Abstract
There has been a long controversy concerning whether the amygdala's response to emotional stimuli is automatic or dependent on attentional load. Using magnoencephalography and an advanced beamformer source localization technique, we found that amygdala automaticity was a function of time: while early amygdala responding to emotional stimuli (40–140 ms) was unaffected by attentional load, later amygdala response (280–410 ms), subsequent to frontoparietal cortex activity, was modulated by attentional load.
Introduction
There has been considerable debate regarding the automaticity of the amygdala response to emotional stimuli. Rodent data indicate that the amygdala responds to emotional stimulation through a fast but coarse subcortical (thalamus–amygdala) processing stream in parallel to a slower, more detail-oriented, cortical pathway (Quirk et al., 1995, 1997; LeDoux, 2000). It has been proposed that these dual routes are present in humans too, and because of the existence of the subcortical route, the amygdala's response to emotional stimuli is largely automatic and can occur independently of attention (de Gelder et al., 2003; Vuilleumier et al., 2003; Whalen et al., 2004; for review, see Dolan and Vuilleumier, 2003; Johnson, 2005). Others have suggested that it is unlikely that representations of emotional stimuli are independent of representational competition and that increased attention to nonemotional stimulus features should significantly reduce the amygdala's response to emotional stimulus features (Pessoa et al., 2002; Bishop et al., 2007; Blair et al., 2007; Mitchell et al., 2008; Pessoa, 2009; for review, see Pessoa and Ungerleider, 2004). It is possible, however, that this debate has been fostered by the limited temporal resolution of blood oxygenation level-dependent (BOLD) functional magnetic resonance imaging (fMRI) data. There may be a rapid, automatic amygdala response to emotional stimuli, possibly via the subcortical route, that is contaminated because of the limited temporal properties of the BOLD response by a slower, attentionally modulated response. If this is true, it can be predicted that there is an early amygdala response that is relatively independent of attentional control and a later amygdala response to the same emotional stimuli that is subject to significant attentional modulation.
To test this hypothesis, it is necessary to use techniques with good temporal and spatial resolution. Magnetoencephalography (MEG), combined with the advanced source analysis technique synthetic aperture magnetometry (SAM) and a sliding window analysis, is ideal for this purpose (for details, see supplemental Methods, available at www.jneurosci.org as supplemental material). While there has been debate regarding the ability of MEG to identify activity in subcortical nuclei (Vrba and Robinson, 2001, 2002), previous work from a variety of laboratories has demonstrated the ability of MEG to detect a signal from deep structures such as hippocampus (Rogers et al., 1991; Ioannides et al., 1995) and amygdala (Ioannides et al., 1995; Streit et al., 2003) even when using evoked field methods. Moreover, SAM uses the second-order covariance between channels rather than single-channel averages and thus is sensitive to spatially correlated activity. As such, it is more sensitive to deep structure activity (Vrba and Robinson, 2001, 2002). Indeed, recent studies using SAM have reported robust amygdala signals over time (Cornwell et al., 2007; Luo et al., 2007, 2009).
We focused on gamma band oscillations as they are considered to be of particular importance for cognition (Singer, 1999; Varela et al., 2001; Bichot et al., 2005; Fries et al., 2007) and emotion (Keil et al., 2001; Oya et al., 2002; Luo et al., 2007; Desmond et al., 2008) and have a demonstrable relationship with the BOLD response (Logothetis et al. 2001; Niessing et al., 2005). An adapted form of an attentional interference paradigm (Erthal et al., 2005) was used in the present study.
Materials and Methods
Task design
Sixteen volunteers (eight males) aged between 22 and 41 years participated. All gave written informed consent and the study was approved by the National Institute of Mental Health Institutional Review Board.
