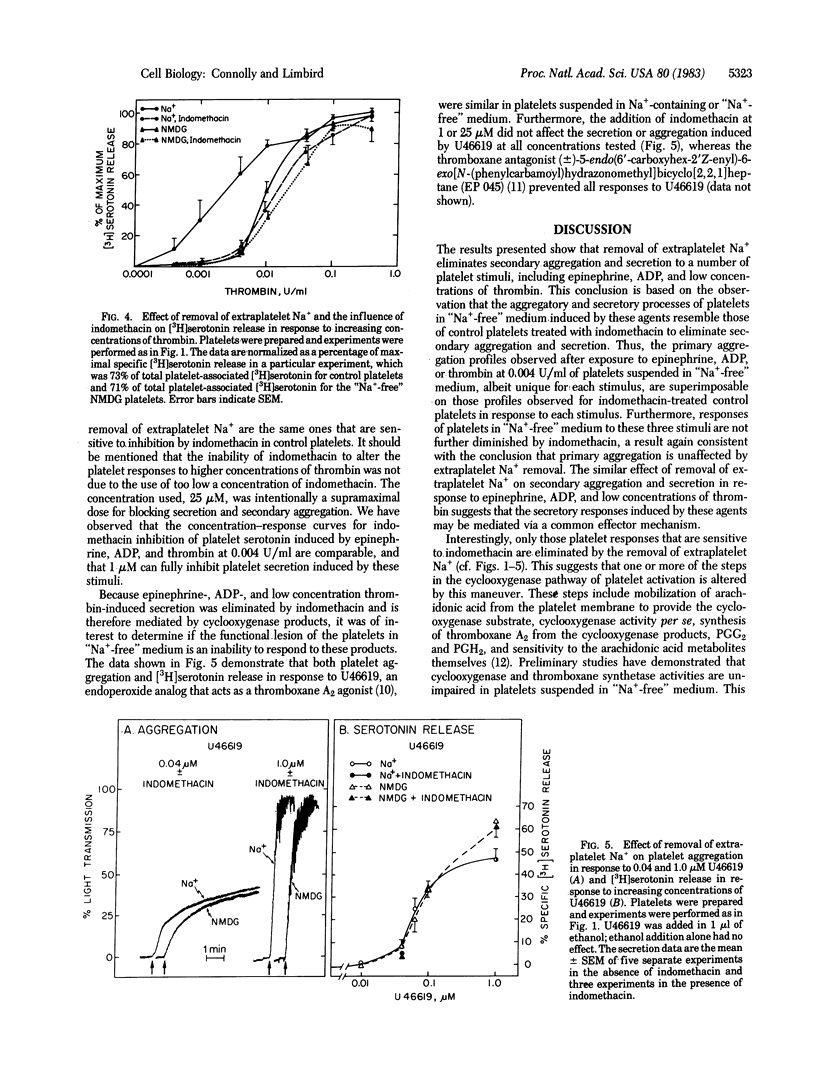
We have previously observed that removal of extraplatelet Na+ markedly diminishes human platelet aggregation and secretion in response to epinephrine. The present studies demonstrate that this effect of the removal of extraplatelet Na+ on platelet function is not unique to activation of platelets by alpha 2-adrenergic agents but represents a phenomenon also evident for other platelet stimuli. Thus, platelet aggregation and secretion in response to maximal concentrations of ADP and lower concentrations of thrombin (less than 0.04 unit/ml) were also markedly reduced in platelets in "Na+-free" medium, suggesting that these agents share an effector mechanism that is similarly inhibited by the removal of extraplatelet Na+. In contrast, platelet aggregation and secretion in response to higher concentrations of thrombin (greater than or equal to 0.04 unit/ml) and to 0.04-1.0 microM (15S)-hydroxy-11 alpha, 9 alpha-(epoxymethano)prosta-5Z,13E-dienoic acid (U46619), an endoperoxide analog, were identical in control platelets and in those suspended in "Na+-free" medium, indicating that platelets suspended in "Na+-free" medium are functionally intact, at least in response to some stimuli. Furthermore, the observation that U46619 can elicit platelet aggregation and secretion independently of extraplatelet Na+ indicates that the loss of platelet responsiveness to epinephrine, ADP, and low concentrations of thrombin cannot be attributed to a loss of sensitivity to the stimulus-provoked secondary mediator(s) of platelet function, endoperoxides or thromboxane A2. Treatment with indomethacin to block the secondary aggregation and secretion pathways of platelets reduced the aggregatory and secretory responses of control platelets induced by epinephrine, ADP, and low concentrations of thrombin to those characteristic of platelets suspended in "Na+-free" medium. In contrast, indomethacin did not alter the functional responses induced by these agents in platelets suspended in "Na+-free" medium, suggesting that "primary" aggregation is intact but that the "secondary" aggregation and secretion mediated by arachidonic acid metabolites are eliminated by removal of extraplatelet Na+. Consistent with this interpretation is the observation that the indomethacin-insensitive aggregation and secretion induced by U46619 and higher concentrations of thrombin were retained in platelets suspended in "Na+-free" medium. Thus, the responses eliminated by removal of extraplatelet Na+ are those eliminated by treating control platelets with indomethacin, suggesting a strong link between the presence of extraplatelet Na+ and the operation of platelet function mediated by the cyclooxygenase pathway.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell R. L., Kennerly D. A., Stanford N., Majerus P. W. Diglyceride lipase: a pathway for arachidonate release from human platelets. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3238–3241. doi: 10.1073/pnas.76.7.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G., Cuatrecasas P. Phospholipase A2 activity specific for phosphatidic acid. A possible mechanism for the production of arachidonic acid in platelets. J Biol Chem. 1981 Jun 10;256(11):5399–5403. [PubMed] [Google Scholar]
- Bills T. K., Smith J. B., Silver M. J. Selective release of archidonic acid from the phospholipids of human platelets in response to thrombin. J Clin Invest. 1977 Jul;60(1):1–6. doi: 10.1172/JCI108745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekman M. J., Ward J. W., Marcus A. J. Phospholipid metabolism in stimulated human platelets. Changes in phosphatidylinositol, phosphatidic acid, and lysophospholipids. J Clin Invest. 1980 Aug;66(2):275–283. doi: 10.1172/JCI109854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly T. M., Limbird L. E. The influence of Na+ on the alpha 2-adrenergic receptor system of human platelets. A method for removal of extraplatelet Na+. Effect of Na+ removal on aggregation, secretion, and cAMP accumulation. J Biol Chem. 1983 Mar 25;258(6):3907–3912. [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam R. J., Davidson M. M., Davies T., Lynham J. A., McClenaghan M. D. Regulation of blood platelet function by cyclic nucleotides. Adv Cyclic Nucleotide Res. 1978;9:533–552. [PubMed] [Google Scholar]
