Abstract
Short-term changes in synaptic gain support information processing throughout the CNS, yet we know little about the developmental regulation of such plasticity. Here we report that auditory experience is necessary for the normal maturation of synaptic inhibitory short-term plasticity (iSTP) in the auditory cortex, and that presynaptic GABAB receptors regulate this development. Moderate or severe hearing loss was induced in gerbils, and iSTP was characterized by measuring inhibitory synaptic current amplitudes in response to repetitive stimuli. We reveal a profound developmental shift of iSTP from depressing to facilitating after the onset of hearing. Even moderate hearing loss prevented this shift. This iSTP change was mediated by a specific class of inhibitory interneurons, the low-threshold spiking cells. Further, using paired recordings, we reveal that presynaptic GABAB receptors at interneuron-pyramidal connections regulate iSTP in an experience-dependent manner. This novel synaptic mechanism may support the emergence of mature temporal processing in the auditory cortex.
Introduction
Early experience coordinates the maturation of synaptic function, thereby establishing adult perceptual skills. Thus, hearing loss may lead to persistent deficits in behavioral performance, including temporal processing and language acquisition (Roberts et al., 2004; Halliday and Bishop, 2006; Moeller et al., 2007). At a mechanistic level, dynamic changes in the strength of synapses, known as short-term plasticity, contribute to temporal processing (Cook et al., 2003). Here, we examined synaptic inhibitory short-term plasticity (iSTP) in the developing auditory cortex (ACx), and show that it is regulated in an experience-dependent manner by presynaptic GABAB receptors.
In vivo studies show that the net synaptic input and spiking responses in the ACx are modified during repetitive sound stimuli separated by tens to hundreds of milliseconds (Calford and Semple, 1995; Lu et al., 2001; Wehr and Zador, 2005; Ter-Mikaelian et al., 2007). Such temporal coding properties are vulnerable to the acoustic rearing environment, including hearing loss (Chang et al., 2005; Aizawa and Eggermont, 2006, 2007), possibly via the disruption of synaptic short-term plasticity. In fact, both moderate and profound hearing loss change the excitatory short-term plasticity in the ACx (Xu et al., 2007).
Inhibitory synapses are particularly susceptible to developmental perturbations. Diminished hearing experience during early life produces widespread changes in inhibition from the brainstem to cortex (Kotak and Sanes, 1996; Vale and Sanes, 2000, 2002; Vale et al., 2003; Kotak et al., 2005). For example, the maturation of GABAA receptor-mediated currents in ACx is arrested by disuse. Developmental hearing loss also alters presynaptic inhibitory properties, as evidenced by an increased frequency of spontaneous currents and increased glutamic acid decarboxylase immunoreactivity (Kotak et al., 2008; Sarro et al., 2008). Given these alterations, we predicted that iSTP would also be disrupted by developmental hearing loss.
Inhibitory synapses display distinct properties, including different forms of iSTP, depending upon the interneuron subtype (Gibson et al., 1999; Gupta et al., 2000; Beierlein et al., 2003). These dynamic properties may be controlled by presynaptic GABAB receptors, which modulate neurotransmitter release by inhibiting voltage-sensitive Ca2+ channels or vesicle priming (Mintz and Bean, 1993; Sakaba and Neher, 2003). In paired-pulse studies, GABA released from the first pulse activates GABAB autoreceptors, which inhibit release on the subsequent pulse. Therefore, these receptors are associated with STP at various inhibitory synapses (Deisz and Prince, 1989; Davies and Collingridge, 1993; Fukuda et al., 1993; Mott et al., 1993; Pearce et al., 1995; Poncer et al., 2000; Kirmse and Kirischuk, 2006). Despite the primacy of GABAB receptors to synaptic transmission, it is unknown whether their development depends on experience.
To examine the experience-dependent development of iSTP, we used single and paired whole-cell recordings in a thalamocortical slice preparation. First, we demonstrate that iSTP undergoes a developmental shift from depression toward facilitation. Second, we show that this shift is disrupted by conductive or sensorineural hearing loss. Third, we determine the specific subset of interneurons that mediates this experience-dependent iSTP. Finally, we identify the presynaptic GABAB receptor as a novel mechanism that regulates GABA release in an experience-dependent manner.
Materials and Methods
Experimental animals.
Gerbils (Meriones unguiculatus) aged postnatal day (P) 8–30 born from breeding pairs (Charles River) were used. This age corresponds to the period during which the auditory system matures (Woolf and Ryan, 1984, 1985; Sanes and Rubel, 1988). Animal care, maintenance and surgeries were in accordance with the guidelines and rules of the Institutional Animal Care and Use Committee, New York University, approved by the Office of Laboratory Animal Welfare, Office of Extramural Research, U.S. National Institutes of Health (Bethesda, MD). The data are drawn from voltage-clamp recordings of 121 pyramidal cells from 60 animals, 368 simultaneous interneuron-pyramidal recordings from 108 animals, and 18 calcium current recordings from 15 animals.
Sensorineural hearing loss and conductive hearing loss.
Both sensorineural hearing loss (SNHL) and conductive hearing loss (CHL) surgeries were performed on gerbils aged P10 when anteroventral cochlear nucleus cell number is unaffected by cochlear ablation (Tierney and Moore, 1997). SNHL was induced using procedures similar to those described previously (Vale and Sanes, 2002). Gerbils were anesthetized with halogenated ethyl methyl ether methoxyflurane (Metofane), an incision was made in the skin over the bulla, and a small hole was made in the cochlear wall. The contents were rapidly cleared with a fine forceps, gel foam was placed in the vacant cochlear cavity, and the wound was closed. The process was repeated for the other ear. CHL was induced by tympanic membrane puncture and malleus extirpation (Xu et al., 2007). The postauricular wound was closed and the procedure repeated on the other side. The success of each surgery was confirmed anatomically after preparing the brain slice.
Brain slice preparation.
Thalamocortical brain slices (500 μm) were generated from gerbils aged P8–30 as described previously (Kotak et al., 2005). The brain was sectioned perihorizontally to preserve the ventral medial geniculate (MGv) and its ascending pathways to the auditory cortex (ACx; Cruikshank et al., 2002). The slices were incubated in artificial CSF (ACSF) at 32°C for 30 min, then at room temperature for 60 min, and transferred to a recording chamber continuously superfused (3 ml/min) with ACSF at 32°C. The ACSF contained (in mm): 125 NaCl, 4 KCl, 1.2 KH2PO4, 1.3 MgSO4, 24 NaHCO3, 15 glucose, 2.4 CaCl2, and 0.4 l-ascorbic acid (pH 7.3 when bubbled with 95% O2/5% CO2). Before whole-cell recordings, the ACx was identified by extracellular field activity recorded in response to MGv stimulation.
Whole-cell recordings for extracellular stimulation.
Whole-cell recordings (PC-501A; Warner Instruments) were obtained from pyramidal neurons in cortical layers (L) 2/3. Recording electrodes were fabricated from borosilicate glass microcapillaries (outer diameter = 1.5 mm) with a micropipette puller (model P-97; Sutter Instruments). The internal solution contained (in mm): 100 KCl, 40 K-gluconate, 8 NaCl, 10 HEPES, 2 MgCl2, 0.1 EGTA, 2 ATP, 0.3 GTP, and 5 lidocaine derivative QX-314 (pH = 7.2 with KOH). The tip resistance of the patch electrode filled with internal solution was 5–10 MΩ. Access resistances were 15–30 MΩ and were compensated by ∼70%. Neurons visually identified as pyramidal-shaped cell bodies under infrared differential interface contrast (IR-DIC) were selected. It is not possible to characterize cells based on discharge properties because intracellular QX-314 blocks sodium channels. However, after breaking into the cell, it was possible to immediately obtain a resting potential (<50 mV) and an overshooting action potential. Recordings were then made using voltage-clamp (VHOLD = −60 mV). Inhibitory postsynaptic currents (IPSCs) were recorded in the presence of ionotropic glutamate receptor blockers, 6,7-dinitroquinoxaline-2,3-dione (DNQX; 20 μm; Sigma) and 2-amino-5-phosphonopentanoic acid (AP-5; 50 μm; Tocris Bioscience), added to the superfusing ACSF. Presynaptic GABAB receptors were blocked with (2S)-(+)5,5-dimethyl-2-morpholineacetic acid (SCH-50911; 10 μm, Tocris Bioscience). The drugs were applied for 15 min before recording IPSCs. Synaptic responses were elicited with electrical stimuli delivered via a stimulus isolator (model BSI-9501; Dagan) to a bipolar stimulating electrode placed on L4. The stimulating electrodes were fabricated from 0.004-inch-diameter Teflon-coated platinum wires (A-M Systems) inserted into a 2-inch-long double-barrel glass electrode. The exposed tip was 0.002 inches in diameter. All stimuli were 100 μs in duration. To determine the stimulus magnitude, incremental stimulus intensities were delivered at 0.05 Hz until an evoked IPSC was discernible from failures (Kotak et al., 2005). A stimulus magnitude of 20% above this threshold was chosen to avoid failures. To assess inhibitory short-term plasticity, 10 sweeps were acquired for every stimulus parameter.
Paired recordings.
Whole-cell current-clamp recordings in interneurons were paired with voltage-clamp recordings in L2/3 pyramidal cells. Interneuron recordings were performed at the layer 4 and 2/3 border. This strategy permitted simultaneous recording of the action potential elicited in the presynaptic interneuron and the IPSC in the postsynaptic neuron. The current-clamp internal solution contained (in mm): 5 KCl, 127.5 K-gluconate, 10 HEPES, 2 MgCl2, 0.6 EGTA, 2 ATP, 0.3 GTP, and 5 phosphocreatine (pH = 7.2 with KOH).
Fast-spiking (FS) and low-threshold spiking (LTS) interneurons were targeted based on the soma shape under IR-DIC and identified by their discharge pattern in response to current injections (1500 ms). FS cells were targeted visually by their morphology and distinguished physiologically by their characteristic narrow spike, deep afterhyperpolarization (AHP), and high discharge (Xiang et al., 1998; Gibson et al., 1999; Beierlein et al., 2000; Amitai et al., 2002; Rose and Metherate, 2005; for review, see Markram et al., 2004). The FS basket cell anatomy was confirmed in a subset of recorded neurons (data not shown; for review, see Markram et al., 2004). LTS cells were targeted visually by a distinct elongated cell body. These cells were distinguished physiologically from excitatory regular-spiking cells by comparing the change in AHP during a depolarizing train. Beierlein et al. (2003) showed that the AHP after the first spike was more hyperpolarized than the AHP after the final spike in all LTS cells. Conversely, the first AHP was more depolarized in all excitatory cells. LTS cells were distinguished from FS cells by their broader spike half-widths, decreased AHP amplitudes, and pronounced spike adaptation (Beierlein et al., 2000, 2003; Wang et al., 2004). The LTS anatomy, including an ovoid-shaped cell body and vertically oriented, bitufted dendritic morphology was confirmed in a subset of recorded neurons (data not shown; Peters et al., 1983; Reyes et al., 1998; Wang et al., 2004; for review, see Markram et al., 2004).