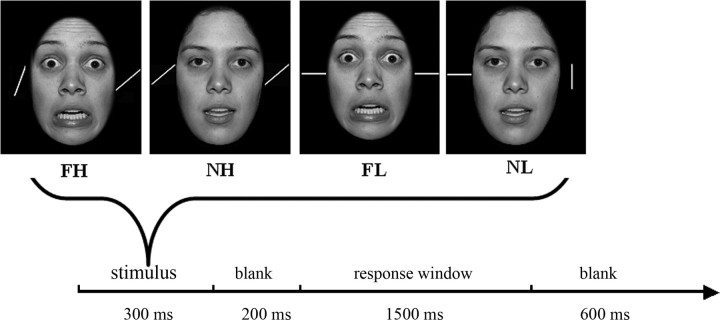
This paradigm, adapted from Erthal et al. (2005), involved a 2 (task load: high, low) × 2 (distracter: fearful, neutral) design. Each trial involved the participant responding to a stimulus array containing a face (fearful or neutral) bracketed between two lines by judging, via button press, whether the lines were parallel or not. Task load was manipulated by varying the angular difference of the bars on nonparallel trials (90° for low-load and 15° for high-load trials). The participant received 208 trials (52 trials in each of the four conditions). The face stimuli (50% male) were selected from the NimStim Face Stimulus Set (http://www.macbrain.org/resource.htm). We cut away the hair and transformed the photos into grayscale.
Each trial lasted 2600 ms (Fig. 1). For the initial 300 ms, the participant received the stimulus: a face bracketed by two lines. There was then a 200 ms blank screen before the response window was presented for 1500 ms. They responded by pressing either the left (S: same) or the right (D: different) button after seeing the response cue (S D). The response window was followed by a second blank screen lasting 600 ms. Stimuli were presented within Presentation software (Neurobehavioral Systems).
Figure 1.
Each stimulus array was presented for 300 ms followed by a 200 ms blank screen. The participant judged whether the bars were parallel during the subsequent 1500 ms response window. The response window was followed by a blank of 600 ms. FH, Fearful expression, high task load; NH. neutral expression, high task load; FL, fearful expression, low task load; NL, neutral expression, low task load.
Data acquisition
MEG data were recorded at 600 Hz using a 275 channel CTF whole-head MEG system in a shielded environment. The CTF MEG system is equipped with synthetic third gradient balancing, an active noise cancellation technique that uses a set of reference channels to subtract background interference. The resulting noise floor is on the order of 5–7 fT above 1 Hz. At the beginning and end of each measurement, the participant's head position was registered with localization coils that were placed at the nasion and the bilateral preauricular points. It was required that head movements did not exceed 0.5 cm. By registration of the head position at these three points, the MEG data could be superimposed on the individual anatomical images with an accuracy of a few millimeters. High-resolution anatomical MRI images were acquired using a T1-weighted, three-dimensional, spoiled GRASS (gradient-recalled acquisition in steady state) imaging sequence (1 × 1 × 1.5 mm3) with a 1.5 tesla GE scanner.
Data processing
Preprocessing.
The VSM/CTF software and software developed at the National Institute of Mental Health (NIMH) MEG Core Facility (Bethesda, MD) together with AFNI (http://afni.nimh.nih.gov/afni/) were used for data processing. Before doing SAM analysis, the data were marked according to the four trial types. A multisphere head model was created for each participant based the anatomical image of each participant. The advantage of using a multisphere over a single-sphere model is that in the former each sphere (one per MEG sensor) is fitted to a small patch of the head model (directly under the sensor) to better model the local return currents.
Time–frequency analysis.
