Abstract
Epithelial cells respond to physico-chemical damage with up-regulation of major histocompatbility complex–like ligands that can activate the cytolytic potential of neighboring intraepithelial T cells by binding the activating receptor, NKG2D. The systemic implications of this lymphoid stress-surveillance response, however, are unknown. We found that antigens encountered at the same time as cutaneous epithelial stress induced strong primary and secondary systemic, T helper 2 (TH2)–associated atopic responses in mice. These responses required NKG2D-dependent communication between dysregulated epithelial cells and tissue-associated lymphoid cells. These data are germane to uncertainty over the afferent induction of TH2 responses and provide a molecular framework for considering atopy as an important component of the response to tissue damage and carcinogenesis.
A conserved feature of T lymphocytes is their subdivision into two main types. One, composed of CD4+ and CD8+ αβ T cells specific for complexes of peptides and major histocompatbility complex (MHC) molecules, has been termed “conventional” and is largely responsible for clonal, pathogen-specific memory responses that are the hallmark of adaptive immunity. The second T cell type, composed of cells expressing the γδ T cell receptor (TCR), is not generally specific for peptide-MHC complexes and may instead recognize cell surface microbial and/or self-encoded moieties, some of which are up-regulated through cellular dysregulation. Although γδ T cells exhibit some properties characteristic of adaptive immunity, a predominant function of these “unconventional” Tcells is thought to be lymphoid stress-surveillance because they do not require major clonal expansion, because their functional potentials are preprogrammed during development, and because some may be activated in vivo independently of the TCR, by cytokines or by ligands for activating natural killer (NK) receptors, such as NKG2D (1). Such cells are major components of large but enigmatic tissue-resident Tcell compartments, which include mouse dendritic epidermal T cells (DETCs) and human or mouse intraepithelial lymphocytes (IELs), among which CD4−CD8β− αβ T cells may also contribute to rapid lymphoid stress-surveillance.
Because NK G2D ligands such as Rae-1 (mouse) and MIC class 1 chain-related protein A (MICA) (human) are activated by DNA damage (2) and are frequently expressed by tumor cells, such T cell responses—which have cytolytic potential—have been associated with local tumor surveillance. Indeed, DETC-deficient mice show increased susceptibility to chemical carcinogenesis (3, 4). However, whether lymphoid stress-surveillance describes a purely local response or whether it affects the systemic immune compartment remains a key question. To investigate this, transgenic Rae-1 expression was induced as described (4) specifically in keratinocytes by a doxycycline (dox)–dependent, bitransgenic (BTg) molecular switch (fig. S1A). This mode of Rae-1 induction avoids pleiotropic effects of applying agents that induce a stressed state within the epidermis. Rae-1 induction on otherwise normal epithelium promoted rapid morphological rearrangements of DETCs and of epidermal Langerhans cells (LCs), which are a tissue-associated dendritic cell (DC) subset lacking NKG2D (figs. S1B and S2) (4). Such changes were not seen in single transgenic (STg) mice that cannot induce dox-dependent Rae-1 up-regulation. Although subject to acute Rae-1 up-regulation, patches containing highly purified ovalbumin (ova, a nominal nonself, non–microbe-associated antigen) or vehicle controls were placed onto skin of mice that had been gently shaved.
