Abstract
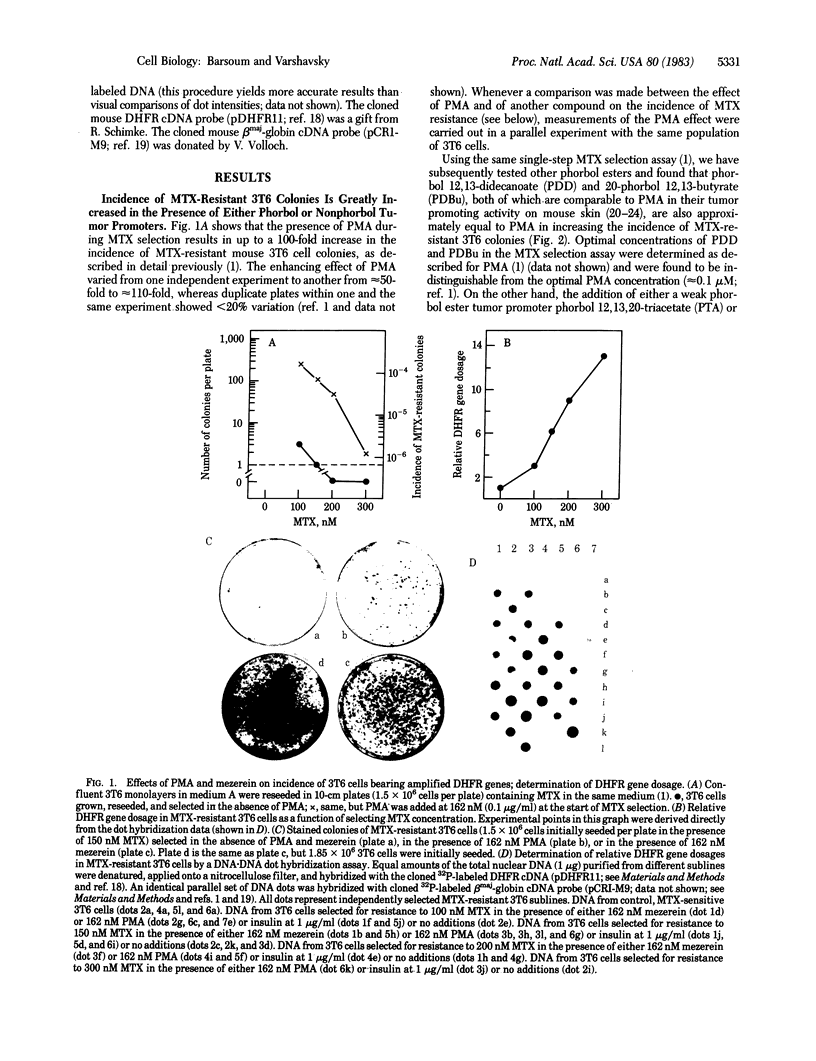
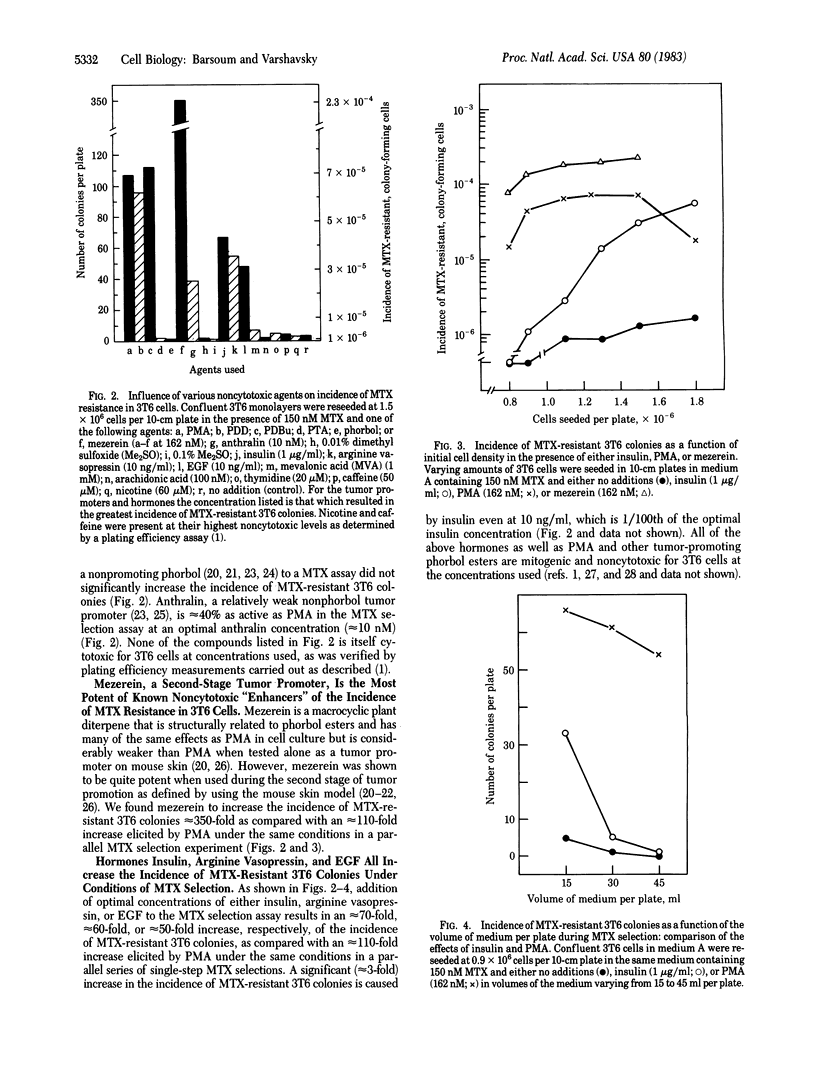
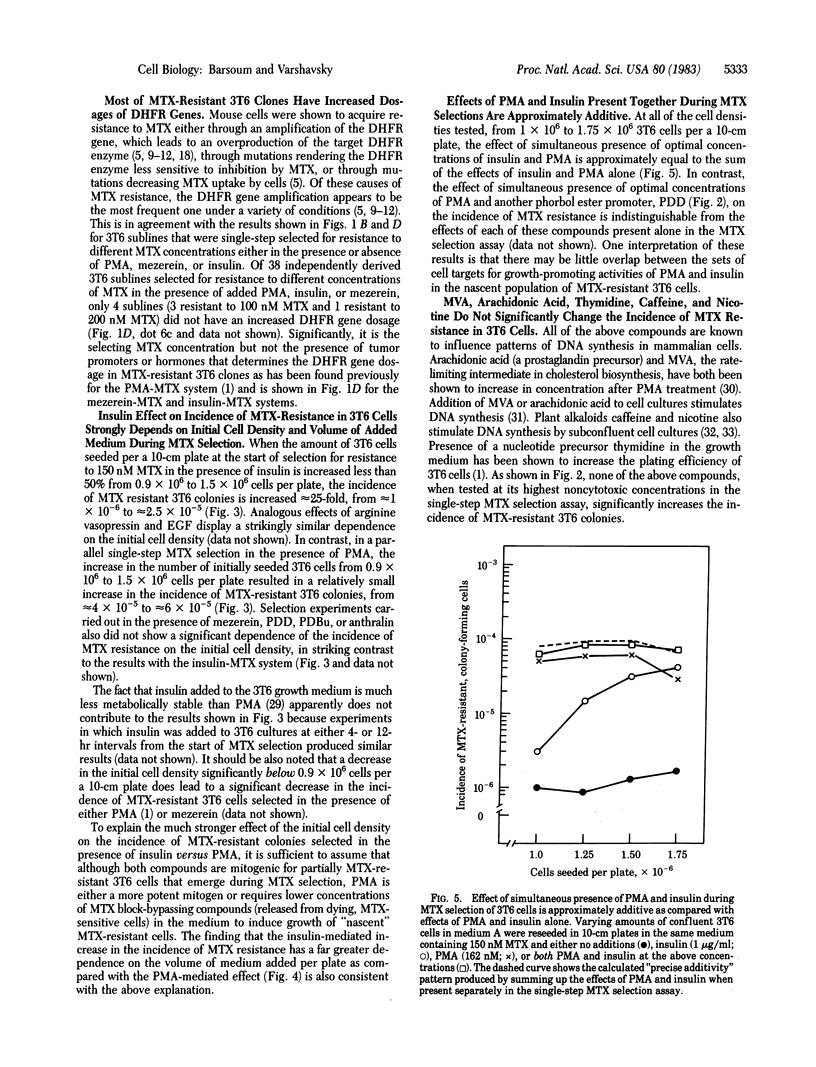
Previous work has shown that the presence of a phorbol ester tumor promoter, phorbol 12-myristate 13-acetate (PMA), during a single-step selection for methotrexate (MTX)-resistant mouse 3T6 cells results in an up to 100-fold increase in the incidence of MTX-resistant, colony-forming cells. MTX resistance of most of these cells is due to amplification of the gene for dihydrofolate reductase (DHFR), the target enzyme for MTX. We show here that other active, noncytotoxic phorbol ester tumor promoters, such as phorbol 12, 13-didecanoate and 20-phorbol 12,13-butyrate, at their optimal concentrations (approximately equal to 0.1 microM) are approximately equal to PMA in increasing the incidence of MTX-resistant 3T6 colonies. Mezerein, a potent second-stage tumor promoter, but a weak complete promoter, increases the incidence of MTX resistance up to 350-fold, the strongest effect for any of the agents so far tested. PMA analogs that are inactive as tumor promoters, such as phorbol or phorbol 12,13,20-triacetate, have no effect on the incidence of MTX-resistant 3T6 colonies. Anthralin, a nonphorbol tumor promoter, is approximately equal to 40% as active as PMA in the MTX selection assay. Remarkably, the hormones insulin, arginine vasopressin, and epidermal growth factor, all of which are mitogenic for 3T6 cells, also exert a strong PMA-like effect on the incidence of MTX-resistant 3T6 colonies under conditions of MTX selection. The effect of insulin at its optimal concentration (approximately equal to 1 microgram/ml) is approximately equal to 70% that of PMA. Although the effect of PMA on the incidence of MTX-resistant 3T6 colonies does not significantly depend on the initial density of seeded cells or volume of the medium added, the analogous effect of insulin is strongly influenced by these parameters. Mevalonic acid, arachidonic acid, thymidine, caffeine, and nicotine, all of which are known to influence patterns of DNA synthesis in mammalian cells, were tested at their highest noncytotoxic concentrations and failed to produce any significant effect on the incidence of MTX-resistant 3T6 colonies. We discuss possible mechanisms of hormone- and tumor promoter-facilitated gene amplification in mammalian cells, relationship of mitogenic hormones to tumor promoters, and also implications of our findings for the problem of drug resistance in cancer chemotherapy.