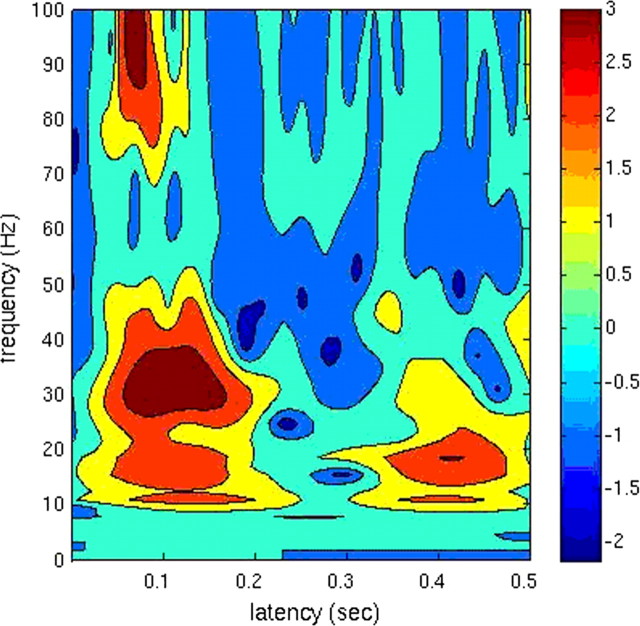
Time–frequency results provide a view of neuromagnetic signals represented over frequency across time. The results would help us to determine the specific frequency range of our analysis in the gamma band. Here, we performed a time–frequency analysis (0–100 Hz) based on averaged signals of the whole brain. The Ctf2st software developed by the NIMH MEG Core Facility (http://kurage.nimh.nih.gov/meglab/Meg/Ctf2st) was adopted. Ctf2st performs Stockwell time–frequency analysis within Matlab. To be consistent with later SAM analyses, the control window was −150 to 0 ms, and the active window was 0 to 500 ms. This was done on the averaged data on all the participants. The group time–frequency results were then contrasted with zero and thresholded at p < 0.05.
Sliding window SAM analysis.
In the present analysis, SAM was then used to analyze task-related activation differences in the gamma frequency band (30–50 Hz). To obtain an image of the dynamic spatiotemporal development of the brain's activity, a sliding window analysis was used in combination with SAM. With a window length of 150 ms and a step of 10 ms, we estimated the signal power in each voxel by using dual-state SAM imaging in which the control state (baseline) was the 150 ms before stimulus onset (or −150 to 0 ms), and the active state was a 150 ms window sliding with a 10 ms step: 150 to 0 ms, −140 to 10 ms, −130 to 20 ms, etc. The dual-state SAM output was the contrast between the active state and the control state. With sliding window SAM, we could obtain information regarding when significant gamma band oscillations emerged, peaked, and offset. For example, if significant gamma band oscillations in a region were not seen in the “−110 to 40 ms” window but seen in the “−100 to 50 ms” window, then we could infer that the onset of gamma band oscillations in this region was between 40 and 50 ms. Fifty dual-state SAM imaging analyses were performed with a spatial resolution of 7 mm. The output results were then concatenated, enabling us to obtain a time course in combination with spatial activation maps across all the time points starting from 150 ms before the stimulus to 500 ms after the stimulus. The time window for button response was not selected for analysis. The high-performance computational capabilities of the NIH Biowulf PC/Linux cluster (http://biowulf.nih.gov) was used to perform the above computation-intensive tasks.
Group analyses.
For group analyses, individual anatomical images were first spatially normalized to the Talairach brain atlas. The SAM results of participants were also normalized (transformed to z-score) and registered to their respective anatomical Talairach images. The group analysis for each of the 50 time windows was performed using a random effects 2 × 2 ANOVA model in AFNI, which generated the gamma band oscillation results. Oscillations in the gamma band of p < 0.005 were considered statistically significant.
Results
Behavioral results
A 2 × 2 ANOVA was conducted on the response time (RT) and error rate data. This revealed a significant effect of task load [F(1,15) = 16.103, p < 0.001]; the participants showed significantly longer RTs for the high-load relative to the low-load trials (423 and 370 ms, respectively). There was no significant effect of distracter emotionality [F(1, 15) = 0.206, p = 0.656] nor distracter emotionality by task load interaction [F(1, 15) = 1.348, p = 0.264]. The results on error rates were similar: a significant effect of task load [F(1, 15) = 25.340, p < 0.001], more errors for the high-load condition relative to the low-load condition (17 and 1%, respectively), but no significant effect of distracter emotionality (F(1, 15) = 0.000, p = 1) or distracter emotionality by task load interaction [F(1, 15) = 0.044, p = 0.837].