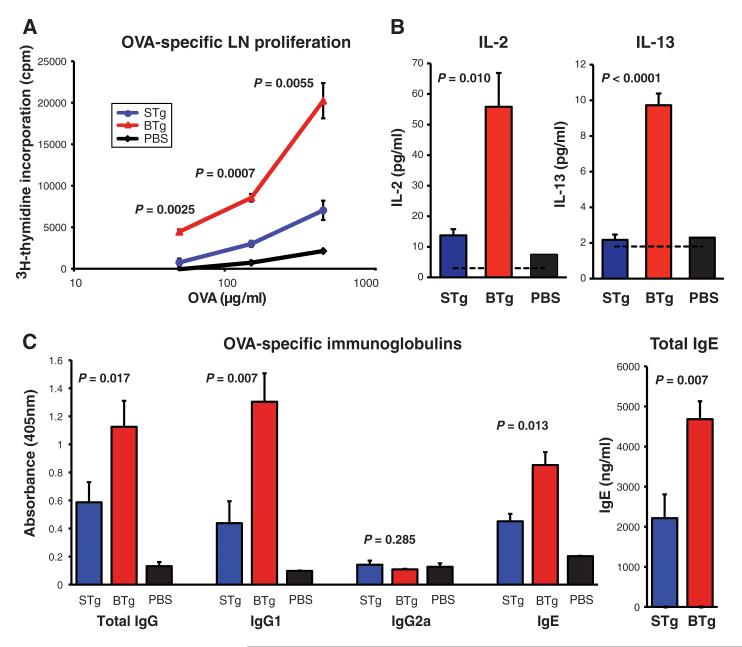
As in previous studies (5, 6) this provoked low but reproducible T helper 2 (TH2) immune responses in STg mice, which were characterized by low levels of interleukin 4 (IL-4) and IL-13 and IL-13–dependent induction of ova-specific and generalized immunoglobulin G1 (IgG1) and IgE (5). In contrast, BTg mice showed significant increases in responsiveness, as judged by ova-specific proliferation of lymph node (LN) and splenic T cells (Fig. 1A and fig. S3); ova-dependent LN IL-2 and IL-13 production (Fig. 1B); ova-specific IgG, IgG1, and IgE; and total IgE, which is customarily and substantially up-regulated in the context of antigen-specific IgE responses (Fig. 1C). Thus, localized epithelial up-regulation of a single self stress-antigen (Rae-1) can powerfully enhance the response to coincidentally encountered antigen. This “adjuvant-effect” amplified but did not dysregulate the immune response because there was no atypical induction of IgG2a.
Fig. 1.
Acute epidermal Rae-1 expression induces systemic immune effects. (A to C) Adjuvant-free ova was administered in phosphate-buffered saline (PBS) in epicutaneous patches to shaved areas of (red) BTg mice and (blue) STg mice dox-treated for 7 days. Ten days later, measurements were made of (A) skin draining LN ova-specific lymphocyte proliferation by means of [3H] thymidine incorporation; (B) of ova-dependent production of IL-2 and IL-13 by cells from draining LN, as measured with Luminex assay; and (C) of levels of serum ova-specific Ig and of total IgE. Black bars and lines show data for mice exposed to patches containing only PBS. Data expressed as mean ± 1 SEM; n = 6 to 8 mice per group, with experiment repeated on four independent occasions with comparable results.
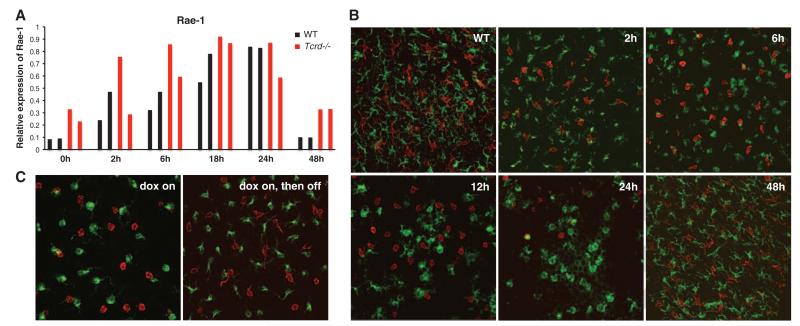
The implication of these data are that lymphoid stress-surveillance of tissue damage may be a substantial driver of TH2 responses developing during cutaneous antigen exposure. To investigate this, mild epidermal abrasion was induced in wild-type (WT) mice by means of tape-stripping, which transiently removes the outer dead cell layer—the stratum corneum—while leaving underlying keratinocytes largely intact. The epidermis returns to normal within 48 hours (fig. S4). Such treatment mimics shaving and depilatory treatments in humans. Tape-stripping is known to activate LCs (6, 7), but whether it also induces a lymphoid stress-surveillance response is unknown. Rae-1 RNA was up-regulated in the epidermis within 2 hours, peaking at 18 to 24 hours, and returning toward baseline by 48 hours, which is consistent with morphological tissue repair (fig. S5). Rae-1 up-regulation was comparable in WT and TCRδ-deficient (Tcrd−/−) mice, as expected for an event upstream of DETC activation (Fig. 2A). Consistent with the consequences of Rae-1 up-regulation seen in BTg mice, tape-stripping was followed by coincident rounding of DETCs and LCs and an aggregate loss of DETCs from the suprabasal and basal layers of the epidermis (Fig. 2B). The cellular changes in tape-stripped and BTg mice were rapidly reversed as Rae-1 expression declined (Fig. 2, B and C). In sum, mild cutaneous abrasion provokes the initial events of lymphoid stress-surveillance.