Voltage-clamp recordings were performed in nearby L2/3 pyramidal cells (intersomatic distance <100 μm). The voltage-clamp internal solution was the same as that used for recordings from individual pyramidal neurons (see above). Data were acquired from pyramidal cells with a resting potential less than −50 mV and an overshooting action potential evoked immediately after cell membrane rupture. IPSCs (VHOLD = −60 mV) in the pyramidal cells were recorded in response to spikes evoked by depolarizing currents steps (5 ms) in the interneuron. IPSCs were recorded in the presence of ionotropic glutamate receptor blockers (AP-5, 50 μm; DNQX, 20 μm). For GABAB experiments, the antagonist (SCH-50911, 10 μm) was also added to the ACSF.
Measure of paired pulse ratio.
Initially, two stimulus pulses were used to examine the paired-pulse ratio (PPR), an assay for iSTP. To examine PPR in dual recordings, the interneuron was stimulated with two current injections (5 ms) to evoke two single spikes, and IPSCs were recorded in the pyramidal cell. Here, PPR is defined as the ratio between the average IPSC amplitude evoked by the second stimulus pulse divided by the average IPSC amplitude evoked by the first stimulus pulse (IPSC2/IPSC1). If the ratio is >1, it represents facilitation, while if the ratio is <1, it represents depression. A subset of the cells used for paired-pulse protocols was also used to assess short-term plasticity during trains of 10 stimuli. For IPSC trains, the average IPSC amplitude evoked by the nth stimulus was divided by the average IPSC amplitude evoked by the first stimulus (IPSCN/IPSC1). IPSC amplitude was measured from baseline just before the response (averaged 1 ms before stimulus). This strategy eliminated the contribution of temporal summation of IPSCs.
Isolated calcium currents in LTS cells.
LTS interneurons were targeted based on the soma shape under IR-DIC and identified by their discharge patterns to a current injection (300 pA, 1500 ms) immediately after cell membrane rupture. Data were only obtained from distinguishable LTS cells (see above; examples shown in supplemental Fig. 1, available at www.jneurosci.org as supplemental material). The internal solution contained (in mm): 127.5 cesium gluconate, 5 KCl, 10 HEPES, 2 MgCl2, 0.6 EGTA, 2 ATP, 0.3 GTP, and 5 phosphocreatine (pH = 7.2 with CsOH). The cells were patched in the same external solution as described above. After physiologically identifying an LTS cell, the external solution was supplemented with channel blockers (in mm): 30 tetraethylammonium-chloride (TEA) and 0.001 tetrodotoxin (TTX). Under voltage clamp (VHOLD = −80 mV), voltage steps were injected (200 ms; from −100 to +20 mV). The resulting inward calcium currents were initiated several milliseconds following the onset of the voltage step, possibly due to an inadequate space clamp. However, the sole purpose of the experiment was to determine the relative effect of GABAB receptor activation in control and hearing loss neurons. The voltage step that elicited the greatest current (between −30 and 0 mV) was used to evaluate the effect of the GABAB receptor agonist, baclofen (100 μm, Tocris Bioscience). After obtaining 5 baseline measures of the evoked calcium current elicited at 30 s intervals, baclofen was applied directly in the recording chamber. After 30 s, 4 calcium currents were recorded at 30 s intervals. Calcium currents were then recorded every 30 s during washout of baclofen, which required several minutes. In some cells, the effects of baclofen were immediately reversed by application of the GABAB receptor antagonist, SCH-50911 (100 μm; data not shown). Calcium current amplitudes were leak-subtracted, based on the leak current evoked by a hyperpolarizing voltage step (−90 mV).
Data acquisition and analysis.
Data were acquired at a sampling rate of 10 kHz using a custom-designed IGOR (version 4.08; WaveMetrics) macro on a Macintosh platform (Apple Computers). A second IGOR macro was used for offline analysis. Any IPSC summated with a spontaneous IPSC was excluded. All data are reported as mean ± SEM. Statistical tests (ANOVA or Student's t test for data distributed normally; Kruskal–Wallis or Wilcoxon's nonparametric tests for data not distributed normally) were performed using statistical software (JMP, SAS Institute). Traces shown are averages of 8–10 IPSCs from single neurons. Stimulus artifacts were decreased.
Biocytin labeling.
To identify recorded neurons, 0.5% biocytin (Sigma) was added to the internal pipette solution. Following recordings, the brain slice was fixed overnight in 4% paraformaldehyde, rinsed in phosphate buffered solution and incubated in 1% H2O2. The slice was preincubated in 0.5% Triton X-100 for 2 h and incubated in ABC-HRP (Vector Laboratories) for 12–18 h. The tissue was reacted in a diaminobenzidine solution with H2O2.
Results
Inhibitory short-term plasticity is modified during postnatal development
To assess developmental characteristics of iSTP, inhibitory postsynaptic currents (IPSCs) were evaluated in the auditory cortex of gerbils aged P8–30. IPSCs recorded in layer 2/3 pyramidal cells were evoked by extracellular stimuli delivered to layer 4. For all neurons, the stimulus intensity was chosen to be 20% above the threshold current (see Materials and Methods).
iSTP was characterized with a paired-pulse paradigm. Figure 1A shows that inhibitory synapses displayed an age-dependent change in the paired-pulse ratio (PPR = IPSC2/IPSC1) for a range of interstimulus intervals (ISI). IPSCs displayed paired-pulse depression (PPD) before the onset of hearing (P8–11) at ISIs from 40 to 2000 ms. However, IPSCs from post-hearing animals (P17–22) matured such that PPD was nearly eliminated and some paired-pulse facilitation (PPF) emerged. PPR remained stable thereafter (P25–30). This change in PPR after hearing onset was significant at intervals ranging from 40 to 500 ms. For example, the PPR at an ISI of 80 ms nearly doubled from pre- to post-hearing animals (pre-hearing: 0.77 ± 0.18, n = 14; post-hearing: 1.39 ± 0.05, n = 23; χ2 = 9.23, p = 0.002). For all groups, no iSTP was observed at an ISI of 3000 ms. These data suggest that the age-dependent change in iSTP is relevant for a range of temporal inputs.
Figure 1.
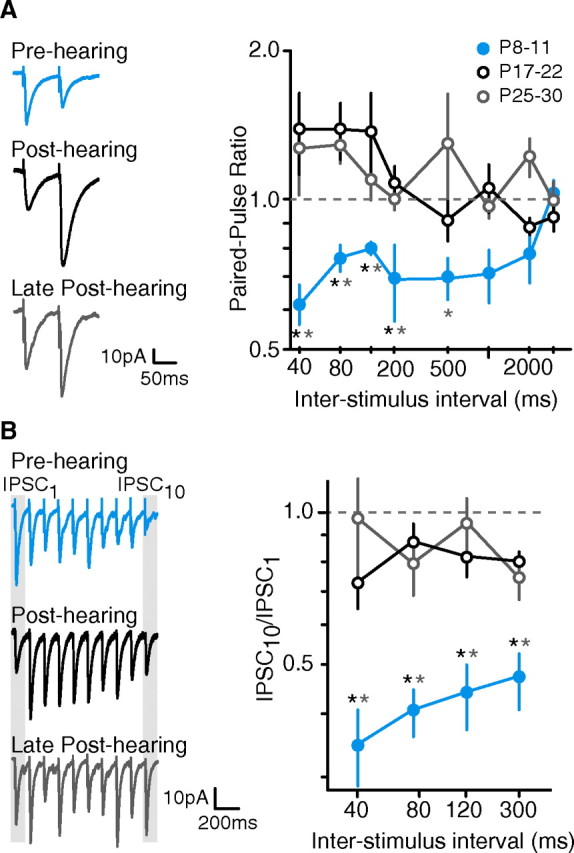
iSTP matures after hearing onset. A, PPR shifts away from depression. Left, Representative IPSCs from a pre-hearing neuron (P10), a post-hearing neuron (P17), and a late post-hearing neuron (P27). IPSCs were evoked in cortical L2/3 pyramidal cells by extracellular stimuli applied to L4 (ISI = 80 ms). Right, Average PPR (means ± SEM) of IPSCs at ISIs ranging from 40 to 3000 ms for neurons recorded from pre-hearing (P8–11), early post-hearing (P17–22), and late post-hearing (P25–30) animals. Pre-hearing neurons displayed depression at ISIs up to 2000 ms while post-hearing neurons did not. Black asterisk indicates p < 0.05 for pre-hearing versus post-hearing; gray asterisk indicates p < 0.05 for pre-hearing versus late post-hearing (number of neurons sampled at ISIs of 40–120, 200–3000: pre-hearing, 14–18, 7–8; early post-hearing, 23–26, 7–14; late post-hearing, 12–15, 8–9). B, iSTP maturation is revealed by stimulus trains. Left, Representative IPSCs from pre-hearing (P9), early post-hearing (P17), and late post-hearing (P27) neurons during a train of 10 stimuli. Right, Average ISPC10/ISPC1 values (means ± SEM) at ISIs from 40 to 300 ms are shown for neurons recorded from pre-hearing, early post-hearing, and late post-hearing animals. IPSCs from pre-hearing neurons are robustly depressed by the end of the 10 pulse train; post-hearing and late post-hearing neurons show significantly less depression. Black asterisk indicates p < 0.05 for pre-hearing versus post-hearing; gray asterisk indicates p < 0.05 for pre-hearing versus late post-hearing (number of neurons sampled: pre-hearing, 6–12; early post-hearing, 8–13; late post-hearing, 13).
To confirm that age-dependent emergence of iSTP was relevant to prolonged stimulation, trains of 10 stimuli were delivered at ISIs of 40–300 ms (Fig. 1B). Here, iSTP was characterized as the ratio of the IPSC amplitude evoked by the tenth pulse to the IPSC amplitude evoked by the first pulse (IPSC10/IPSC1). Pre-hearing neurons displayed robust depression by the end of the train at each ISI tested. In contrast, both post-hearing and late post-hearing neurons displayed significantly less depression. Together, these results show that mature iSTP properties emerge only after the onset of hearing.