Time–frequency results
The result showed that within the gamma band there was strong power at 30–50 Hz (Fig. 2). This range was therefore used in the present analysis. Two other reasons for focusing on 30–50 Hz are as follows. (1) Within the gamma band, oscillations around 40 Hz are to date the most established frequencies associated with cognition (Singer, 1999; Varela et al., 2001). (2) Our previous MEG studies have shown that 30–50 Hz is modulated by emotion (Luo et al., 2007, 2009).
Figure 2.
Time–frequency results. Time–frequency change from 0 to100 Hz (the y-axis) over time from 0 to 500 ms (the x-axis) after stimulus onset based on averaged signals of the whole brain (p < 0.05).
MEG-SAM results in the gamma band
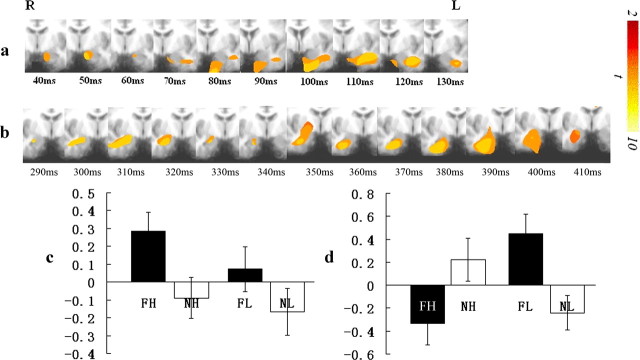
Our overall aim was to determine whether the amygdala response to emotional stimuli is under different levels of attentional control as a function of time. Our first goal was to determine whether participants showed an early amygdala response to emotional stimuli that is independent of attention, i.e., that is not modulated by task load. Consistent with our hypothesis, a significant main effect of distracter was seen within the left amygdala; increased gamma band activity was seen in response to fearful relative to neutral expressions very rapidly after stimulus onset (30–40 ms) and lasted until 50–60 ms (p < 0.005; at a more lenient threshold of p < 0.05 this effect lasted until 140 ms). There was no significant task load or task load-by-distracter interaction within the amygdala for this time period; i.e., task load did not modulate the early amygdala response (Fig. 3a,c).
Figure 3.
Spatiotemporal profiles of amygdala responses in the gamma band. a, Main effect of distracter emotionality within the left amygdala. R, Right; L, left. b, The task load-by-distracter interaction within the right amygdala. c, Power changes relative to the baseline by condition for the amygdala regions showing a main effect of distracter emotionality. d, Power changes relative to the baseline by condition for the amygdala region showing the task load-by-distracter emotionality interaction. FH, Fearful expression, high task load; NH, neutral expression, high task load; FL, fearful expression, low task load; NL, neutral expression, low task load.
Our second goal was to determine whether later amygdala responding is modulated by task load. Consistent with our hypothesis, a significant task load-by-distracter interaction was seen within the right amygdala starting at 280–290 ms and lasting until 330–340 ms (p < 0.005; at p < 0.05 this effect lasted until 410 ms). Notably this interaction became significant after a significant main effect of task load was seen within regions implicated in top-down attentional control (Desimone and Duncan, 1995) (lateral frontal [BA8] and parietal cortices [BA7]; 120–130 to 370–380 ms and 200–210 to 290–300 ms, respectively). Under high-load conditions, there was no significant effect of distracter emotionality on the amygdala response. In contrast, under low-load conditions, there was significantly greater gamma band activity within the amygdala to fearful relative to neutral expressions (p < 0.005) (Fig. 3b,d).
Discussion
Our results demonstrated an early (40–140 ms) amygdala response to emotional information that was independent of attentional modulation and a later (290–410 ms) amygdala response that showed significant attentional modulation. In short, these data indicate that the degree of automaticity of the amygdala response is a function of time.