Fig. 2.
Epidermal Rae-1 expression and local immune activation characterize the response to mild tissue abrasion. (A) Expression of endogenous Rae-1 mRNA in (black) WT and (red) Tcrd−/− epidermis after tissue abrasion by tape-stripping. Quantitative reverse transcription polymerase chain reaction (RT-PCR) was performed on isolated epidermis; each bar represents an individual mouse; data are expressed relative to the control gene cyclophilin. Six WT and four Trcd−/− mice were analyzed per time-point in three separate experiments. (B) Representative confocal images show freshly isolated epidermal sheets from WT fxxxxxx vxxxxxxxxx bxxxxxxx mice at various timepoints after tape-stripping: (red) DETCs and (green) LCs. (C) Confocal images show (red) DETCs and (green) LCs after 5 days on dox (left) and 3 days after withdrawal of dox (right). Original magnification was ×63 for (B) and (C). Micrographs are representative of analysis of 4 to 10 mice per condition.
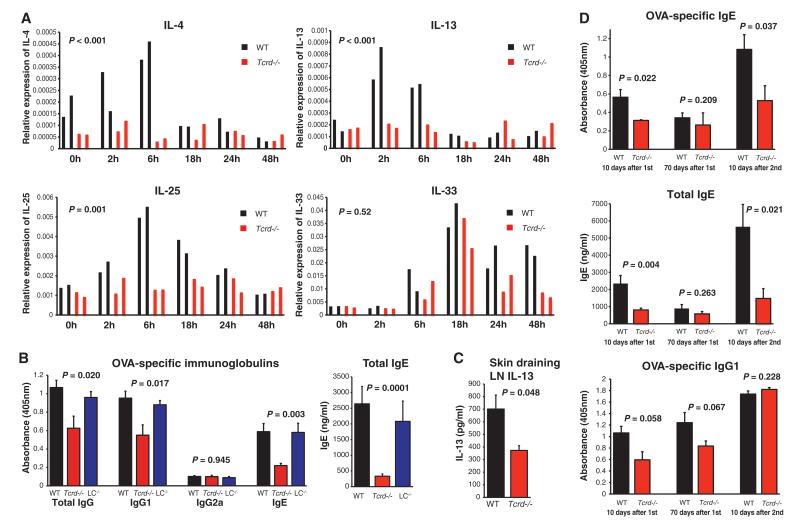
To define abrasion-induced lymphoid stress-surveillance, we analyzed local gene expression changes in sets of age- and gender-matched WT and Tcrd−/− mice. As reported, after epithelial stress (8, 9) several cytokine mRNAs commonly attributed to epithelial cells were up-regulated, including IL-1, IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) (Fig. 3A and fig. S6). mRNAs for some Tcell–associated interleukins— notably IL-4, IL-13, interferon-γ (IFNγ), and IL-10—were also up-regulated. Such up-regulation of Rae-1 and TH2 cytokines was likewise observed after careful shaving of mice (fig. S7).
Fig. 3.