Both moderate and severe hearing loss disrupt the maturation of iSTP
Since iSTP shifted from depression to facilitation soon after hearing onset, we asked whether auditory experience was necessary. To test this, two forms of developmental hearing loss were examined: conductive hearing loss (CHL) and sensorineural hearing loss (SNHL). CHL was induced by bilateral removal of a middle ear bone, the malleus. CHL leads to higher thresholds, attenuating sound by 34–46 dB (Xu et al., 2007). SNHL was induced by bilateral cochlear ablation, and results in complete deafness (Vale and Sanes, 2002; Kotak et al., 2005). Both surgeries were performed at P10, and the animals were compared with post-hearing age-matched controls at P17–22. Therefore, the manipulated pups were either partially or completely deprived of auditory experience while control animals received normal sound exposure.
Figure 2A shows the average PPR for neurons from control, CHL, and SNHL animals at ISIs ranging from 40 to 3000 ms. IPSCs from both CHL and SNHL animals displayed a significantly reduced PPR compared with post-hearing controls at ISIs from 40 to 500 ms. For example, the PPR at an ISI of 80 ms for post-hearing animals was nearly double that of age-matched CHL and SNHL animals (post-hearing: 1.39 ± 0.05, n = 23; CHL: 0.77 ± 0.06, n = 10; SNHL: 0.71 ± 0.05, n = 27; ANOVA, F = 9.68, p = 0.0002; post-hearing vs CHL, χ2 = 7.96, p = 0.005; post-hearing vs SNHL, t = 3.90, p = 0.0003). This effect persisted across multiple stimuli, with CHL and SNHL neurons showing consistent depression by the end of a 10-pulse train (Fig. 2B). Therefore, CHL and SNHL neurons displayed PPD that was similar to that observed in pre-hearing neurons, suggesting that the maturation of iSTP depends on auditory experience.
Figure 2.
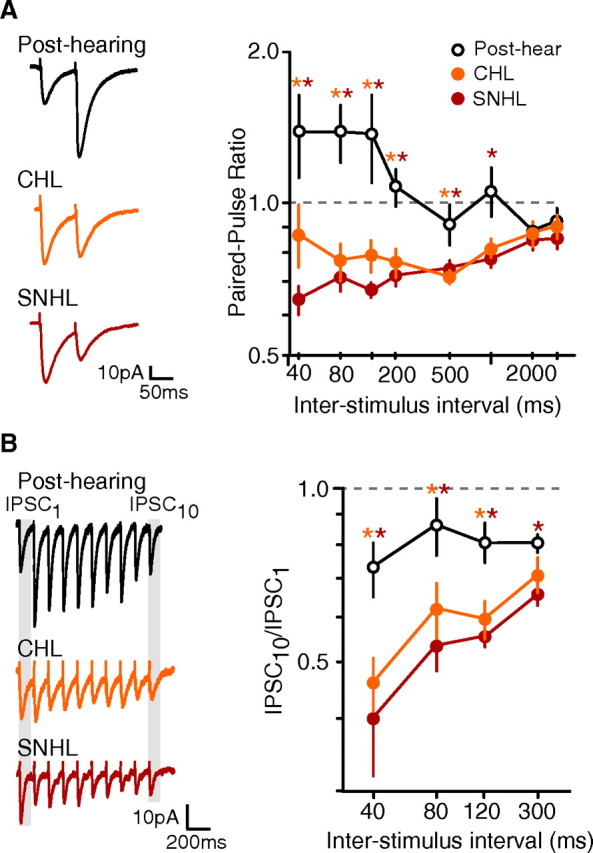
Maturation of iSTP depends on auditory experience. A, Hearing loss leads to a decrease in PPR. Left, Representative IPSCs from a control post-hearing (P19), a CHL (P21), and a SNHL neuron (P19). IPSCs were evoked by paired extracellular stimuli applied to L4 and recorded in cortical L2/3 pyramidal cells (ISI = 80 ms). Right, Average PPR (means ± SEM) at ISIs ranging from 40 to 3000 ms for post-hearing (P17–22), CHL (P17–22), and SNHL (P17–22) neurons. SNHL and CHL neurons displayed depression at a range of ISIs. Orange asterisk indicates p < 0.05 for post-hearing versus CHL; red asterisk indicates p < 0.05 for post-hearing versus SNHL (number of neurons sampled at ISIs of 40–120, 200–3000: post-hearing, 23–26, 7–14; CHL, 8–12, 6–9; SNHL, 27–42, 12–19). B, Disrupted iSTP is revealed by stimulus trains. Left, Representative IPSCs from post-hearing (P17), CHL (P20), and SNHL (P18) neurons during a train of 10 stimuli. Right, Average ISPC10/ISPC1 (means ± SEM) at ISIs from 40 to 300 ms for neurons recorded from post-hearing, CHL, and SNHL neurons. IPSCs from SNHL and CHL neurons were depressed by the end of the 10 pulse train; post-hearing animals displayed significantly less depression. Orange asterisk indicates p < 0.05 for post-hearing versus CHL; red asterisk indicates p < 0.05 for post-hearing versus SNHL (number of neurons: post-hearing, 8–13; CHL, 6–11; SNHL, 8–12).
Experience-dependent reduction of presynaptic GABAB receptor function
Presynaptic GABAB receptors modulate the release of transmitters, both at excitatory as well as inhibitory synaptic terminals. Therefore, we asked whether GABAB receptors localized on inhibitory presynaptic terminals were responsible for the alteration of iSTP. Postsynaptic GABAB receptors are blocked with QX-314 in the recording pipette (Nathan et al., 1990; Andrade, 1991; Deisz et al., 1997), permitting for the selective assessment of presynaptic GABAB receptor function with the antagonist, SCH-50911 (10 μm).
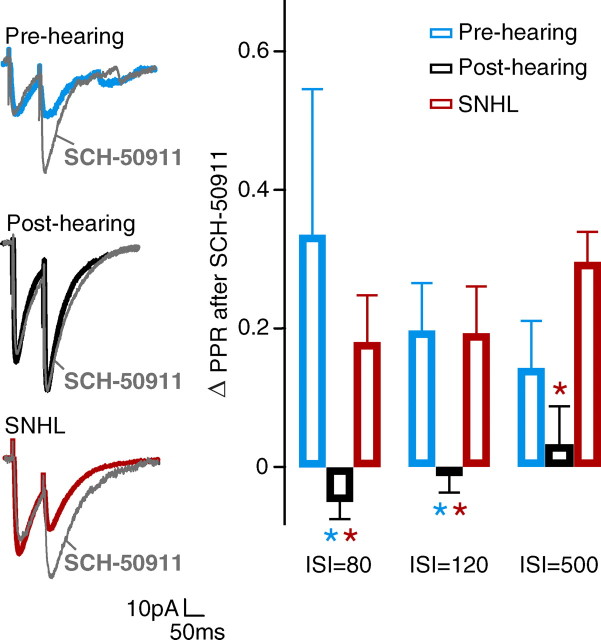
To assess whether presynaptic GABAB receptor signaling emerges during development, the effect of SCH-50911 was compared in neurons from pre-hearing and post-hearing animals. The magnitude of change induced by the drug was calculated as the change in PPR value (ΔPPR = PPRSCH50911 − PPRPRE-DRUG). In pre-hearing neurons, the GABAB receptor antagonist increased the PPR value, even reversing the depression in some cells (Fig. 3). However, in post-hearing neurons, SCH-50911 had no effect. Thus, there was a significant difference in ΔPPR between pre-hearing and post-hearing neurons (at ISI = 80 ms, ΔPPR pre-hearing: 0.33 ± 0.21, n = 6; ΔPPR post-hearing: −0.05 ± 0.03, n = 8; χ2 = 4.82, p = 0.03). This result suggests that presynaptic GABAB receptor function declines following the onset of hearing.
Figure 3.
Experience-dependent reduction of presynaptic GABAB receptors. Left, Effects of the GABAB receptor antagonist, SCH-50911 (10 μm), on IPSCs from representative pre-hearing (P9), post-hearing (P18), and SNHL (P18) neurons. PPR of IPSCs is increased by SCH-50911 in both the pre-hearing and SNHL neurons, but not in the post-hearing neuron. Gray traces were obtained after application of SCH-50911. Right, Bar graph showing the average effect of SCH-50911 on neurons recorded from pre-hearing, post-hearing, and SNHL neurons. The magnitude of the drug effect is represented as ΔPPR (PPRSCH50911 − PPRPRE-DRUG) (means ± SEM). This effect was greater in pre-hearing and SNHL animals compared with post-hearing at ISIs of 80, 120, and 500 ms. Blue asterisk indicates p < 0.05 for pre-hearing versus post-hearing; red asterisk indicates p < 0.05 for SNHL versus post-hearing (number of neurons: pre-hearing, 5–6; post-hearing, 6–9; SNHL, 5–12).
To determine whether the development of presynaptic GABAB receptor signaling depends on auditory experience, the effect of SCH-50911 was assessed following SNHL. In neurons from SNHL animals (P17–22), SCH-50911 produced a significant increase in PPR values, similar to that in neurons from pre-hearing animals (at ISI = 80 ms, ΔPPR post-hearing: −0.05 ± 0.03, n = 8; ΔPPR SNHL: 0.17 ± 0.07, n = 12; χ2 = 5.72, p = 0.02). The increased PPR observed in pre-hearing and SNHL neurons occurred at ISIs that are consistent with those reported to produce a GABAB antagonist effect (Caillard et al., 1998; Poncer et al., 2000). These results suggest that the experience-dependent maturation of iSTP depends, in part, on presynaptic GABAB receptors.
Experience-dependent development of iSTP occurs at LTS-pyramidal connections
The results described above reveal a global change in the STP of inhibitory inputs to cortical pyramidal cells during development and following hearing loss. However, the presynaptic population of neurons that is activated by extracellular stimulation is unknown, and this limits interpretation of the results. For example, with extracellular stimulation, we do not know whether the same afferents are recruited to spike in response to all stimuli in a train. Variability or adaptation in spiking during trains may lead to a nonsynaptic basis for STP changes. Moreover, it is not possible to know which types of interneurons are evoked to spike during extracellular stimulation. The sensory cortex is composed of a variety of interneuron subtypes that have distinct anatomical and physiological properties, and may serve disparate roles in the cortical network. Therefore, it was necessary to determine whether a specific inhibitory circuit mediated the experience-dependent changes in iSTP. To address these issues, paired recordings were obtained between connected interneurons and pyramidal cells.