Previous work has identified early gamma band activity in the amygdala in response to emotional expressions (Luo et al., 2007, 2009), and work with rodents has indicated that auditory fearful signals can reach the amygdala at a short latency of 12 ms (Quirk et al., 1995; LeDoux, 1998). The current data suggest that this early amygdala response is not modulated by attentional load because it occurs before task-related activity is seen within lateral frontal and parietal cortices. Later amygdala activity, which occurred after task related activity within these regions, showed a significant emotion-by-task load interaction. We hypothesize that this interaction reflects the impact of top-down attentional mechanisms. Specifically, stronger representation of task-relevant information during high-load trials via top-down attention should result in reduced representation of emotional distracter information caused by representational competition (Desimone and Duncan, 1995; Kastner and Ungerleider, 2000; Marois et al., 2004). We believe that the limited temporal resolution of BOLD data results in the contamination of the rapid, automatic amygdala response to emotional stimuli by the slower, attentionally modulated response.
Our results can be considered consistent with suggestions of a dual-route model to amygdala activation (LeDoux, 2000; de Gelder et al., 2003; Dolan and Vuilleumier, 2003; Ohman et al., 2007). Considerable animal work has demonstrated that conditioned stimulus (CS) inputs can reach the amygdala via this subcortical route (Quirk et al., 1995, 1997; LeDoux, 2000). Moreover, the human amygdala has been reported to show activity changes during conditioning to a masked CS that correlate with activity in the thalamus but not the cortex (Morris et al., 1999). Early findings of automatic amygdala activity that was not reduced by reduced attention were thought to reflect activity via this subcortical route (Vuilleumier et al., 2001; Anderson et al., 2003). Later fMRI work, however, indicated that increased attention to nonemotional stimulus features did significantly reduce the amygdala's response to emotional stimulus features (e.g., Pessoa et al., 2002; Bishop et al., 2007; Blair et al., 2007; Mitchell et al., 2008; Pessoa, 2009). It is possible that the early amygdala activity seen in the current study that was independent of attentional modulation was a result of stimulation of the amygdala via the subcortical route. The later amygdala activity that showed significant attentional modulation may reflect the interaction of lateral frontal and parietal regions implicated in top-down attentional control priming competing task-relevant representations within the cortical route.
The apparent laterality of our findings was not predicted. The left amygdala showed an early response to emotional information that was independent of attentional modulation, while the right amygdala showed a later response to emotional information that was significantly modulated by attention. However, it should be noted that, at a more lenient significance threshold (p < 0.05), the left amygdala also showed a later response to emotional information that was significantly modulated by attention. This started at 180–190 ms and lasted until 210–220 ms. While there was no indication of an early right amygdala response to emotional information in the current study, this has been reported in previous work (Luo et al., 2007). In short, on the basis of the current literature, we do not believe that the apparent laterality of our findings reflects genuine processing/connectivity differences between the left and right amygdala.
It is worth considering whether the current results are specific for emotional expressions or whether they might generalize to other stimulus categories. Most work considering the degree of attentional modulation of amygdala activity and the potential subcortical route has involved emotional expression stimuli (e.g., Pessoa et al., 2002; Vuilleumier et al., 2003; de Gelder et al., 2003; Whalen et al., 2004; Mitchell et al., 2008), although not all has done so (Blair et al., 2007). However, the potential evolutionary importance of the subcortical route (allowing the very rapid processing of potential threats) (Ohman et al., 2007) might suggest that similar findings should be seen for other biologically relevant threat stimuli (e.g., snakes, spiders, and snarling animals), although not for evolutionarily more recent threats (e.g., pointed guns). In this regard, it is interesting to note recent findings indicating altered processing of biologically relevant threat stimuli in a patient with pulvinar damage (Ward et al., 2007). Our current work explores these predictions.
In summary, our results suggest that the early amygdala response to emotional distracters may be automatic. We argue that this reflects the reduced time for frontoparietal cortex to augment the representational strength of task-relevant stimuli and thus, following representational competition, reduce the representational strength of emotional information. In contrast, later amygdala response is modulated by task load because frontoparietal cortex has had sufficient time to augment the representation of task-relevant information. In short, amygdala automaticity is a matter of timing.
Footnotes
This research was supported by the National Institute of Mental Health Intramural Research Program.