Local stress-surveillance responses to mild abrasion are linked to γδ T cell–dependent initiation of Type-2 responses. (A) Quantitative RT-PCR analysis of mRNA expression for the indicated cytokines over a 48-hour period after tape-stripping in (black) WT and (red) Tcrd−/− epidermis. Data are expressed relative to the control gene cyclophilin; P values refer throughout the figure to statistically significant differences between WT and Tcrd−/− mice. Two representative mice of each genotype are shown per timepoint. The entire experiment was repeated three times for WT mice (n = 6 mice per timepoint) and two times for Tcrd−/− mice (n = 4 mice per timepoint). (B and C) After shaving, ova was applied as in Fig. 1 to dorsal skin of (black) WT, (red) Tcrd−/−, or (blue) LC-deficient (LC−/−, Langerin-DTA mice) mice. The Ig response (B) was assayed 10 days after primary immunzation and IL-13 secretion by means of skin draining LN (C) after further 72 hours in vitro restimulation with ova, all performed by means of enzyme-linked immunosorbent assay (ELISA). Data are expressed as mean ± 1 SEM, and n = 6 to 8 mice per group. Experiments (B) and (C) were repeated independently six times for Tcrd−/− and WT mice and twice for LC−/− mice, with comparable results. (D) Tcrd−/− (red) and WT (black) mice were rested for 70 days after primary epicutaneous antigen exposure; re-bled; reexposed to an ova-containing patch as described; and re-bled 10 days later. Ig responses were measured with ELISA. Data expressed as mean ± 1 SEM, and n = 8 mice per group.
The response to tape-stripping was radically different in Tcrd−/− mice: Whereas IL-10 and TSLP up-regulation were unaffected, IL-4, IL-13, and IL-25 RNAs were not induced, and those of IL-33 and IL-1β less durable (Fig. 3A and fig. S6). Collectively, these data establish that γδ T cells are required for the normal local induction of specific cytokines commonly associated with TH2 responses to antigens encountered at epithelial surfaces. This conclusion was in part supported by newly generated lines of BTg mice with dox-inducible expression of either Rae-1 or H60c, another NKG2D ligand (10). When TCRγδ+ DETCs, LCs, and keratinocytes were purified from post-induction epidermis, rapid increases in IL-13 mRNA were specifically shown by DETCs (fig. S8), attesting to the rapid functional activation of the local T cell compartment in vivo. Moreover, because NKG2D ligands are in this case only transiently up-regulated on suprabasal keratinocytes in the absence of any other perturbation, local IL-13 up-regulation can be attributed to intrinsic lymphoid stress-surveillance, rather than to obligate disruption of the epidermal barrier, as is often invoked (7, 11).
To determine whether such responses of γδ Tcells translated to effects on systemic immunity, shaved mice were exposed to ova-containing patches, as described. As before, there was significant induction of ova-specific IgG, IgG1, and IgE, and of total IgE. However, whereas such responses were enhanced in BTg mice, they were significantly reduced in Tcrd−/− mice that cannot mount epidermal lymphoid stress-surveillance (Fig. 3B). Again, there was no atypical induction of IgG2a.
Consistent with this, skin-draining LN cells from Tcrd−/− mice produced significantly less ova-dependent IL-13 than did WT mice (Fig. 3C). Parallel treatment of other strains showed as predicted that αβ T cells were required for IgG and IgE specific for antigen encountered during mild cutaneous abrasion, whereas less predictably congenital LC deletion had negligible impact (Fig. 3B and fig. S9). As more experiments were undertaken, it became clear that the dependence of ova-specific IgG and IgG1 on γδ T cells was less than the dependence on αβ T cells and did not always reach statistical significance (Fig. 3D and fig. S10); conversely, γδ T cells were invariably important for IgE induced in this manner. Moreover, the characteristic induction of nonspecific IgE was fully ablated in Tcrd−/− mice and in Tcrb−/−Tcrd−/− double-knockout mice (fig. S9). That γδ T cells are a driving force for such IgE production was evident in the finding that Tcrb−/− mice carry abnormally high levels of non-specific IgE, as was previously reported for Tcra−/− mice (12). Thus, the γδ T cell compartment has a profound effect on the production of antigen-specific and total IgE after antigen exposure at mildly stressed epithelium.
TH2 in comparison with Th1 cell differentiation is often regarded as de facto irreversible because of the positive feedback loop established by GATA3 expression (13). Hence, the impact of stress-surveillance on primary TH2-IgE responses may have long-term implications for TH2-immunity. To examine this, WT and Tcrd−/− mice were exposed to ova as before and then reexposed 70 days after initial challenge. Again, γδ T cells were required for WT levels of ova-specific IgE induction (Fig. 3D). After 70 days, ova-specific and total IgE levels in WT mice had returned to normal. Although they were substantially boosted in mice reexposed to antigen at day 70 and reexamined 10 days later, this secondary response was greatly impaired in Tcrd−/− mice (Fig. 3D). In contrast, ova-specific IgG1 had remained elevated 70 days after challenge and was boosted comparably in WT and Tcrd−/− mice, again segregating the impact of lymphoid stress-surveillance on IgE and IgG1, respectively. In sum, the magnitude of antigen-specific IgE responses to antigen repetitively encountered at the skin is regulated by γδ T cells.