This study focused on the IPSCs evoked from two distinct classes of interneurons: fast-spiking (FS) and low-threshold spiking (LTS) cells. These cells were initially identified by the shape of their cell bodies observed under IR-DIC optics. Subsequently, each interneuron was characterized by its passive and firing properties in response to depolarizing and hyperpolarizing current pulses (1500 ms; see Materials and Methods) (Fig. 4A). Single action potentials were then elicited in the interneuron by a brief (5 ms) depolarizing current injection while an IPSC was recorded in a nearby pyramidal cell (VHOLD = −60 mV).
Figure 4.
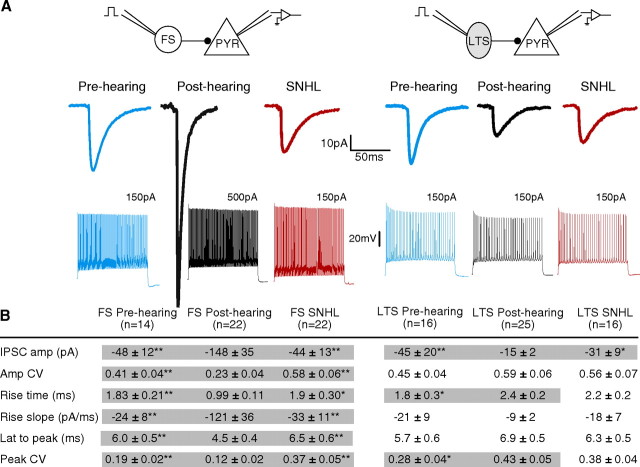
Experience-dependent development of IPSCs evoked by FS and LTS interneurons. A, IPSCs at FS- and LTS-pyramidal connections from pre-hearing, post-hearing, and SNHL animals. IPSCs were evoked by FS or LTS interneuron spikes and recorded in pyramidal cells (VHOLD = −60 mV). Representative spiking responses of FS and LTS interneurons to current injection (1500 ms) are shown below. FS interneurons displayed characteristic tonic spiking and LTS interneurons showed characteristic spike adaptation in response to suprathreshold current injection. Amplitude of current injection is indicated above traces. B, Table of synaptic properties of FS- and LTS-pyramidal connections from pre-hearing (P8–12), post-hearing (P17–22), and SNHL animals (P17–22) (means ± SEM). FS-evoked IPSC amplitudes showed a robust increase in amplitude and decrease in amplitude variance during development, but this was prevented by SNHL. In addition, SNHL FS-evoked IPSCs showed immature kinetics compared with post-hearing controls, including a significantly smaller rising slope, a longer rise time and latency to peak, and larger variance of the latency to peak. Conversely, at LTS-pyramidal connections, IPSC amplitudes significantly decreased during development, but not in SNHL animals. *p < 0.05, **p < 0.01. IPSC amp, IPSC amplitude; Amp CV, amplitude coefficient of variation; Rise time, IPSC 20–80% rising time; Rise slope, IPSC 20–80% rising slope; Lat to peak, latency from spike to IPSC peak; Peak CV, latency to peak coefficient of variation. The numbers of connections sampled (n) are indicated in the table.
A total of 368 interneuron-pyramidal cell simultaneous recordings were tested, yielding 115 connections. For FS interneurons, the probabilities of connection were 33% for pre-hearing animals, 55% for post-hearing animals and 42% for SNHL animals. For LTS interneurons, the probabilities of connection were 21% for pre-hearing animals, 31% for post-hearing animals and 21% for SNHL animals.
We have previously shown that SNHL arrests the development of synaptic inhibition (Kotak et al., 2008). Therefore, we asked whether experience-dependent regulation of IPSCs occurred at both FS- and LTS-pyramidal connections. To test this, single IPSCs from pre-hearing (P8–12), post-hearing (P17–22), and SNHL (P17–22) animals were compared. FS-evoked IPSC amplitudes profoundly increased across postnatal development, but this increase was prevented by SNHL (Fig. 4; IPSC amp). Further, the variation of IPSC amplitude at single connections was significantly greater in both pre-hearing and SNHL neurons. The kinetics of FS-evoked IPSCs were also slower in pre-hearing and SNHL animals. For example, IPSC rising slopes were shallower and 20–80% rise times were longer. Finally, the average latency to peak was longer and displayed greater variation in pre-hearing and SNHL neurons. In contrast to these results, LTS-evoked IPSCs displayed a developmental decrease in amplitude, which did not occur in animals with SNHL. These changes demonstrate that auditory experience has a significant impact on the normal development of both FS- and LTS-evoked IPSCs.
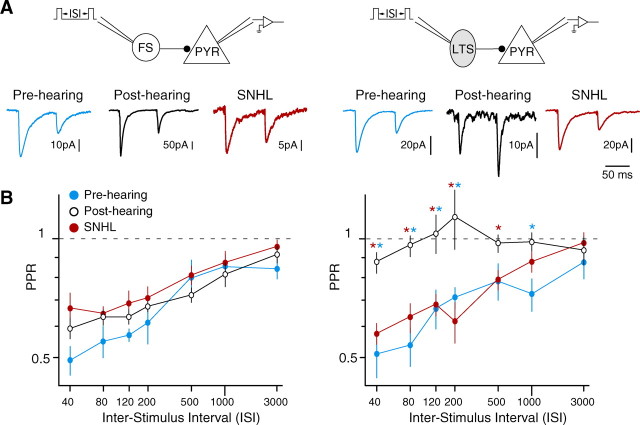
To evaluate the effects of SNHL on the maturation of iSTP at FS- and LTS-pyramidal connections, pairs of spikes were evoked in each interneuron with brief current pulses. As shown in Figure 5, the PPR of FS-evoked IPSCs did not change across development. Depression was observed at ISIs up to 1000 ms for both pre-hearing and post-hearing animals. Moreover, SNHL did not produce a significant effect on the PPR of FS-evoked IPSCs. In contrast, there was a main effect of group on LTS-mediated iSTP (ISI = 80; ANOVA; F = 15.12, df = 2, p < 0.0001). These connections displayed a profound increase in PPR across development at intervals ranging from 40 to 1000 ms (Fig. 5). For example, at an ISI of 80 ms, PPR showed a developmental increase from 0.54 ± 0.06 in pre-hearing (P8–12; n = 13) to 0.96 ± 0.06 in post-hearing (P17–22; n = 21, t = 4.75, p < 0.0001). However, PPR in SNHL animals (P17–22; n = 14) was significantly reduced to 0.63 ± 0.05 compared with age-matched post-hearing controls (t = 3.86, p = 0.0005).
Figure 5.
Experience-dependent development of iSTP occurs at LTS-pyramidal connections. A, IPSCs recorded at FS- and LTS-pyramidal connections evoked by two spikes in pre-hearing, post-hearing, and SNHL neurons (ISI = 80 ms). B, Average PPR (means ± SEM) of IPSCs evoked by FS or LTS interneurons from pre-hearing (P8–12), post-hearing (P17–22), and SNHL (P17–22) animals at a range of ISIs. At FS-pyramidal connections, pre-hearing, post-hearing and SNHL neurons showed similar paired-pulse depression across all ISIs (number of connections sampled: pre-hearing, 12; control, 16–22; SNHL, 12–14). At LTS-pyramidal connections, pre-hearing and SNHL neurons showed a significant reduction in PPR compared with post-hearing neurons at ISIs from 40 to 500 ms (number of connections sampled at ISIs of 40–120, 200–3000: pre-hearing, 12–13, 12; post-hearing, 17–21, 12–14; SNHL, 13–14, 12–13). Blue asterisk indicates p < 0.02 for pre-hearing versus post-hearing; red asterisk indicates p < 0.01 for SNHL versus post-hearing.
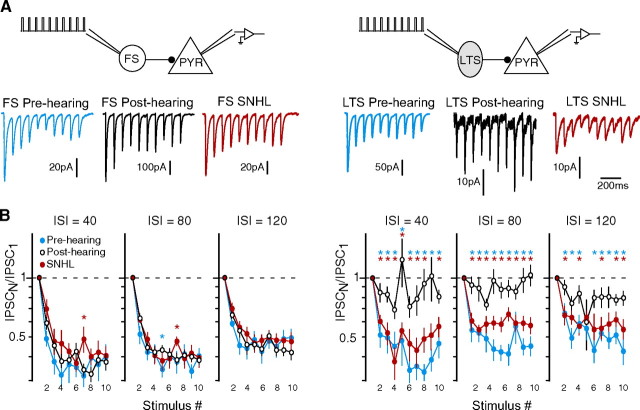
A similar finding was obtained when trains of IPSCs evoked by 10 interneuron spikes were examined. FS-evoked IPSCs from pre-hearing, post-hearing and SNHL animals depressed robustly during the course of the train at all ISIs tested (Fig. 6). However, LTS-evoked IPSCs in post-hearing animals showed little change in relative amplitude during the train compared with the first IPSC (IPSCN/IPSC1). In pre-hearing and SNHL neurons, IPSCs displayed more depression, evidenced by a significant reduction in this relative amplitude across the train.
Figure 6.
Altered iSTP at LTS-pyramidal connections revealed by stimulus trains. A, IPSCs recorded at FS- and LTS-pyramidal connections evoked by trains of 10 spikes in pre-hearing, post-hearing, and SNHL neurons (ISI = 80 ms). B, Average IPSC amplitude (means ± SEM) normalized to the first IPSC (ISPCN/ISPC1) evoked by each of 10 stimuli at FS- and LTS-pyramidal connections (ISI = 40, 80, and 120 ms as indicated). At FS-pyramidal connections from pre-hearing (P8–12), post-hearing (P17–22), and SNHL (P17–22) animals, IPSCs became depressed during the 10-spike train (number of connections sampled: pre-hearing, 12; post-hearing, 16–20; SNHL, 12–13). At LTS-pyramidal connections, IPSCs from post-hearing animals were generally not depressed during the 10-spike train (number of connections sampled, 9–12). In contrast, IPSCs from pre-hearing and SNHL animals showed robust depression, corresponding to a significant decrease in the ISPCN/ISPC1 ratio (number of connections sampled: pre-hearing, 12–13; SNHL, 10–12). Blue asterisk indicates p < 0.05 for pre-hearing versus post-hearing; red asterisk indicates p < 0.05 for SNHL versus post-hearing.