- Holmsen H. Prostaglandin endoperoxide--thromboxane synthesis and dense granule secretion as positive feedback loops in the propagation of platelet responses during "the basic platelet reaction". Thromb Haemost. 1977 Dec 15;38(4):1030–1041. [PubMed] [Google Scholar]
- Johnson J. D., Epel D. Intracellular pH and activation of sea urchin eggs after fertilisation. Nature. 1976 Aug 19;262(5570):661–664. doi: 10.1038/262661a0. [DOI] [PubMed] [Google Scholar]
- Jones R. L., Wilson N. H., Armstrong R. A., Peesapati V., Smith G. M. Effects of thromboxane antagonist EP 045 on platelet aggregation. Adv Prostaglandin Thromboxane Leukot Res. 1983;11:345–350. [PubMed] [Google Scholar]
- Kinlough-Rathbone R. L., Packham M. A., Reimers H. J., Cazenave J. P., Mustard J. F. Mechanisms of platelet shape change, aggregation, and release induced by collagen, thrombin, or A23,187. J Lab Clin Med. 1977 Oct;90(4):707–719. [PubMed] [Google Scholar]
- Knapp H. R., Oates J. A. Monovalent cations and renomedullary prostaglandin release. Adv Prostaglandin Thromboxane Res. 1980;7:1027–1032. [PubMed] [Google Scholar]
- Korchak H. M., Weissmann G. Stimulus-response coupling in the human neutrophil. Transmembrane potential and the role of extracellular Na+. Biochim Biophys Acta. 1980 Sep 2;601(1):180–194. doi: 10.1016/0005-2736(80)90523-4. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Chandrabose K. A., Cuatrecasas P. Ionophore A-23187- and thrombin-induced platelet aggregation: independence from cycloxygenase products. Proc Natl Acad Sci U S A. 1978 Feb;75(2):818–822. doi: 10.1073/pnas.75.2.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbird L. E., Speck J. L., Smith S. K. Sodium ion modulates agonist and antagonist interactions with the human platelet alpha 2-adrenergic receptor in membrane and solubilized preparations. Mol Pharmacol. 1982 May;21(3):609–617. [PubMed] [Google Scholar]
- Marcus A. J. The role of lipids in platelet function: with particular reference to the arachidonic acid pathway. J Lipid Res. 1978 Sep;19(7):793–826. [PubMed] [Google Scholar]
- Neufeld E. J., Majerus P. W. Arachidonate release and phosphatidic acid turnover in stimulated human platelets. J Biol Chem. 1983 Feb 25;258(4):2461–2467. [PubMed] [Google Scholar]
- Packham M. A., Guccione M. A., Greenberg J. P., Kinlough-Rathbone R. L., Mustard J. F. Release of 14C-serotonin during initial platelet changes induced by thrombin, collagen, or A23187. Blood. 1977 Nov;50(5):915–926. [PubMed] [Google Scholar]
- Prescott S. M., Majerus P. W. Characterization of 1,2-diacylglycerol hydrolysis in human platelets. Demonstration of an arachidonoyl-monoacylglycerol intermediate. J Biol Chem. 1983 Jan 25;258(2):764–769. [PubMed] [Google Scholar]
- Reeves J. P., Sutko J. L. Sodium-calcium ion exchange in cardiac membrane vesicles. Proc Natl Acad Sci U S A. 1979 Feb;76(2):590–594. doi: 10.1073/pnas.76.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse-Simmons S. Production of diglyceride from phosphatidylinositol in activated human platelets. J Clin Invest. 1979 Apr;63(4):580–587. doi: 10.1172/JCI109339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel E. G., Wollheim C. B., Renold A. E., Sharp G. W. Evidence for the involvement of Na/Ca exchange in glucose-induced insulin release from rat pancreatic islets. J Clin Invest. 1980 Nov;66(5):996–1003. doi: 10.1172/JCI109969. [DOI] [PMC free article] [PubMed] [Google Scholar]


