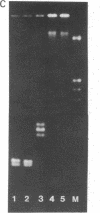
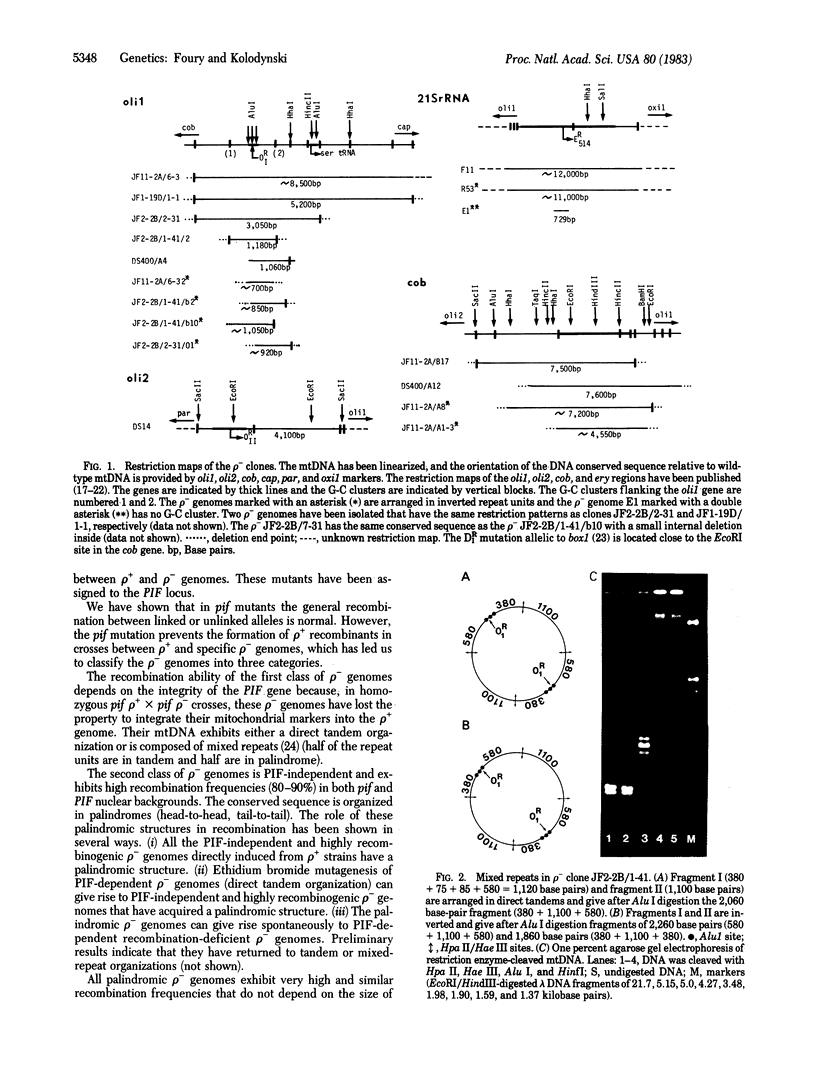
Abstract
Three allelic nuclear mutants affected in the recombination of mtDNA have been characterized in Saccharomyces cerevisiae and assigned to the PIF locus. In the mutants, the general recombination measured by the recombination frequency between linked or unlinked alleles is normal. However, the pif mutations prevent the integration into the rho+ genome of the markers (oli1, oli2, diu1, ery, oxi1, oxi2) of those rho- genomes that have tandemly arrayed repeat units. Therefore, these rho- genomes characterize a PIF-dependent recombination system. The pif mutations have also revealed the existence of a PIF-independent recombination system used by those rho- genomes that have an inverted organization of their repeat units. The markers of such palindromic rho- genomes exhibit high integration frequency into the rho+ genome even in the presence of the pif mutation. In addition, the pif mutations greatly increase suppressiveness in crosses between pif rho+ strains and PIF-dependent as well as PIF-independent rho- clones. We conclude that the recombination between rho+ and rho- genomes involves at least two distinct systems that depend on the organization of the rho- genome.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianchi L., Foury F. Antimutators of mitochondrial and nuclear DNA in Saccharomyces cerevisiae. Relationship with gamma-ray sensitivity. Mol Gen Genet. 1982;185(3):418–423. doi: 10.1007/BF00334133. [DOI] [PubMed] [Google Scholar]
- Blanc H., Dujon B. Replicator regions of the yeast mitochondrial DNA responsible for suppressiveness. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3942–3946. doi: 10.1073/pnas.77.7.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boltin-Fukuhara M., Fukuhara H. Modified recombination and transmission of mitochondrial genetic markers in rho minus mutants of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4608–4612. doi: 10.1073/pnas.73.12.4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calos M. P., Miller J. H. Transposable elements. Cell. 1980 Jul;20(3):579–595. doi: 10.1016/0092-8674(80)90305-0. [DOI] [PubMed] [Google Scholar]
- Colson A. M., Slonimski P. P. Genetic localization of diuron- and mucidin-resistant mutants relative to a group of loci of the mitochondrial DNA controlling coenzyme QH2-cytochrome c reductase in Saccharomyces cerevisiae. Mol Gen Genet. 1979 Jan 2;167(3):287–298. doi: 10.1007/BF00267421. [DOI] [PubMed] [Google Scholar]
- Conde J., Fink G. R. A mutant of Saccharomyces cerevisiae defective for nuclear fusion. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3651–3655. doi: 10.1073/pnas.73.10.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujon B. Sequence of the intron and flanking exons of the mitochondrial 21S rRNA gene of yeast strains having different alleles at the omega and rib-1 loci. Cell. 1980 May;20(1):185–197. doi: 10.1016/0092-8674(80)90246-9. [DOI] [PubMed] [Google Scholar]
- Dujon B., Slonimski P. P., Weill L. Mitochondrial genetics IX: A model for recombination and segregation of mitochondrial genomes in saccharomyces cerevisiae. Genetics. 1974 Sep;78(1):415–437. doi: 10.1093/genetics/78.1.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancashire W. E., Mattoon J. R. Cytoduction: a tool for mitochondrial genetic studies in yeast. Utilization of the nuclear-fusion mutation kar 1-1 for transfer of drug r and mit genomes in Saccharomyces cerevisiae. Mol Gen Genet. 1979 Mar 5;170(3):333–344. doi: 10.1007/BF00267067. [DOI] [PubMed] [Google Scholar]
- Lazowska J., Slonimski P. P. Site-specific recombination in "petite colony" mutants of Saccharomyces cerevisiae. I. Electron microscopic analysis of the organization of recombinant DNA resulting from end to end joining of two mitochondrial segments. Mol Gen Genet. 1977 Nov 14;156(2):163–175. doi: 10.1007/BF00283489. [DOI] [PubMed] [Google Scholar]
