Abstract
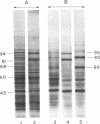
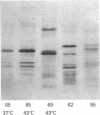
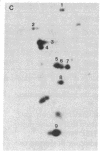
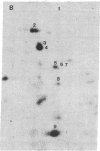
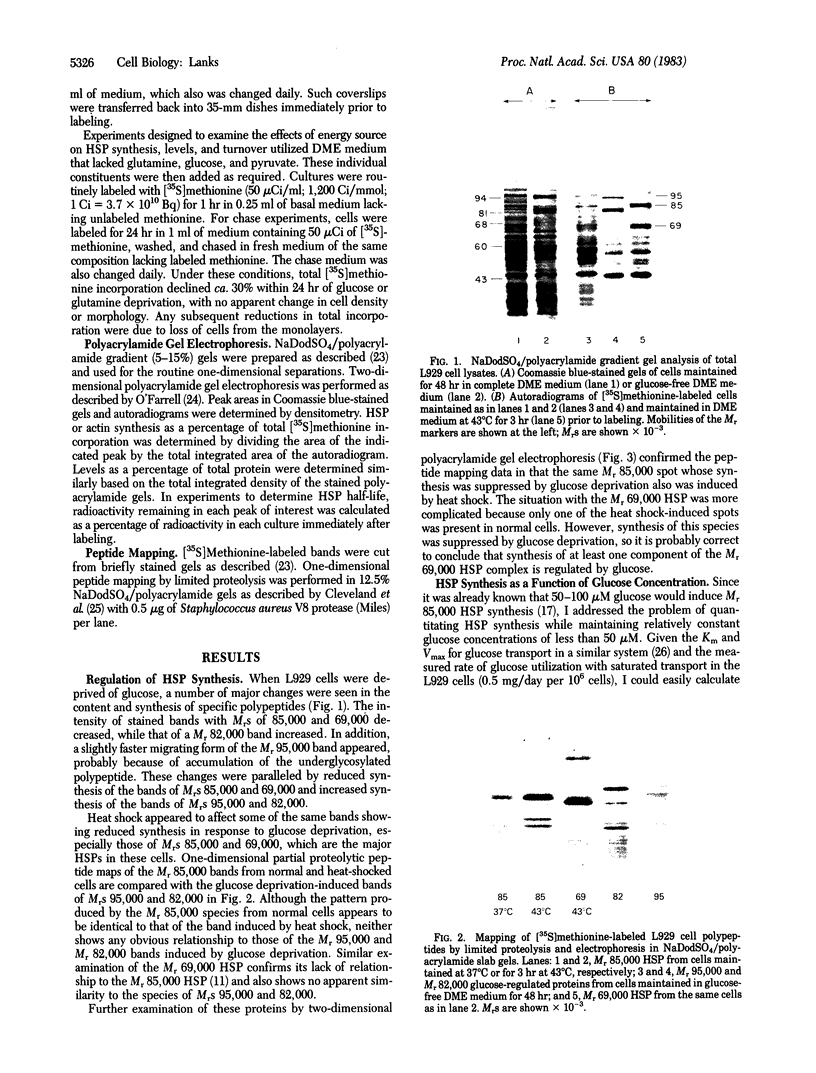
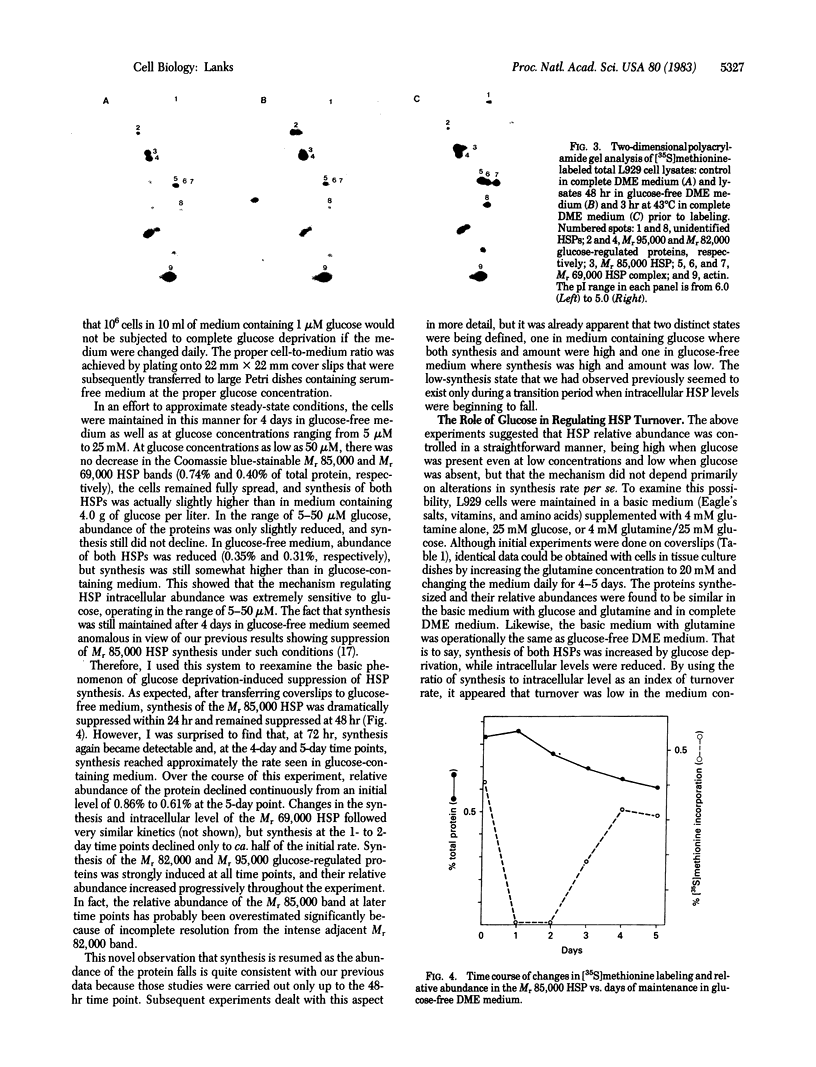
When murine L929 cells are briefly exposed to elevated temperature (43 degrees C) they preferentially synthesize heat shock proteins (HSPs) of Mrs 85,000 and 69,000. By the criteria of two-dimensional polyacrylamide gel electrophoresis and partial proteolytic peptide mapping, the Mr 85,000 HSP is indistinguishable from the major cytoplasmic protein whose synthesis and intracellular level were shown previously to be suppressed by glucose deprivation. The mechanism regulating the Mr 85,000 HSP levels is quite sensitive, operating in the range of 0-50 microM glucose. In glucose-free medium, synthesis is at first suppressed but returns to a high level after 3 days as levels of the protein decrease. Synthesis and level of the Mr 69,000 HSP also were affected by glucose deprivation, but this protein appeared to be a complex of several isoelectric and Mr species, so the effects were not as dramatic. Chase experiments show that the half-lives of both the Mr 85,000 and Mr 69,000 HSPs are reduced by a factor of 2.0-2.5 after 5 days of glucose deprivation. The half-life of the Mr 69,000 HSP also was reduced by glutamine deprivation, whereas that of the Mr 85,000 HSP was essentially unaffected. This increase in turnover appears to be sufficient to account for the reduced intracellular level, thus suggesting that glucose sustains high HSP levels mainly by decreasing degradation of the proteins. Although the function of the HSPs is not known, these data support the concept that they have important roles in the general cellular economy and do not function merely as "stress" proteins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Chin N. W., Lanks K. W. Use of immobilized lactoperoxidase to label L cell proteins involved in adhesion to polystyrene. J Cell Biol. 1980 May;85(2):402–413. doi: 10.1083/jcb.85.