Experience-dependent development of GABAB receptor function occurs at LTS-pyramidal connections
Our experiments using extracellular stimulation suggested that modification of presynaptic GABAB receptor function could underlie the experience-dependent maturation of iSTP. We predicted that this effect was due to the maturation of GABAB receptor function at LTS-pyramidal connections. To test this, the effects of the GABAB receptor antagonist, SCH-50911 (10 μm), on PPR were evaluated at both FS- and LTS-pyramidal connections (Fig. 7). The magnitude of change induced by drug was calculated as the change in PPR (ΔPPR = PPRSCH50911 − PPRPRE-DRUG). The PPR displayed by FS-evoked IPSCs was not affected by SCH-50911 in pre-hearing, post-hearing or SNHL animals. In contrast, there was a main effect of group for ΔPPR at LTS-pyramidal connections (ISI = 80 ms; Kruskal–Wallis test, χ2 = 7.26, p = 0.03). ΔPPR was significantly greater in pre-hearing and SNHL neurons compared with post-hearing control neurons (ISI = 80 ms, ΔPPR pre-hearing: 0.26 ± 0.03, n = 9; ΔPPR post-hearing: −0.03 ± 0.07, n = 7; ΔPPR SNHL: 0.30 ± 0.12, n = 7; pre-hearing vs post-hearing: χ2 = 5.18, p = 0.02; SNHL vs post-hearing: χ2 = 5.59, p = 0.02). Recovery of PPR by washout of SCH-50911 was verified in a subset of connections (data not shown). Thus, the developmental downregulation of GABAB receptor function at LTS terminals depends on normal hearing, and accounts for the proper expression of iSTP at LTS-pyramidal connections.
Figure 7.
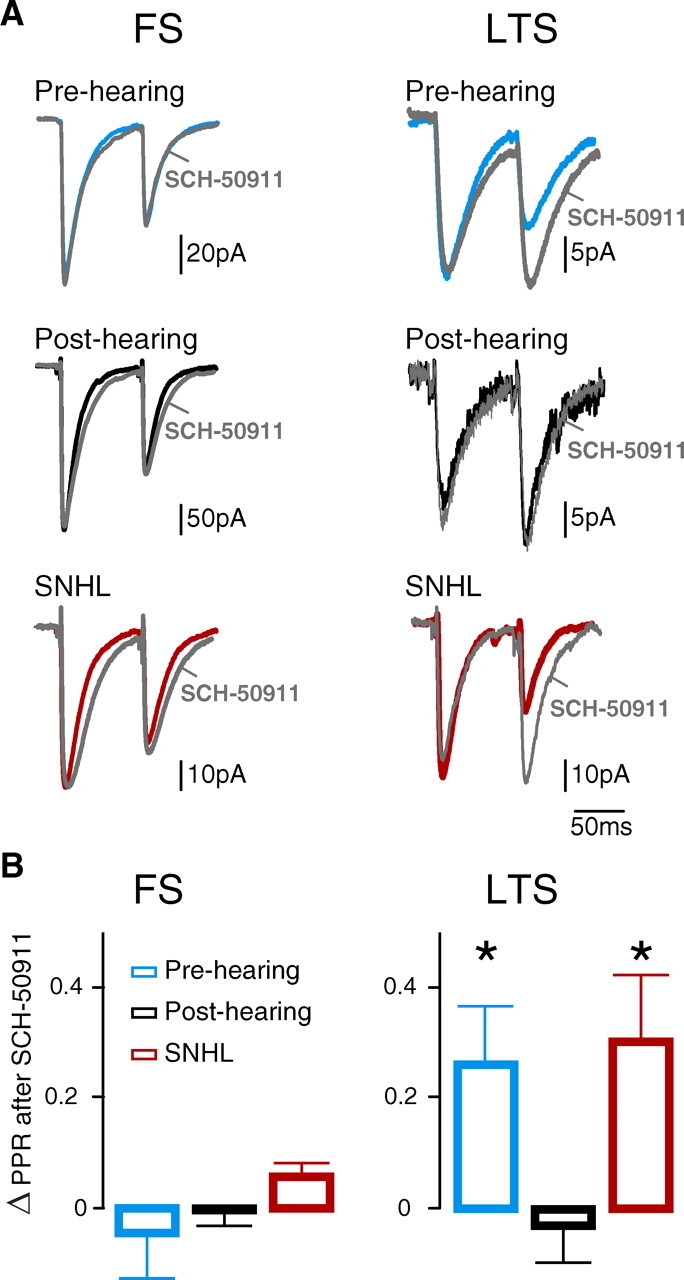
Experience-dependent development of GABAB receptor function occurs at LTS-pyramidal connections. A, Effect of the GABAB receptor antagonist, SCH-50911 (10 μm), on IPSCs at FS- and LTS-pyramidal connections from pre-hearing, post-hearing, and SNHL neurons (ISI = 80 ms). PPR of IPSCs was not affected by SCH-50911 at FS-pyramidal connections. PPR of IPSCs was increased by SCH-50911 at LTS-pyramidal connections from pre-hearing and SNHL animals. This effect was not seen at LTS-pyramidal connections from post-hearing animals. Gray traces were obtained after the application of SCH-50911. B, The average magnitude (means ± SEM) of the drug effect, ΔPPR (PPRSCH50911 − PPRPRE-DRUG) on IPSCs from pre-hearing (P8–12), post-hearing (P17–22), and SNHL animals (P17–22; ISI = 80 ms). ΔPPR was significantly greater in pre-hearing and SNHL animals compared with post-hearing animals at LTS-pyramidal connections (number of connections sampled: pre-hearing, 9; post-hearing, 7; SNHL, 7; *p < 0.05), but not FS-pyramidal connections (number of connections sampled: pre-hearing, 8; post-hearing, 12; SNHL, 10).
Auditory experience regulates GABAB receptor-mediated inhibition of calcium currents
The enhanced sensitivity to the GABAB receptor antagonist at LTS-pyramidal connections in SNHL animals could be explained by several mechanisms. For example, changes in the release probability of GABA or the efficiency of GABA reuptake, both developmentally regulated, may underlie this effect (Caillard et al., 1998; Murthy et al., 2001; Conti et al., 2004; Kotak et al., 2008; Sarro et al., 2008). Alternatively, there may be changes in the function of the presynaptic GABAB receptor. One way to assess presynaptic GABAB receptor function is to focally apply the agonist, baclofen, to the pyramidal cell soma and observe its effects on IPSC amplitude (Kruglikov and Rudy, 2008). However, LTS interneurons form inhibitory synapses on the dendrites of pyramidal cells, making it difficult to find a target for focal application. To circumvent this issue, the effect of baclofen on isolated calcium currents in LTS cells was evaluated. Although the effect was assessed using somatic voltage-clamp recordings, mechanisms underlying GABAB receptor-inhibition of calcium channels at the soma may mirror those at the inhibitory terminals (Lambert and Wilson, 1996; Carter and Mynlieff, 2004).
To isolate calcium currents, the external and internal solutions were supplemented with channel blockers (see Materials and Methods). Inward calcium currents were then generated by depolarizing voltage steps (VHOLD = −80 mV). The currents were confirmed as calcium by blocking them with nickel chloride (1 mm, Sigma) (data not shown). Activation of GABAB receptors by a saturating concentration of the agonist, baclofen (100 μm), produced a significantly greater decrease in the average amplitude of the peak calcium current in animals with SNHL compared with post-hearing animals (Fig. 8; percentage decrease by baclofen, SNHL: 13.6 ± 2.5, n = 6; post-hearing: 0.3 ± 3.9, n = 12; χ2 = 5.05, p = 0.02). Although GABAB receptor function may be independently regulated at the terminal and somatodendritic subcellular domains (Wang and Lambert, 2000; Pérez-Garci et al., 2006), these results suggest a possible mechanism for the increased depression of LTS-evoked IPSCs following SNHL: an upregulation of GABAB receptor-mediated inhibition of calcium currents.
Figure 8.
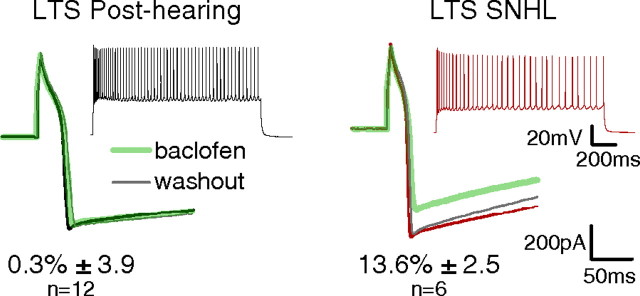
Auditory experience regulates GABAB receptor-mediated inhibition of calcium currents. Effects of the GABAB receptor agonist, baclofen (100 μm; green trace), on calcium currents evoked by voltage steps (200 ms) in LTS cells. Left, Baclofen produced no effect on the peak calcium current in LTS cells from post-hearing animals. Right, Baclofen decreased the amplitude of the peak calcium current in LTS cells from SNHL animals. Insets show spiking responses of LTS interneurons from post-hearing and SNHL neurons to current injection (1500 ms) obtained immediately after rupture. Numbers indicate the average magnitude of baclofen-induced suppression of calcium currents (means ± SEM). The percentage decrease in the peak calcium currents by baclofen was significantly greater in SNHL LTS cells compared with post-hearing LTS cells (number of LTS cells sampled: post-hearing, 12, SNHL, 6; p = 0.02).
Discussion
The maturation of excitatory synapses has long been tied to experience-dependent mechanisms. While it is recognized that inhibitory synapses display similar mechanisms, key issues are unresolved. Chief among these is the role played by presynaptic GABAB receptors, which are known to regulate iSTP. Here, we found that the maturation of iSTP emerges after the onset of hearing and requires auditory experience. Importantly, our results demonstrate that this regulation of iSTP is mediated by a specific interneuron subtype. Moreover, the underlying mechanism depends on an experience-dependent reduction of presynaptic GABAB receptor function. Below, we explore the potential ramifications for auditory processing during development and following hearing loss.
The maturation of iSTP depends on hearing
A profound developmental switch of iSTP from depression to facilitation occurs at an age when gerbils first hear airborne sound (Figs. 1, 5). This is in accord with the developmental alterations in both excitatory and inhibitory STP reported in many brain regions (Reyes and Sakmann, 1999; Iwasaki and Takahashi, 2001; Kumar and Huguenard, 2001; Frick et al., 2007; Ingram et al., 2008; Oswald and Reyes, 2008).
The shift toward facilitating iSTP after hearing onset suggested that this property could be influenced by auditory experience. Our results demonstrate that even a moderate elevation of auditory thresholds (CHL) resulted in the failure of iSTP to shift from depression to facilitation, and this effect was nearly identical to removal of both cochleae (SNHL) (Fig. 2). Comparable effects were also observed for excitatory thalamocortical synapses, where short-term depression became more pronounced following moderate or severe developmental hearing loss (Xu et al., 2007). Moreover, the experience-dependent maturation of iSTP in auditory cortex (ACx) is consistent with reports from other auditory regions and sensory cortices. In the auditory midbrain, SNHL leads to decreased paired-pulse facilitation of IPSCs (Vale and Sanes, 2000). An alteration of iSTP was observed in the deprived region of the visual cortex following monocular eye suture or dark rearing (Maffei et al., 2004; Tang et al., 2007). Despite procedural differences between our experiment and these studies (e.g., cortical layer, duration of manipulation), the effect on iSTP was similar. Excitatory STP is also experience-dependent, but may involve distinct mechanisms. For example, altered excitatory STP in the somatosensory cortex requires competition between undeprived and deprived synapses; complete sensory deprivation does not lead to a change (Finnerty et al., 1999; Finnerty and Connors, 2000).