References
- Anderson AK, Christoff K, Panitz D, De Rosa E, Gabrieli JD. Neural correlates of the automatic processing of threat facial signals. J Neurosci. 2003;23:5627–5633. doi: 10.1523/JNEUROSCI.23-13-05627.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichot N, Rossi A, Desimone R. Parallel and serial neural mechanisms for visual search in macaque area V4. Science. 2005;308:529–534. doi: 10.1126/science.1109676. [DOI] [PubMed] [Google Scholar]
- Bishop SJ, Jenkins R, Lawrence AD. Neural processing of fearful faces: effects of anxiety are gated by perceptual capacity limitations. Cereb Cortex. 2007;17:1595–1603. doi: 10.1093/cercor/bhl070. [DOI] [PubMed] [Google Scholar]
- Blair KS, Smith BW, Mitchell DG, Morton J, Vythilingam M, Pessoa L, Fridberg D, Zametkin A, Sturman D, Nelson EE, Drevets WC, Pine DS, Martin A, Blair RJ. Modulation of emotion by cognition and cognition by emotion. Neuroimage. 2007;35:430–440. doi: 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell BR, Baas JM, Johnson L, Holroyd T, Carver FW, Lissek S, Grillon C. Neural responses to auditory stimulus deviance under threat of electric shock revealed by spatially-filtered magnetoencephalography. Neuroimage. 2007;37:282–289. doi: 10.1016/j.neuroimage.2007.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gelder B, Frissen I, Barton J, Hadjikhani N. A modulatory role for facial expressions in prosopagnosia. Proc Natl Acad Sci U S A. 2003;100:13105–13110. doi: 10.1073/pnas.1735530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Rev. Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Desmond J, Oathes DJ, Ray WJ, Yamasaki AS, Borkovec TD, Castonguay LG, Newman MG, Nitschke J. Worry, generalized anxiety disorder, and emotion: evidence from the EEG gamma band. Biol Psychol. 2008;79:165–170. doi: 10.1016/j.biopsycho.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan RJ, Vuilleumier P. Amygdala automaticity in emotional processing. Ann N Y Acad Sci. 2003;985:348–355. doi: 10.1111/j.1749-6632.2003.tb07093.x. [DOI] [PubMed] [Google Scholar]
- Erthal FS, de Oliveira L, Mocaiber I, Pereira MG, Machado-Pinheiro W, Volchan E, Pessoa L. Load-dependent modulation of affective picture processing. Cogn Affect Behav Neurosci. 2005;5:388–395. doi: 10.3758/cabn.5.4.388. [DOI] [PubMed] [Google Scholar]
- Fries P, Nikolić D, Singer W. The gamma cycle. Trends Neurosci. 2007;30:309–316. doi: 10.1016/j.tins.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Ioannides AA, Liu MJ, Liu LC, Bamidis PD, Hellstrand E, Stephan KM. Magnetic field tomography of cortical and deep processes: examples of ‘real-time mapping’ of averaged and single trial MEG signals. Int J Psychophysiol. 1995;20:161–175. doi: 10.1016/0167-8760(95)00031-3. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Sub-cortical face processing. Nat Rev Neurosci. 2005;6:766–774. doi: 10.1038/nrn1766. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider L. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Keil A, Müller MM, Gruber T, Wienbruch C, Stolarova M, Elbert T. Effects of emotional arousal in the cerebral hemispheres: a study of oscillatory brain activity and event-related potentials. Clin Neurophysiol. 2001;112:2057–2068. doi: 10.1016/s1388-2457(01)00654-x. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. The emotional brain. New York: Sïmon and Schuster; 1998. [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Luo Q, Holroyd T, Jones M, Hendler T, Blair J. Neural dynamics for facial threat processing as revealed by gamma band synchronization using MEG. Neuroimage. 2007;34:839–847. doi: 10.1016/j.neuroimage.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q, Mitchell D, Cheng X, Mondillo K, Mccaffrey D, Holroyd T, Carver F, Coppola R, Blair RJ. Visual awareness, emotion, and gamma band synchronization. Cereb Cortex. 2009;19:1896–1904. doi: 10.1093/cercor/bhn216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marois R, Chun MM, Gore JC. A common parieto-frontal network is recruited under both low visibility and high perceptual interference conditions. J Neurophysiol. 2004;92:2985–2992. doi: 10.1152/jn.01061.2003. [DOI] [PubMed] [Google Scholar]