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher C. W., Morgan R. A. Are lysosomes involved in hexose transport regulation? Turnover of hexose carriers and the activity of thiol cathepsins are arrested by cyanate and ammonia. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4416–4420. doi: 10.1073/pnas.78.7.4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Hammond G. L., Lai Y. K., Markert C. L. Diverse forms of stress lead to new patterns of gene expression through a common and essential metabolic pathway. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3485–3488. doi: 10.1073/pnas.79.11.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser L., Levy-Wilson B. Kinetic changes in protein synthesis in response to a sublethal heat shock in starved Tetrahymena thermophila. J Biol Chem. 1981 Apr 25;256(8):3612–3614. [PubMed] [Google Scholar]
- Kasambalides E. J., Lanks K. W. Effects of low molecular weight nutrients on the pattern of proteins synthesized by non-proliferating murine L cells. Exp Cell Res. 1981 Mar;132(1):31–39. doi: 10.1016/0014-4827(81)90079-3. [DOI] [PubMed] [Google Scholar]
- Kasambalides E. J., Lanks K. W. Patterns of proteins synthesized by non-proliferating murine L cells. Exp Cell Res. 1979 Feb;118(2):269–275. doi: 10.1016/0014-4827(79)90152-6. [DOI] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. The effect of amino acid analogues and heat shock on gene expression in chicken embryo fibroblasts. Cell. 1978 Dec;15(4):1277–1286. doi: 10.1016/0092-8674(78)90053-3. [DOI] [PubMed] [Google Scholar]
- Lanks K. W., Kasambalides E. J., Chinkers M., Brugge J. S. A major cytoplasmic glucose-regulated protein is associated with the Rous sarcoma virus pp60src protein. J Biol Chem. 1982 Aug 10;257(15):8604–8607. [PubMed] [Google Scholar]