FS and LTS interneurons respond differentially to hearing loss
FS-pyramidal connections displayed robust experience-dependent alterations in synaptic properties, becoming weaker, slower and more variable following SNHL (Fig. 4). Conversely, LTS-pyramidal connections became stronger. IPSCs from SNHL animals resembled those from pre-hearing animals, suggesting an arrest in development. Our findings are in general agreement with studies on visual and somatosensory cortices showing that FS and LTS interneurons respond distinctly to a reduction in activity (Maffei et al., 2004; Bartley et al., 2008). Moreover, these results indicate that FS cells account for the smaller and slower IPSCs following SNHL that we have previously observed (Kotak et al., 2008).
Only the LTS-pyramidal connections displayed a dramatic change in STP, producing more depression following hearing loss (Figs. 5, 6). This depression resembled that observed in pre-hearing animals, suggesting that the normal developmental regulation of STP at LTS synapses depends on experience. The near-minimum extracellular stimulation that we used may have been biased in recruiting LTS cells, which have lower spike thresholds. Consistent with this, extracellular-evoked IPSCs showed amplitudes and kinetics comparable to LTS-evoked IPSCs (data not shown). FS cells did not display a decrease in PPR following SNHL, and therefore could not have explained the observed changes in iSTP examined by extracellular stimuli.
GABAB receptor function is regulated by auditory experience
The central hypothesis examined in this study is that GABAB receptor function serves as a mechanism for experience-dependent maturation of iSTP. At LTS-pyramidal connections, presynaptic GABAB receptor blockade reversed inhibitory paired-pulse depression in pre-hearing, but not in post-hearing neurons (Fig. 7). Normal auditory experience is required for this transition, because SNHL prevented the reduction in presynaptic GABAB receptor function. This antagonist had no effect on the PPR at FS-evoked IPSCs, supporting the hypothesis that changes in GABAB receptor-mediated iSTP are interneuron subtype-specific. Together, our findings support the novel idea that presynaptic GABAB receptors serve as a key experience-dependent mechanism in the maturation of iSTP.
The present study reveals an enhanced GABAB receptor-mediated inhibition of calcium currents in SNHL LTS cells. Although changes in GABA release probability and GABA uptake could also be involved (Caillard et al., 1998; Murthy et al., 2001; Conti et al., 2004; Kotak et al., 2008; Sarro et al., 2008), the GABAB receptor mechanism is sufficient to explain our results. Changes in GABAB receptor function could be due to altered expression. In support of this, expression of GABAB(1a), the putative presynaptic isoform, is highest in the rat brain during the first postnatal week and rapidly declines thereafter (Fritschy et al., 2001; Pérez-Garci et al., 2006; Vigot et al., 2006). Alternatively, a transient overexpression of targets of GABAB receptor modulation, such as the N-type calcium channel, during early life has been shown to increase presynaptic GABAB receptor function (Iwasaki et al., 2000; Ishikawa et al., 2005; Inchauspe et al., 2007). While here we selectively examined presynaptic GABAB receptors, additional insights may be gained by studying the developmental regulation of postsynaptic GABAB receptors, which may modulate network activity in ACx (Oswald et al., 2009).
Implications for regulation of cortical development
During early postnatal life, the constraint of GABA release by presynaptic GABAB receptors may enhance network excitability and be advantageous for development of the cortical network. In fact, differences between excitatory and inhibitory STP may shift the balance of excitatory and inhibitory input to cortical pyramidal cells (Galarreta and Hestrin, 1998; Gibson et al., 1999; Varela et al., 1999; Beierlein et al., 2003, Gabernet et al., 2005), and presynaptic GABAB receptors may underlie such differences (Magnusson et al., 2008). Here, the persistence of presynaptic GABAB receptor activity on inhibitory terminals after SNHL may contribute to the heightened network excitability. Generally, the numerous changes in the ACx after SNHL favor excitability. For example, excitatory pyramidal cells show increased intrinsic excitability, less spike adaptation, stronger glutamatergic synapses, and weaker GABAergic synapse (Kotak et al., 2005, 2008; Xu et al., 2007; Sarro et al., 2008). Here, we reveal that the inhibitory input to excitatory cells is also more depressing, which may lead to amplified excitation during prolonged stimulation.
The net amplification of excitation by inhibitory short-term depression also favors long-term plasticity (LTP) mechanisms. For example, disinhibition via presynaptic GABAB receptors enhances NMDA receptor activation during repetitive stimulation and can lead to long-term changes in excitatory strength (Davies et al., 1991; Mott and Lewis, 1991; Metherate and Ashe, 1994; Wagner and Alger, 1995). In fact, knock-out mice lacking presynaptic GABAB receptors show compromised excitatory LTP (Vigot et al., 2006). Thus, disinhibition by elevated activity of the presynaptic GABAB receptors may play a permissive role in excitatory plasticity during the period when environmental activity shapes cortical function. By disrupting the maturation of these receptors, hearing loss may alter LTP mechanisms in the ACx (Kotak et al., 2007). Interestingly, critical period plasticity may be regulated by cortical GABA release (Hensch et al., 1998). Here, we discover an endogenous mechanism that may achieve this regulation, the presynaptic GABAB receptor.
Implications for auditory processing and hearing loss
Many in vivo studies suggest that the modification of inhibitory synapse function is responsible for changes in auditory coding (Kitzes and Semple, 1985; Calford et al., 1993; Szczepaniak and Møller, 1995; McAlpine et al., 1997; Rajan, 1998; Kimura and Eggermont, 1999; Salvi et al., 2000; Wang et al., 2002; Noreña et al., 2003; Chang et al., 2005; Razak et al., 2008). Here, we propose that developmental changes in presynaptic GABAB receptors may affect temporal responses by modulating iSTP of LTS-evoked IPSCs (Fig. 5), which are thought to entrain pyramidal cell spiking (Deans et al., 2001; Goldberg et al., 2004; Long et al., 2005). The facilitating iSTP in the mature ACx may underlie the adaptation of spiking responses to acoustic stimuli. This mechanism may permit the encoding of relevant temporal features of sound (Lu et al., 2001; Liang et al., 2002; Malone et al., 2007; Ter-Mikaelian et al., 2007). In fact, synaptic short-term plasticity, including iSTP, has been implicated theoretically in amplitude-modulation processing (Eggermont, 1999; Elhilali et al., 2004).
Abnormal temporal processing in the human ACx may underlie perceptual deficits associated with hearing impairments. For example, hearing loss produces deficits in psychoacoustic measures of temporal processing, such as gap detection and forward masking (Kidd et al., 1984; Gagné, 1988; Festen and Plomp, 1990; Nelson and Thomas, 1997; Snell and Frisina, 2000). The disruption of iSTP after hearing loss could contribute to such deficits. Further, our results provide evidence for an underlying synaptic mechanism: presynaptic GABAB receptors are crucial for the normal development of iSTP. Targeting these receptors pharmacologically may restore processing mechanisms in the auditory cortex following developmental hearing loss.
Footnotes
This work was supported by National Institutes of Health Grants DC006864 (D.H.S., V.C.K.) and DC008920 (A.E.T.). We thank Margaret Bezrutczyk and Neeti Sharma for processing and photographing the biocytin-filled neurons.