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreis P. G., Whitfield J. F., Armato U. Stimulation of DNA synthesis and mitosis of hepatocytes in primary cultures of neonatal rat liver by arachidonic acid and prostaglandins. Exp Cell Res. 1981 Aug;134(2):265–272. doi: 10.1016/0014-4827(81)90425-0. [DOI] [PubMed] [Google Scholar]
- Barker P. E. Double minutes in human tumor cells. Cancer Genet Cytogenet. 1982 Feb;5(1):81–94. doi: 10.1016/0165-4608(82)90043-7. [DOI] [PubMed] [Google Scholar]
- Barker P. E., Hsu T. C. Are double minutes chromosomes? Exp Cell Res. 1978 May;113(2):456–458. doi: 10.1016/0014-4827(78)90391-9. [DOI] [PubMed] [Google Scholar]
- Barrett J. C., Wong A., McLachlan J. A. Diethylstilbestrol induces neoplastic transformation without measurable gene mutation at two loci. Science. 1981 Jun 19;212(4501):1402–1404. doi: 10.1126/science.6262919. [DOI] [PubMed] [Google Scholar]
- Baskin F., Rosenberg R. N., Dev V. Correlation of double-minute chromosomes with unstable multidrug cross-resistance in uptake mutants of neuroblastoma cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3654–3658. doi: 10.1073/pnas.78.6.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedler J. L., Spengler B. A. Metaphase chromosome anomaly: association with drug resistance and cell-specific products. Science. 1976 Jan 16;191(4223):185–187. doi: 10.1126/science.942798. [DOI] [PubMed] [Google Scholar]
- Boutwell R. K. The function and mechanism of promoters of carcinogenesis. CRC Crit Rev Toxicol. 1974 Jan;2(4):419–443. doi: 10.3109/10408447309025704. [DOI] [PubMed] [Google Scholar]
- Colburn N. H., Wendel E. J., Abruzzo G. Dissociation of mitogenesis and late-stage promotion of tumor cell phenotype by phorbol esters: mitogen-resistant variants are sensitive to promotion. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6912–6916. doi: 10.1073/pnas.78.11.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan K. H., Goldsmith M. E., Levine R. M., Aitken S. C., Douglass E., Clendeninn N., Nienhuis A. W., Lippman M. E. Dihydrofolate reductase gene amplification and possible rearrangement in estrogen-responsive methotrexate-resistant human breast cancer cells. J Biol Chem. 1982 Dec 25;257(24):15079–15086. [PubMed] [Google Scholar]