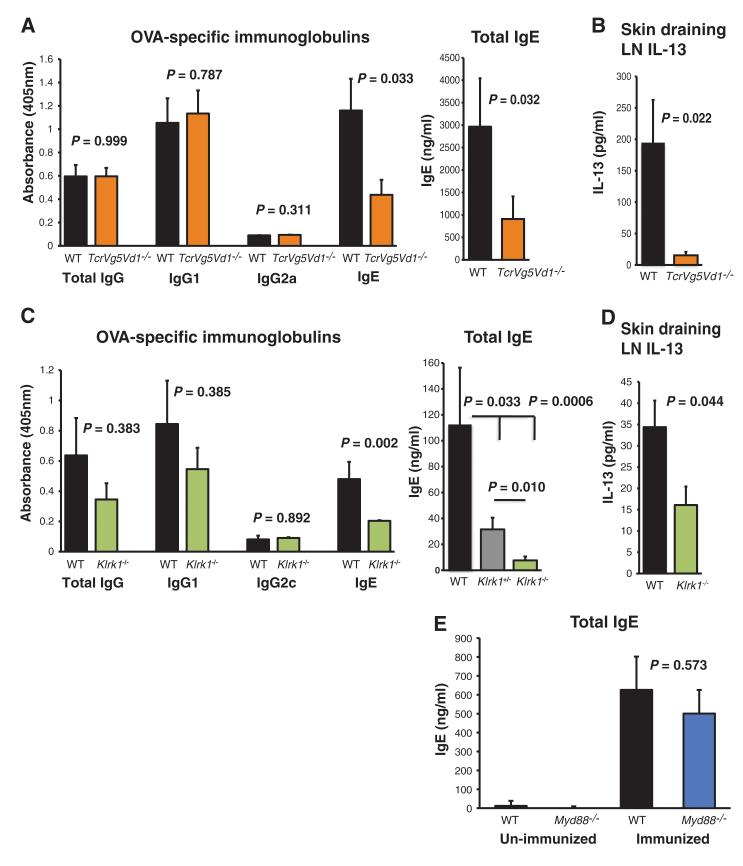
To test whether this dependence of IgE up-regulation on γδ T cells reflects its origin in lymphoid stress-surveillance, the studies were repeated in mice deficient in TCRVδ1Vδ5 that lack only the normal DETC repertoire (4). Such mice showed substantially impaired ova-specific and total IgE; consistent with which, almost no ova-dependent IL-13 production could be evoked from skin-draining LN cells (Fig. 4, A and B). In these studies, there was no substantial impact on IgG1. Moreover, germane to the greater impact of local T cells on IgE versus IgG1, total IgE was already greatly up-regulated in WT mice (but not Tcrd−/− mice) by day 7, whereas IgG1 up-regulation was not seen until day 10 (fig. S10). Consistent with IgE induction being substantially a product of lymphoid stress-surveillance, its up-regulation was significantly impaired in mice lacking the NKG2D receptor that mediates DETC responses to local Rae-1 up-regulation. Again, there was less impairment of IgG and IgG1 responses. There was an intermediate impairment of IgE responses in mice heterozygous for the gene that encodes NKG2D (Klrk1) (Fig. 4C). IL-13 production from skin-draining LN was also greatly reduced in Klrk1−/− mice (Fig. 4D). Complementing these deficiencies in IgE production in NKG2D-deficient mice, IgE was readily detected in WT mice in squamous cell carcinomas that overexpress Rae-1 (fig. S11) (3, 4).
Fig. 4.