References
- Aizawa N, Eggermont JJ. Effects of noise-induced hearing loss at young age on voice onset time and gap-in-noise representations in adult cat primary auditory cortex. J Assoc Res Otolaryngol. 2006;7:71–81. doi: 10.1007/s10162-005-0026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa N, Eggermont JJ. Mild noise-induced hearing loss affects temporal modulation transfer functions in adult cat primary auditory cortex. Hear Res. 2007;223:71–82. doi: 10.1016/j.heares.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Amitai Y, Gibson JR, Beierlein M, Patrick SL, Ho AM, Connors BW, Golomb D. The spatial dimensions of electrically coupled networks of interneurons in the neocortex. J Neurosci. 2002;22:4142–4152. doi: 10.1523/JNEUROSCI.22-10-04142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade R. Blockade of neurotransmitter-activated K+ conductance by QX-314 in the rat hippocampus. Eur J Pharmacol. 1991;199:259–262. doi: 10.1016/0014-2999(91)90467-5. [DOI] [PubMed] [Google Scholar]
- Bartley AF, Huang ZJ, Huber KM, Gibson JR. Differential activity-dependent, homeostatic plasticity of two neocortical inhibitory circuits. J Neurophysiol. 2008;100:1983–1994. doi: 10.1152/jn.90635.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat Neurosci. 2000;3:904–910. doi: 10.1038/78809. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol. 2003;90:2987–3000. doi: 10.1152/jn.00283.2003. [DOI] [PubMed] [Google Scholar]
- Caillard O, McLean HA, Ben-Ari Y, Gaïarsa JL. Ontogenesis of presynaptic GABAB receptor-mediated inhibition in the CA3 region of the hippocampus. J Neurophysiol. 1998;79:1341–1348. doi: 10.1152/jn.1998.79.3.1341. [DOI] [PubMed] [Google Scholar]
- Calford MB, Semple MN. Monaural inhibition in the cat auditory cortex. J Neurophysiol. 1995;73:1876–1891. doi: 10.1152/jn.1995.73.5.1876. [DOI] [PubMed] [Google Scholar]
- Calford MB, Rajan R, Irvine DR. Rapid changes in the frequency tuning of neurons in cat auditory cortex resulting from pure-tone-induced temporary threshold shift. Neuroscience. 1993;55:953–964. doi: 10.1016/0306-4522(93)90310-c. [DOI] [PubMed] [Google Scholar]
- Carter TJ, Mynlieff M. γ-Aminobutyric acid type B receptors facilitate L-type and attenuate N-type Ca2+ currents in isolated hippocampal neurons. J Neurosci Res. 2004;76:323–333. doi: 10.1002/jnr.20085. [DOI] [PubMed] [Google Scholar]
- Chang EF, Bao S, Imaizumi K, Schreiner CE, Merzenich MM. Development of spectral and temporal response selectivity in the auditory cortex. Proc Natl Acad Sci U S A. 2005;102:16460–16465. doi: 10.1073/pnas.0508239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F, Minelli A, Melone M. GABA transporters in the mammalian cerebral cortex: localization, development and pathological implications. Brain Res Rev. 2004;45:196–212. doi: 10.1016/j.brainresrev.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Cook DL, Schwindt PC, Grande LA, Spain WJ. Synaptic depression in the localization of sound. Nature. 2003;421:66–70. doi: 10.1038/nature01248. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Rose HJ, Metherate R. Auditory thalamocortical synaptic transmission in vitro. J Neurophysiol. 2002;87:361–384. doi: 10.1152/jn.00549.2001. [DOI] [PubMed] [Google Scholar]
- Davies CH, Collingridge GL. The physiological regulation of synaptic inhibition by GABAB autoreceptors in rat hippocampus. J Physiol. 1993;472:245–265. doi: 10.1113/jphysiol.1993.sp019945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CH, Collingridge GL. Regulation of EPSPs by the synaptic activation of GABAB autoreceptors in rat hippocampus. J Physiol. 1996;496:451–470. doi: 10.1113/jphysiol.1996.sp021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CH, Starkey SJ, Pozza MF, Collingridge GL. GABAB autoreceptors regulate the induction of LTP. Nature. 1991;349:609–611. doi: 10.1038/349609a0. [DOI] [PubMed] [Google Scholar]
- Deans MR, Gibson JR, Sellitto C, Connors BW, Paul DL. Synchronous activity of inhibitory networks in neocortex requires electrical synapses containing connexin36. Neuron. 2001;31:477–485. doi: 10.1016/s0896-6273(01)00373-7. [DOI] [PubMed] [Google Scholar]
- Deisz RA, Prince DA. Frequency-dependent depression of inhibition in guinea-pig neocortex in vitro by GABAB receptor feed-back on GABA release. J Physiol. 1989;412:513–541. doi: 10.1113/jphysiol.1989.sp017629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisz RA, Billard JM, Zieglgänsberger W. Presynaptic and postsynaptic GABAB receptors of neocortical neurons of the rat in vitro: differences in pharmacology and ionic mechanisms. Synapse. 1997;25:62–72. doi: 10.1002/(SICI)1098-2396(199701)25:1<62::AID-SYN8>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. The magnitude and phase of temporal modulation transfer functions in cat auditory cortex. J Neurosci. 1999;19:2780–2788. doi: 10.1523/JNEUROSCI.19-07-02780.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhilali M, Fritz JB, Klein DJ, Simon JZ, Shamma SA. Dynamics of precise spike timing in primary auditory cortex. J Neurosci. 2004;24:1159–1172. doi: 10.1523/JNEUROSCI.3825-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festen JM, Plomp R. Effects of fluctuation noise and interfering speech-reception threshold for impaired and normal hearing. J Acoust Soc Am. 1990;88:1725–1736. doi: 10.1121/1.400247. [DOI] [PubMed] [Google Scholar]
- Finnerty GT, Connors BW. Sensory deprivation without competition yields modest alterations of short-term synaptic dynamics. Proc Natl Acad Sci U S A. 2000;97:12864–12868. doi: 10.1073/pnas.230175697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerty GT, Roberts LS, Connors BW. Sensory experience modifies the short-term dynamics of neocortical synapses. Nature. 1999;400:367–371. doi: 10.1038/22553. [DOI] [PubMed] [Google Scholar]
- Frick A, Feldmeyer D, Sakmann B. Postnatal development of synaptic transmission in local networks of L5A pyramidal neurons in rat somatosensory cortex. J Physiol. 2007;585:103–116. doi: 10.1113/jphysiol.2007.141788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Meskenaite V, Weinmann O, Honer M, Benke D, Mohler H. GABAB-receptor splice variants GB1a and GB1b in rat brain: developmental regulation, cellular distribution and extrasynaptic localization. Eur J Neurosci. 2001;11:761–768. doi: 10.1046/j.1460-9568.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- Fukuda A, Mody I, Prince DA. Differential ontogenesis of presynaptic and postsynaptic GABAB inhibition in rat somatosensory cortex. J Neurophysiol. 1993;70:448–452. doi: 10.1152/jn.1993.70.1.448. [DOI] [PubMed] [Google Scholar]
- Gabernet L, Jadhav SP, Feldman DE, Carandini M, Scanziani M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron. 2005;48:315–327. doi: 10.1016/j.neuron.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Gagné JP. Excess masking among listeners with a sensorineural hearing loss. J Acoust Soc Am. 1988;83:2311–2321. doi: 10.1121/1.396362. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Frequency-dependent synaptic depression and the balance of excitation and inhibition in the neocortex. Nat Neurosci. 1998;1:587–594. doi: 10.1038/2822. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Goldberg JH, Lacefield CO, Yuste R. Global dendritic calcium spikes in mouse layer 5 low threshold spiking interneurones: implications for control of pyramidal cell bursting. J Physiol. 2004;558:465–478. doi: 10.1113/jphysiol.2004.064519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Wang Y, Markram H. Organizing principles for a diversity of GABAergic interneurons and synapses in the neocortex. Science. 2000;287:273–278. doi: 10.1126/science.287.5451.273. [DOI] [PubMed] [Google Scholar]
- Halliday LF, Bishop DV. Is poor frequency modulation detection linked to literacy problems? A comparison of specific reading disability and mild to moderate sensorineural hearing loss. Brain Lang. 2006;97:200–213. doi: 10.1016/j.bandl.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inchauspe CG, Forsythe ID, Uchitel OD. Changes in synaptic transmission properties due to the expression of N-type calcium channels at the calyx of Held synapse of mice lacking P/Q-type calcium channels. J Physiol. 2007;584:835–851. doi: 10.1113/jphysiol.2007.139683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram RA, Fitzgerald M, Baccei ML. Developmental changes in the fidelity and short-term plasticity of GABAergic synapses in the neonatal rat dorsal horn. J Neurophysiol. 2008;99:3144–3150. doi: 10.1152/jn.01342.2007. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Kaneko M, Shin HS, Takahashi T. Presynaptic N-type and P/Q – type Ca2+ channels mediating synaptic transmission at the calyx of Held of mice. J Physiol. 2005;568:199–209. doi: 10.1113/jphysiol.2005.089912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S, Takahashi T. Developmental regulation of transmitter release at the calyx of Held in rat auditory brainstem. J Physiol. 2001;534:861–871. doi: 10.1111/j.1469-7793.2001.00861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S, Momiyama A, Uchitel OD, Takahashi T. Developmental changes in calcium channel types mediating central synaptic transmission. J Neurosci. 2000;20:59–65. doi: 10.1523/JNEUROSCI.20-01-00059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd G, Jr, Mason CR, Feth LL. Temporal integration of forward masking in listeners having sensorineural hearing loss. J Acoust Soc Am. 1984;75:937–944. doi: 10.1121/1.390558. [DOI] [PubMed] [Google Scholar]
- Kimura M, Eggermont JJ. Effects of acute pure tone induced hearing loss on response properties in three auditory cortical fields in cat. Hear Res. 1999;135:146–162. doi: 10.1016/s0378-5955(99)00104-5. [DOI] [PubMed] [Google Scholar]
- Kirmse K, Kirischuk S. Ambient GABA constrains the strength of GABAergic synapses at Cajal-Retzius cells in the developing visual cortex. J Neurosci. 2006;26:4216–4227. doi: 10.1523/JNEUROSCI.0589-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzes LM, Semple MN. Single-unit responses in the inferior colliculus: effects of neonatal unilateral cochlear ablation. J Neurophysiol. 1985;53:1483–1500. doi: 10.1152/jn.1985.53.6.1483. [DOI] [PubMed] [Google Scholar]
- Kotak VC, Sanes DH. Developmental influence of glycinergic transmission: regulation of NMDA receptor-mediated EPSPs. J Neurosci. 1996;16:1836–1843. doi: 10.1523/JNEUROSCI.16-05-01836.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Fujisawa S, Lee FA, Karthikeyan O, Aoki C, Sanes DH. Hearing loss raises excitability in the auditory cortex. J Neurosci. 2005;25:3908–3918. doi: 10.1523/JNEUROSCI.5169-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Breithaupt AD, Sanes DH. Developmental hearing loss eliminates long-term potentiation in the auditory cortex. Proc Natl Acad Sci U S A. 2007;104:3550–3555. doi: 10.1073/pnas.0607177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Takesian AE, Sanes DH. Hearing loss prevents the maturation of GABAergic transmission in the auditory cortex. Cereb Cortex. 2008;18:2098–2108. doi: 10.1093/cercor/bhm233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruglikov I, Rudy B. Perisomatic GABA release and thalamocortical integration onto neocortical excitatory cells are regulation by neuromodulators. Neuron. 2008;58:911–924. doi: 10.1016/j.neuron.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SS, Huguenard JR. Properties of excitatory synaptic connections mediated by the corpus collosum in the developing rat neocortex. J Neurophysiol. 2001;86:2973–2985. doi: 10.1152/jn.2001.86.6.2973. [DOI] [PubMed] [Google Scholar]