- Cowell J. K. Double minutes and homogeneously staining regions: gene amplification in mammalian cells. Annu Rev Genet. 1982;16:21–59. doi: 10.1146/annurev.ge.16.120182.000321. [DOI] [PubMed] [Google Scholar]
- De Young L. M., Helmes C. T., Chao W. R., Young J. M., Miller V. Paradoxical effect of anthralin on 12-O-tetradecanoylphorbol-13-acetate-induced mouse epidermal ornithine decarboxylase activity, proliferation, and tumor promotion. Cancer Res. 1981 Jan;41(1):204–208. [PubMed] [Google Scholar]
- Dexter D. L., Calabresi P. Intraneoplastic diversity. Biochim Biophys Acta. 1982 Dec 21;695(2):97–112. doi: 10.1016/0304-419x(82)90019-1. [DOI] [PubMed] [Google Scholar]
- Diamond L., O'Brien T. G., Baird W. M. Tumor promoters and the mechanism of tumor promotion. Adv Cancer Res. 1980;32:1–74. doi: 10.1016/s0065-230x(08)60360-7. [DOI] [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Phorbol esters and vasopressin stimulate DNA synthesis by a common mechanism. Nature. 1980 Oct 16;287(5783):607–612. doi: 10.1038/287607a0. [DOI] [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Tumour promoter induces proliferation of 3T6 cells in serum-free medium, as do polypeptide growth factors. Exp Cell Res. 1980 Dec;130(2):474–477. doi: 10.1016/0014-4827(80)90030-0. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Kochenburger R. J., Castagna M., Blumberg P. M. Kinetics and subcellular localization of specific [3H]phorbol 12, 13-dibutyrate binding by mouse brain. Cancer Res. 1981 Jul;41(7):2640–2647. [PubMed] [Google Scholar]
- Emerit I., Cerutti P. A. Tumour promoter phorbol-12-myristate-13-acetate induces chromosomal damage via indirect action. Nature. 1981 Sep 10;293(5828):144–146. doi: 10.1038/293144a0. [DOI] [PubMed] [Google Scholar]
- Enomoto T., Sasaki Y., Shiba Y., Kanno Y., Yamasaki H. Tumor promoters cause a rapid and reversible inhibition of the formation and maintenance of electrical cell coupling in culture. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5628–5632. doi: 10.1073/pnas.78.9.5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganss M., Seemann D., Fürstenberger G., Marks F. Calcium-dependent release of arachidonic acid from a murine epidermal cell line induced by the tumor promoter TPA or ionophore A 23187: dissection of ionophoretic and phospholipase A2-stimulating activity. FEBS Lett. 1982 Jun 1;142(1):54–58. doi: 10.1016/0014-5793(82)80218-4. [DOI] [PubMed] [Google Scholar]