IgE is regulated by intraepidermal γδ T cells and is dependent on NKG2D. (A and B) Total and ova-specific serum Ig induction (A) and IL-13 secretion in the skin draining LN (B) of (orange) TcrgV5−/−TcrdV1−/− relative to (black) WT mice after epicutaneous immunization with ova, as described in Fig. 1. (C) Serum ova-specific Ig and total IgE responses after epicutaneous immunization with ova, as described in Fig. 1, in (black) WT and (green) Klrk1−/− mice as measured with ELISA. (D) ova-dependent IL-13 production in skin draining LNs from (green) Klrk1−/− and (black) WT mice, after epicutaneous immunization and 72-hour in vitro restimulation with ova, determined with ELISA. Data are expressed as mean ± 1 SEM, and n = 6 to 10 mice per group. All experiments [(A) to (D)] were repeated twice with comparable results. (E) Total serum IgE response in (black) WT C57BL/6 and (blue) Myd88−/− mice 10 days after primary epicutaneous immunization, as described in Fig. 1, or in unimmunized mice. Data are expressed as mean ± 1 SEM, and n = 4 unimmunized mice and n = 7 immunized mice per group.
Although epithelial dysregulation has previously been linked to atopy—as in the genetic association of atopic dermatitis with mutations in the epidermal structural protein, filaggrin (14)—this has most often been attributed to the effects of microbes gaining access to local DCs, such as LCs, by way of barrier disruption (7, 11). However, shaving and ova-patching of WT mice and mice lacking MyD88, which transduces signals from bacteria-responsive Toll-like receptors (TLRs), induced comparable levels of Rae-1, IL-25, IL-13, and IgE (Fig. 4E and fig. S12), which is consistent with the proposal that these responses are initiated by epithelial dysregulation per se.
Given the critical role of IL-13 in epicutaneously induced TH2 responses, and its strong association with clinical allergies, it is noteworthy that in comparison to conventionally polarized TCRαβ+ TH2 cells, DETC activated in vitro or in vivo express an excess of IL-13 mRNA relative to IL-4 mRNA (fig. S13). Moreover, such cells’ regulation of stress-dependent epithelial expression of IL-25, IL-33, and IL-1 seems also germane to the afferent promotion of TH2 responses. Given that NKG2D-expressing mouse and human systemic γδ cells and NKT cells also co-produce IL-13 and IFNγ (15, 16), and have been locally implicated in human allergic disease (17, 18), there may be multiple cellular mechanisms for stress-initiated TH2 responses.
Although the same factors regulate B cell class-switching from IgM to IgE or to IgG1 (mouse) or IgG4 (human), an alternative, recently elucidated pathway favoring IgE class-switching (19) might accommodate the preferential induction of IgE by NKG2D-dependent stress-surveillance shown here and the germinal center-independent induction of IgE in αβ T cell–deficient and germfree mice (12, 20). In parallel, increasing tissue damage would expose DCs to IL-1 cytokines and/or to microbes, promoting conventional CD4+ αβ T cell help for antigen-specific IgG1 responses. This can explain prior implications of TLRs in atopy (11, 21); the γδ T cell-independence of IgG responses in multiply shaved and tape-stripped mice repeatedly exposed to antigen (22); and the reduced induction of antigen-specific IgG in shaved and ova-patched Myd88−/− mice (fig. S14).
The humoral component of stress-surveillance may limit tissue damage by targeting foreign moieties, such as toxins, that are root causes of tissue dysregulation. The IgE effector response could promote toxin expulsion and (by reducing blood pressure) limit their systemic dissemination, the collateral cost being the IgE response to benign antigens co-encountered with tissue disruption (23). This linkage of lymphoid stress-surveillance and atopy evokes a long-standing implication in allergy of tumor-responsive NKT cells (24) and a large epidemiologic meta-analysis asserting an inverse relationship, albeit weak, between IgE production and carcinogenesis (25). It also raises the possibility that increasing frequencies of atopic allergy might result from increased exposure to environmental toxins, rather than simply a paucity of environmental microbes.