- Lambert NA, Wilson WA. High-threshold Ca2+ currents in rat hippocampal interneurons and their selective inhibition by activation of GABA(B) receptor. J Physiol. 1996;492:115–127. doi: 10.1113/jphysiol.1996.sp021294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Lu T, Wang X. Neural representations of sinusoidal amplitude and frequency modulations in the primary auditory cortex of awake primates. J Neurophysiol. 2002;87:2237–2261. doi: 10.1152/jn.2002.87.5.2237. [DOI] [PubMed] [Google Scholar]
- Long MA, Cruikshank SJ, Jutras MJ, Connors BW. Abrupt maturation of a spike-synchronizing mechanism in neocortex. J Neurosci. 2005;25:7309–7316. doi: 10.1523/JNEUROSCI.0375-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Liang L, Wang X. Temporal and rate representations of time-varying signals in the auditory cortex of awake primates. Nat Neurosci. 2001;4:1131–1138. doi: 10.1038/nn737. [DOI] [PubMed] [Google Scholar]
- Maffei A, Nelson SB, Turrigiano GG. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat Neurosci. 2004;7:1353–1359. doi: 10.1038/nn1351. [DOI] [PubMed] [Google Scholar]
- Magnusson AK, Park TJ, Pecka M, Grothe B, Koch U. Retrograde GABA signaling adjusts sound localization by balancing excitation and inhibition in the brainstem. Neuron. 2008;59:911–924. doi: 10.1016/j.neuron.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Malone BJ, Scott BH, Semple MN. Dynamic amplitude coding in the auditory cortex of awake rhesus macaques. J Neurophysiol. 2007;98:1451–1474. doi: 10.1152/jn.01203.2006. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- McAlpine D, Martin RL, Mossop JE, Moore DR. Response properties of neurones in the inferior colliculus of the monaurally-deafened ferret to acoustic stimulation of the intact ear. J Neurophysiol. 1997;78:767–779. doi: 10.1152/jn.1997.78.2.767. [DOI] [PubMed] [Google Scholar]
- Metherate R, Ashe JH. Facilitation of an NMDA receptor-mediated EPSP by paired-pulse stimulation in rat neocortex via depression of GABAergic IPSPs. J Physiol. 1994;481:331–348. doi: 10.1113/jphysiol.1994.sp020443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz IM, Bean BP. GABAB receptor inhibition of P-type Ca2+ channels in central neurons. Neuron. 1993;10:889–898. doi: 10.1016/0896-6273(93)90204-5. [DOI] [PubMed] [Google Scholar]
- Moeller MP, Tomblin JB, Yoshinaga-Itano C, Connor CM, Jerger S. Current state of knowledge: language and literacy of children with hearing impairment. Ear Hear. 2007;28:740–753. doi: 10.1097/AUD.0b013e318157f07f. [DOI] [PubMed] [Google Scholar]
- Mott DD, Xie CW, Wilson WA, Swartzwelder HS, Lewis DV. GABAB autoreceptors mediate activity-dependent disinhibition and enhance signal transmission in the dentate gyrus. J Neurophysiol. 1993;69:674–691. doi: 10.1152/jn.1993.69.3.674. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Schikorski T, Stevens CF, Zhu Y. Inactivity produces increases in neurotransmitter release and synapse size. Neuron. 2001;32:673–682. doi: 10.1016/s0896-6273(01)00500-1. [DOI] [PubMed] [Google Scholar]
- Nathan T, Jensen MS, Lambert JD. The slow inhibitory postsynaptic potential in rat hippocampal CA1 neurones is blocked by intracellular injection of QX-314. Neurosci Lett. 1990;110:309–313. doi: 10.1016/0304-3940(90)90865-7. [DOI] [PubMed] [Google Scholar]
- Nelson PB, Thomas SD. Gap detection as a function of stimulus loudness for listeners with and without hearing loss. J Speech Lang Hear Res. 1997;40:1387–1394. doi: 10.1044/jslhr.4006.1387. [DOI] [PubMed] [Google Scholar]
- Noreña AJ, Tomita M, Eggermont JJ. Neural changes in cat auditory cortex after a transient pure-tone trauma. J Neurophysiol. 2003;90:2387–2401. doi: 10.1152/jn.00139.2003. [DOI] [PubMed] [Google Scholar]
- Oswald AM, Reyes AD. Maturation of intrinsic and synaptic properties of layer 2/3 pyramidal neurons in mouse auditory cortex. J Neurophysiol. 2008;99:2998–3008. doi: 10.1152/jn.01160.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce RA, Grunder SD, Faucher LD. Different mechanisms for use-dependent depression of two GABAA-mediated IPSCs in rat hippocampus. J Physiol. 1995;482:425–435. doi: 10.1113/jphysiol.1995.sp020675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Garci E, Gassmann M, Bettler B, Larkum ME. The GABAB1b isoform mediates long-lasting inhibition of dendritic Ca2+ spikes in layer 5 somatosensory pyramidal neurons. Neuron. 2006;50:603–616. doi: 10.1016/j.neuron.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Peters A, Miller M, Kimerer LM. Cholecystokinin-like immunoreactive neurons in rat cerebral cortex. Neuroscience. 1983;8:431–448. doi: 10.1016/0306-4522(83)90190-2. [DOI] [PubMed] [Google Scholar]
- Poncer JC, McKinney RA, Gähwiler BH, Thompson SM. Differential control of GABA release at synapses from distinct interneurons in rat hippocampus. J Physiol. 2000;528:123–130. doi: 10.1111/j.1469-7793.2000.00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan R. Receptor organ damage causes loss of cortical surround inhibition without topographic map plasticity. Nat Neurosci. 1998;1:138–143. doi: 10.1038/388. [DOI] [PubMed] [Google Scholar]
- Razak KA, Richardson MD, Fuzessery ZM. Experience is required for the maintenance and refinement of FM sweep selectivity in the developing auditory cortex. Proc Natl Acad Sci U S A. 2008;105:4465–4470. doi: 10.1073/pnas.0709504105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A, Sakmann B. Developmental switch in the short-term modification of unitary EPSPs evoked in layer 2/3 and layer 5 pyramidal neurons of rat neocortex. J Neurosci. 1999;19:3827–3835. doi: 10.1523/JNEUROSCI.19-10-03827.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nat Neurosci. 1998;1:279–285. doi: 10.1038/1092. [DOI] [PubMed] [Google Scholar]
- Roberts J, Hunter L, Gravel J, Rosenfeld R, Berman S, Haggard M, Hall J, Lannon C, Moore D, Vernon-Feagans L, Wallace I. Otitis media, hearing loss, and language learning: controversies and current research. J Dev Behav Pediatr. 2004;25:110–122. doi: 10.1097/00004703-200404000-00007. [DOI] [PubMed] [Google Scholar]
- Rose HJ, Metherate R. Auditory thalamocortical transmission is reliable and temporally precise. J Neurophysiol. 2005;94:2019–2030. doi: 10.1152/jn.00860.2004. [DOI] [PubMed] [Google Scholar]
- Sakaba T, Neher E. Direct modulation of synaptic vesicle priming by GABA(B) receptor activation at a glutamatergic synapse. Nature. 2003;424:775–778. doi: 10.1038/nature01859. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hear Res. 2000;147:261–274. doi: 10.1016/s0378-5955(00)00136-2. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Rubel EW. The ontogeny of inhibition and excitation in the gerbil lateral superior olive. J Neurosci. 1988;8:682–700. doi: 10.1523/JNEUROSCI.08-02-00682.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarro EC, Kotak VC, Sanes DH, Aoki C. Hearing loss alters the subcellular distribution of presynaptic GAD and postsynaptic GABAA receptors in the auditory cortex. Cereb Cortex. 2008;18:2855–2867. doi: 10.1093/cercor/bhn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell KB, Frisina DR. Relationships among age-related differences in gap detection and word recognition. J Acoust Soc Am. 2000;107:1615–1626. doi: 10.1121/1.428446. [DOI] [PubMed] [Google Scholar]
- Szczepaniak WS, Møller AR. Evidence of decreased GABAergic influence on temporal integration in the inferior colliculus following acute noise exposure. Neurosci Lett. 1995;196:77–80. doi: 10.1016/0304-3940(95)11851-m. [DOI] [PubMed] [Google Scholar]
- Tang AH, Chai Z, Wang SQ. Dark rearing alters the short-term synaptic plasticity in visual cortex. Neurosci Lett. 2007;422:49–53. doi: 10.1016/j.neulet.2007.05.053. [DOI] [PubMed] [Google Scholar]
- Ter-Mikaelian M, Sanes DH, Semple MN. Transformation of temporal properties between auditory midbrain and cortex in the awake Mongolian gerbil. J Neurosci. 2007;27:6091–6102. doi: 10.1523/JNEUROSCI.4848-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney TS, Moore DR. Naturally occurring neuron death during postnatal development of the gerbil ventral cochlear nucleus begins at the onset of hearing. J Comp Neurol. 1997;387:421–429. [PubMed] [Google Scholar]
- Vale C, Sanes DH. Afferent regulation of inhibitory synaptic transmission in the developing auditory midbrain. J Neurosci. 2000;20:1912–1921. doi: 10.1523/JNEUROSCI.20-05-01912.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale C, Sanes DH. The effect of bilateral deafness on excitatory and inhibitory synaptic strength in the inferior colliculus. Eur J Neurosci. 2002;16:2394–2404. doi: 10.1046/j.1460-9568.2002.02302.x. [DOI] [PubMed] [Google Scholar]
- Vale C, Schoorlemmer J, Sanes DH. Deafness disrupts chloride transporter function and inhibitory synaptic transmission. J Neurosci. 2003;23:7516–7524. doi: 10.1523/JNEUROSCI.23-20-07516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela JA, Song S, Turrigiano GG, Nelson SB. Differential depression at excitatory and inhibitory synapses in visual cortex. J Neurosci. 1999;19:4293–4304. doi: 10.1523/JNEUROSCI.19-11-04293.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigot R, Barbieri S, Bräuner-Osborne H, Turecek R, Shigemoto R, Zhang YP, Luján R, Jacobson LH, Biermann B, Fritschy JM, Vacher CM, Müller M, Sansig G, Guetg N, Cryan JF, Kaupmann K, Gassmann M, Oertner TG, Bettler B. Differential compartmentalization and distinct functions of GABAB receptor variants. Neuron. 2006;50:589–601. doi: 10.1016/j.neuron.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ding D, Salvi RJ. Functional reorganization in chinchilla inferior colliculus associated with chronic and acute cochlear damage. Hear Res. 2002;168:238–249. doi: 10.1016/s0378-5955(02)00360-x. [DOI] [PubMed] [Google Scholar]
- Wang X, Lambert NA. GABA(B) receptors couple to potassium and calcium channels on identified lateral perforant pathway projection neurons. J Neurophysiol. 2000;83:1073–1078. doi: 10.1152/jn.2000.83.2.1073. [DOI] [PubMed] [Google Scholar]
- Wang Y, Toledo-Rodriguez M, Gupta A, Wu C, Silberberg G, Luo J, Markram H. Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. J Physiol. 2004;561:65–90. doi: 10.1113/jphysiol.2004.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Synaptic mechanisms of forward suppression in rat auditory cortex. Neuron. 2005;47:437–445. doi: 10.1016/j.neuron.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Woolf NK, Ryan AF. The development of auditory function in the cochlea of the Mongolian gerbil. Hear Res. 1984;13:277–283. doi: 10.1016/0378-5955(84)90081-9. [DOI] [PubMed] [Google Scholar]
- Woolf NK, Ryan AF. Ontogeny of neural discharge patterns in the ventral cochlear nucleus of the Mongolian gerbil. Brain Res. 1985;349:131–147. doi: 10.1016/0165-3806(85)90138-5. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Huguenard JR, Prince DA. Cholinergic switching within neocortical inhibitory networks. Science. 1998;281:985–988. doi: 10.1126/science.281.5379.985. [DOI] [PubMed] [Google Scholar]
- Xu H, Kotak VC, Sanes DH. Conductive hearing loss disrupts synaptic and spike adaptation in developing auditory cortex. J Neurosci. 2007;27:9417–9426. doi: 10.1523/JNEUROSCI.1992-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]