- Lanks K. W., Kasambalides E. J. Purification and characterization of a major component from the cytoplasmic matrix of cultured murine L cells. Biochim Biophys Acta. 1979 May 23;578(1):1–12. doi: 10.1016/0005-2795(79)90106-5. [DOI] [PubMed] [Google Scholar]
- Lazo P. A. Amino acids and glucose utilization by different metabolic pathways in ascites-tumour cells. Eur J Biochem. 1981 Jun;117(1):19–25. doi: 10.1111/j.1432-1033.1981.tb06297.x. [DOI] [PubMed] [Google Scholar]
- Li G. C., Werb Z. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc Natl Acad Sci U S A. 1982 May;79(10):3218–3222. doi: 10.1073/pnas.79.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S. Regulation of protein synthesis during heat shock. Nature. 1981 Sep 24;293(5830):311–314. doi: 10.1038/293311a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oppermann H., Levinson W., Bishop J. M. A cellular protein that associates with the transforming protein of Rous sarcoma virus is also a heat-shock protein. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1067–1071. doi: 10.1073/pnas.78.2.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitzer L. J., Wice B. M., Kennell D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem. 1979 Apr 25;254(8):2669–2676. [PubMed] [Google Scholar]
- Tsukeda H., Maekawa H., Izumi S., Nitta K. Effect of heat shock on protein synthesis by normal and malignant human lung cells in tissue culture. Cancer Res. 1981 Dec;41(12 Pt 1):5188–5192. [PubMed] [Google Scholar]
- Voellmy R., Bromley P. A. Massive heat-shock polypeptide synthesis in late chicken embryos: convenient system for study of protein synthesis in highly differentiated organisms. Mol Cell Biol. 1982 May;2(5):479–483. doi: 10.1128/mcb.2.5.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuyama S., Zimmerman A. M. RNA synthesis in Tetrahymena. Temperature-pressure studies. Exp Cell Res. 1972 Mar;71(1):193–203. doi: 10.1016/0014-4827(72)90278-9. [DOI] [PubMed] [Google Scholar]