- Macino G., Tzagoloff A. Assembly of the mitochondrial membrane system. The DNA sequence of a mitochondrial ATPase gene in Saccharomyces cerevisiae. J Biol Chem. 1979 Jun 10;254(11):4617–4623. [PubMed] [Google Scholar]
- Macino G., Tzagoloff A. Assembly of the mitochondrial membrane system: partial sequence of a mitochondrial ATPase gene in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Jan;76(1):131–135. doi: 10.1073/pnas.76.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macino G., Tzagoloff A. Assembly of the mitochondrial membrane system: sequence analysis of a yeast mitochondrial ATPase gene containing the oli-2 and oli-4 loci. Cell. 1980 Jun;20(2):507–517. doi: 10.1016/0092-8674(80)90637-6. [DOI] [PubMed] [Google Scholar]
- Michaelis G., Michel F., Lazowska J., Slonimski P. P. Recombined molecules of mitochondrial DNA obtained from crosses between cytoplasmic petite mutants of Saccharomyces cerevisiae: the stoichiometry of parental DNA repeats within the recombined molecule. Mol Gen Genet. 1976 Dec 8;149(2):125–130. doi: 10.1007/BF00332879. [DOI] [PubMed] [Google Scholar]
- Michaelis G., Petrochilo E., Slonimski P. P. Mitochondrial genetics. 3. Recombined molecules of mitochondrial DNA obtained from crosses between cytoplasmic petite mutants of Saccharomyces cerevisiae: physical and genetic characterization. Mol Gen Genet. 1973;123(1):51–65. doi: 10.1007/BF00282988. [DOI] [PubMed] [Google Scholar]
- Michel F., Grandchamp C., Dujon B. Genetic and physical characterization of a segment of yeast mitochondrial DNA involved in the control of genetic recombination. Biochimie. 1979;61(9):985–1010. doi: 10.1016/s0300-9084(80)80254-9. [DOI] [PubMed] [Google Scholar]
- Moustacchi E., Perlman P. S., Mahler H. R. A novel class of Saccharomyces cerevisiae mutants specifically UV-sensitive to "petite" induction. Mol Gen Genet. 1976 Nov 17;148(3):251–261. doi: 10.1007/BF00332899. [DOI] [PubMed] [Google Scholar]
- Nagley P., Linnane A. W. Expression of mitochondrial DNA in Saccharomyces cerevisiae: the construction of sets of isonuclear haploid strains containing different specified mitochondrial genomes. Biochem Biophys Res Commun. 1978 Nov 29;85(2):585–592. doi: 10.1016/0006-291x(78)91203-2. [DOI] [PubMed] [Google Scholar]
- Nobrega F. G., Tzagoloff A. Assembly of the mitochondrial membrane system. DNA sequence and organization of the cytochrome b gene in Saccharomyces cerevisiae D273-10B. J Biol Chem. 1980 Oct 25;255(20):9828–9837. [PubMed] [Google Scholar]
- Perlman P. S. Genetic analysis of petite mutants of Saccharomyces cerevisiae: transmissional types. Genetics. 1976 Apr;82(4):645–663. doi: 10.1093/genetics/82.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slonimski P. P., Tzagoloff A. Localization in yeast mitochondrial DNA of mutations expressed in a deficiency of cytochrome oxidase and/or coenzyme QH2-cytochrome c reductase. Eur J Biochem. 1976 Jan 2;61(1):27–41. doi: 10.1111/j.1432-1033.1976.tb09994.x. [DOI] [PubMed] [Google Scholar]
- Sor F., Fukuhara H. Complete DNA sequence coding for the large ribosomal RNA of yeast mitochondria. Nucleic Acids Res. 1983 Jan 25;11(2):339–348. doi: 10.1093/nar/11.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sor F., Fukuhara H. Unequal excision of complementary strands is involved in the generation of palindromic repetitions of rho- mitochondrial DNA in yeast. Cell. 1983 Feb;32(2):391–396. doi: 10.1016/0092-8674(83)90458-0. [DOI] [PubMed] [Google Scholar]
- de Zamaroczy M., Marotta R., Faugeron-Fonty G., Goursot R., Mangin M., Baldacci G., Bernardi G. The origins of replication of the yeast mitochondrial genome and the phenomenon of suppressivity. Nature. 1981 Jul 2;292(5818):75–78. doi: 10.1038/292075a0. [DOI] [PubMed] [Google Scholar]