Supplementary Material
Acknowledgements
We thank M. Girardi, M. L. Michel, and M. Wencker for materials and advice; A. Yates for statistical assistance; P. Vantourout, G. Turchinovich, and R. Tigelaar for discussions; S. Diebold and C. Reis e Sousa for mice; and for funding, a Wellcome Trust Programme grant (A.H. and J.S.), a National Institute of Health Research cBRC grant (A.H. and O.S.), and Croatian Ministry of Science, Education and Sports grant 062-0621261-1271 (B.Z. and B.P.). The data reported in this paper are tabulated in the main paper and the supporting online material.
Footnotes
Supporting Online Material www.sciencemag.org/cgi/content/full/[vol]/[issue no.]/[page]/ DC1 Materuials and Methods Figs. S1 to S14 References (26–34)
References and Notes
- 1.Hayday AC. Immunity. 2009;31:184. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Gasser S, Orsulic S, Brown EJ, Raulet DH. Nature. 2005;436:1186. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Girardi M, et al. Science. 2001;294:605. [Google Scholar]
- 4.Strid J, et al. Nat. Immunol. 2008;9:146. doi: 10.1038/ni1556. [DOI] [PubMed] [Google Scholar]
- 5.Herrick CA, Xu L, McKenzie AN, Tigelaar RE, Bottomly K. J. Immunol. 2003;170:2488. doi: 10.4049/jimmunol.170.5.2488. [DOI] [PubMed] [Google Scholar]
- 6.Strid J, Hourihane J, Kimber I, Callard R, Strobel S. Eur. J. Immunol. 2004;34:2100. doi: 10.1002/eji.200425196. [DOI] [PubMed] [Google Scholar]
- 7.Nishijima T, et al. J. Invest. Dermatol. 1997;109:175. doi: 10.1111/1523-1747.ep12319282. [DOI] [PubMed] [Google Scholar]
- 8.Wood LC, Jackson SM, Elias PM, Grunfeld C, Feingold KR. J. Clin. Invest. 1992;90:482. doi: 10.1172/JCI115884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oyoshi MK, Larson RP, Ziegler SF, Geha RS. J. Allergy Clin. Immunol. 2010;126:976, 984, e1. doi: 10.1016/j.jaci.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takada A, et al. J. Immunol. 2008;180:1678. doi: 10.4049/jimmunol.180.3.1678. [DOI] [PubMed] [Google Scholar]
- 11.Le TA, et al. Int. Arch. Allergy Immunol. 2011;151:31. doi: 10.1159/000318679. [DOI] [PubMed] [Google Scholar]
- 12.Wen L, et al. Nature. 1994;369:654. doi: 10.1038/369654a0. [DOI] [PubMed] [Google Scholar]
- 13.Ouyang W, et al. Immunity. 2000;12:27. doi: 10.1016/s1074-7613(00)80156-9. [DOI] [PubMed] [Google Scholar]
- 14.Palmer CN, et al. Nat. Genet. 2006;38:441. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 15.Vemijlen D, et al. J. Immunol. 2007;178:4304. doi: 10.4049/jimmunol.178.7.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bendelac A, Savage PB, Teyton L. Annu. Rev. Immunol. 2007;25:297. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 17.Wu WH, et al. J. Allergy Clin. Immunol. 2010;126:290, 299, e1. doi: 10.1016/j.jaci.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 18.Iwamura C, Nakayama T. Curr. Opin. Immunol. 2010;22:807. doi: 10.1016/j.coi.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Zhang T, et al. Proc. Natl. Acad. Sci. U.S.A. 2010;107:3040. [Google Scholar]
- 20.McCoy KD, et al. Immunity. 2006;24:329. doi: 10.1016/j.immuni.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Hammad H, et al. Nat. Med. 2009;15:410. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodward AL, et al. J. Allergy Clin. Immunol. 2001;107:359. doi: 10.1067/mai.2001.112695. [DOI] [PubMed] [Google Scholar]
- 23.Profet M. Q. Rev. Biol. 1991;66:23. doi: 10.1086/417049. [DOI] [PubMed] [Google Scholar]
- 24.Lisbonne M, et al. J. Immunol. 2003;171:1637. doi: 10.4049/jimmunol.171.4.1637. [DOI] [PubMed] [Google Scholar]
- 25.Van Hemelrijc M, et al. Cancer Causes Control. 2010;21:1657. doi: 10.1007/s10552-010-9594-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.