- Guernsey D. L., Ong A., Borek C. Thyroid hormone modulation of X ray-induced in vitro neoplastic transformation. Nature. 1980 Dec 11;288(5791):591–592. doi: 10.1038/288591a0. [DOI] [PubMed] [Google Scholar]
- Hamlin J. L., Biedler J. L. Replication pattern of a large homogenously staining chromosome region in antifolate-resistant Chinese hamster cell lines. J Cell Physiol. 1981 Apr;107(1):101–114. doi: 10.1002/jcp.1041070112. [DOI] [PubMed] [Google Scholar]
- Harrison J., Auersperg N. Epidermal growth factors enhances viral transformation of granulosa cells. Science. 1981 Jul 10;213(4504):218–219. doi: 10.1126/science.6264597. [DOI] [PubMed] [Google Scholar]
- Horowitz A. D., Greenebaum E., Weinstein I. B. Identification of receptors for phorbol ester tumor promoters in intact mammalian cells and of an inhibitor of receptor binding in biologic fluids. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2315–2319. doi: 10.1073/pnas.78.4.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kletzien R. F., Day P. Modulation of the G0 to S phase transit time by insulin: potential involvement of protein phosphorylation. J Cell Physiol. 1980 Dec;105(3):533–539. doi: 10.1002/jcp.1041050318. [DOI] [PubMed] [Google Scholar]
- Lavi S. Carcinogen-mediated amplification of viral DNA sequences in simian virus 40-transformed Chinese hamster embryo cells. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6144–6148. doi: 10.1073/pnas.78.10.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levan A., Levan G. Have double minutes functioning centromeres? Hereditas. 1978;88(1):81–92. doi: 10.1111/j.1601-5223.1978.tb01606.x. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Cancer metastasis. Organ colonization and the cell-surface properties of malignant cells. Biochim Biophys Acta. 1982 Dec 21;695(2):113–176. doi: 10.1016/0304-419x(82)90020-8. [DOI] [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell P. C. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Nunberg J. H., Kaufman R. J., Chang A. C., Cohen S. N., Schimke R. T. Structure and genomic organization of the mouse dihydrofolate reductase gene. Cell. 1980 Feb;19(2):355–364. doi: 10.1016/0092-8674(80)90510-3. [DOI] [PubMed] [Google Scholar]