- Mitchell D, Luo Q, Mondillo K, Vythilingam M, Finger E, Blair RJ. The interference of operant task performance by emotional distracters: an antagonistic relationship between the amygdala and frontoparietal cortices. Neuroimage. 2008;40:859–868. doi: 10.1016/j.neuroimage.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. A subcortical pathway to the right amygdala mediating “unseen” fear. Proc Natl Acad Sci U S A. 1999;96:1680–1685. doi: 10.1073/pnas.96.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RAW. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309:948–951. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- Ohman A, Carlsson K, Lundqvist D, Ingvar M. On the unconscious subcortical origin of human fear. Physiol Behav. 2007;10:180–185. doi: 10.1016/j.physbeh.2007.05.057. [DOI] [PubMed] [Google Scholar]
- Oya H, Kawasaki H, Howard MA, III, Adolphs R. Electrophysiological responses in the human amygdala discriminate emotion categories of complex visual stimuli. J Neurosci. 2002;22:9502–9512. doi: 10.1523/JNEUROSCI.22-21-09502.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive control? Trends Cogn Sci. 2009;13:160–166. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Ungerleider LG. Neuroimaging studies of attention and the processing of emotion-laden stimuli. Prog Brain Res. 2004;144:171–182. doi: 10.1016/S0079-6123(03)14412-3. [DOI] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proc Natl Acad Sci U S A. 2002;99:11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Armony JL, LeDoux JE. Fear conditioning enhances different temporal components of toned-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997;19:613–624. doi: 10.1016/s0896-6273(00)80375-x. [DOI] [PubMed] [Google Scholar]
- Rogers RL, Papanicolaou AC, Baumann SB, Bourbon TW, Alagarsamy S, Eisenberg HM. Localization of P3 sources using magnetoencephalography and magnetic resonance imaging. Electroencephalogr Clin Neurophysiol. 1991;79:308–321. doi: 10.1016/0013-4694(91)90126-o. [DOI] [PubMed] [Google Scholar]
- Singer W. Neurobiology: striving for coherence. Nature. 1999;397:391–393. doi: 10.1038/17021. [DOI] [PubMed] [Google Scholar]
- Streit M, Dammers J, Simsek-Kraues S, Brinkmeyer J, Wolwer W, Ioannides AA. Time course of regional brain activations during facial emotion recognition in humans. Neurosci Lett. 2003;342:101–104. doi: 10.1016/s0304-3940(03)00274-x. [DOI] [PubMed] [Google Scholar]
- Varela F, Lachaux J, Rodriguez E, Martinerie J. The brain web: phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Vrba J, Robinson SE. Signal processing in magnetoencephalography. Methods Mol Med. 2001;25:249–271. doi: 10.1006/meth.2001.1238. [DOI] [PubMed] [Google Scholar]
- Vrba J, Robinson SE. SQUID sensor array configurations for magnetoencephalography applications. Supercond Sci Technol. 2002;15:R51–R89. [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron. 2001;30:829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nat Neurosci. 2003;6:624–631. doi: 10.1038/nn1057. [DOI] [PubMed] [Google Scholar]
- Ward R, Calder AJ, Parker M, Arend I. Emotion recognition following human pulvinar damage. Neuropsychologia. 2007;45:1973–1978. doi: 10.1016/j.neuropsychologia.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Kagan J, Cook RG, Davis FC, Kim H, Polis S, McLaren DG, Somerville LH, McLean AA, Maxwell JS, Johnstone T. Human amygdala responsivity to masked fearful eye whites. Science. 2004;306:2061. doi: 10.1126/science.1103617. [DOI] [PubMed] [Google Scholar]