- Painter R. B. Effect of caffeine on DNA synthesis in irradiated and unirradiated mammalian cells. J Mol Biol. 1980 Nov 5;143(3):289–301. doi: 10.1016/0022-2836(80)90191-6. [DOI] [PubMed] [Google Scholar]
- Poste G., Fidler I. J. The pathogenesis of cancer metastasis. Nature. 1980 Jan 10;283(5743):139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- Rochaix J. D., Rougeon F., Mach B. Electron microscope analysis of mouse and rabbit globin and immunoglobulin gene sequences. Gene. 1978 Feb;3(1):9–16. doi: 10.1016/0378-1119(78)90003-3. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., Brown P. C., Kaufman R. J., McGrogan M., Slate D. L. Chromosomal and extrachromosomal localization of amplified dihydrofolate reductase genes in cultured mammalian cells. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):785–797. doi: 10.1101/sqb.1981.045.01.097. [DOI] [PubMed] [Google Scholar]
- Schnös M., Inman R. B. Caffeine-induced re-initiation of phage lambda DNA replication. J Mol Biol. 1982 Aug 15;159(3):457–465. doi: 10.1016/0022-2836(82)90294-7. [DOI] [PubMed] [Google Scholar]
- Slaga T. J., Fischer S. M., Nelson K., Gleason G. L. Studies on the mechanism of skin tumor promotion: evidence for several stages in promotion. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3659–3663. doi: 10.1073/pnas.77.6.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. On the possibility of metabolic control of replicon "misfiring": relationship to emergence of malignant phenotypes in mammalian cell lineages. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3673–3677. doi: 10.1073/pnas.78.6.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. Phorbol ester dramatically increases incidence of methotrexate-resistant mouse cells: possible mechanisms and relevance to tumor promotion. Cell. 1981 Aug;25(2):561–572. doi: 10.1016/0092-8674(81)90074-x. [DOI] [PubMed] [Google Scholar]
- Woodcock D. M., Cooper I. A. Aberrant double replication of segments of chromosomal DNA following DNA synthesis inhibition by cytosine arabinoside. Exp Cell Res. 1979 Oct 1;123(1):157–166. doi: 10.1016/0014-4827(79)90432-4. [DOI] [PubMed] [Google Scholar]