Abstract
Ebola virus (EBOV) is one of the most lethal filoviruses, with mortality rates of up to 90% in humans. Previously, we demonstrated 100% and 50% survival of EBOV-infected cynomologus macaques with a combination of 3 EBOV-GP-specific monoclonal antibodies (ZMAb) administered at 24 or 48 hours post-exposure, respectively. The survivors demonstrated EBOV-GP–specific humoral and cell-mediated immune responses. In order to evaluate whether the immune response induced in NHPs during the ZMAb treatment and EBOV challenge is sufficient to protect survivors against a subsequent exposure, animals that survived the initial challenge were rechallenged at 10 or 13 weeks after the initial challenge. The animals rechallenged at 10 weeks all survived whereas 4 of 6 animals survived a rechallenge at 13 weeks. The data indicate that a robust immune response was generated during the successful treatment of EBOV-infected NHPs with EBOV, which resulted in sustained protection against a second lethal exposure.
Ebola virus is a member of the family Filoviridae1. It causes severe hemorrhagic fevers in primates and respiratory disease in pigs2. Of the five Ebolavirus species, Zaire ebolavirus (EBOV) is the most lethal in humans, with a mortality rate approaching 90%. While EBOV is not currently a major burden on public health, the lack of an approved vaccine and post-exposure treatment raises concerns in the event of a possible outbreak. Several vaccines (reviewed in Falzarano et al.3), and post-exposure therapeutics have been developed with mixed success4. Many promising vaccines are moving through pre-clinical or clinical trials, but mass immunization is unlikely due to the localized and sporadic nature of EBOV infections. Post-exposure interventions are therefore necessary for the treatment of cases as they occur, but have been harder to develop as death from an EBOV infection typically occurs within 6–9 days in non-human primates (NHPs)5,6. This leaves a very short window for the treatment to reduce virus replication until the immune response expands sufficiently to control the infection. Currently, the majority of proposed post-exposure therapeutics needs to be administered within one hour in order to fully protect experimentally infected animals6,7,8.
The initial treatments evaluated against EBOV were more supportive in nature with a focus on remediating the coagulation abnormalities induced by the virus9,10,11,12,13. A study using the VSVΔG/ZEBOVGP vaccine as a post-exposure intervention showed that this strategy was more efficacious than previous interventions, protecting 50% of the infected animals if administered within 30 minutes after exposure6. More recently, antisense therapy has been applied to EBOV infection with success. Modified phosphorodiamidate morpholino oligomers (PMOplus) were found to protect 62.5% of the infected animals when administered daily for 10–14 days8. Small interfering RNAs (siRNAs) were shown to be fully protective in NHPs when administered daily for seven days7. In both cases, treatment began within 30–60 minutes of exposure.
Passive immunization constitutes another strategy to treat EBOV infections and was first attempted during the initial outbreak in 1976, where an infected laboratory employee was successfully treated with interferon and convalescent serum14. During the 1995 outbreak, convalescent serum was administered to eight infected individuals, seven of which survived15. However, evaluations of passive therapies in animal models have had mixed success. The administration of immunoglobulin G (IgG) purified from EBOV-hypervaccinated horses failed to protect macaques against an EBOV challenge16,17 and the neutralizing human monoclonal antibody KZ52, isolated from a survivor of EBOV infection, also failed to protect macaques18. However, polyclonal IgG from rhesus macaques which survived an EBOV challenge was shown to protect naïve rhesus macaques when administered up to 48 hours after infection19. In 2012, two groups demonstrated partial protection in rhesus macaques following treatment with monoclonal antibodies at 24 hours after infection20,21 and one group reported complete protection in cynomolgus macaques with another monoclonal antibody treatment (ZMAb) also initiated 24 hours after infection22. In addition, the ZMAb treatment protected two of four macaques when initiated 48 hours after exposure. In 2013, the treatment window was extended by using an adenovirus-vectored consensus human IFNα (Ad-IFN)23 administered with or before ZMAb24. While the precise mechanism of protection remains unclear, it was shown that the monoclonal antibody-based treatment did not completely inhibit EBOV replication, leading to the development of a host immune response against EBOV22,24.
In order to evaluate whether the immune response induced in NHPs during the ZMAb treatment and EBOV challenge is of sufficient quality to protect survivors against a subsequent exposure, ZMAb-treated animals that survived an initial challenge22,24 were rechallenged with EBOV either 10 or 13 weeks after the initial challenge and the memory immune responses were evaluated before and during the rechallenge.
Results
Clinical findings
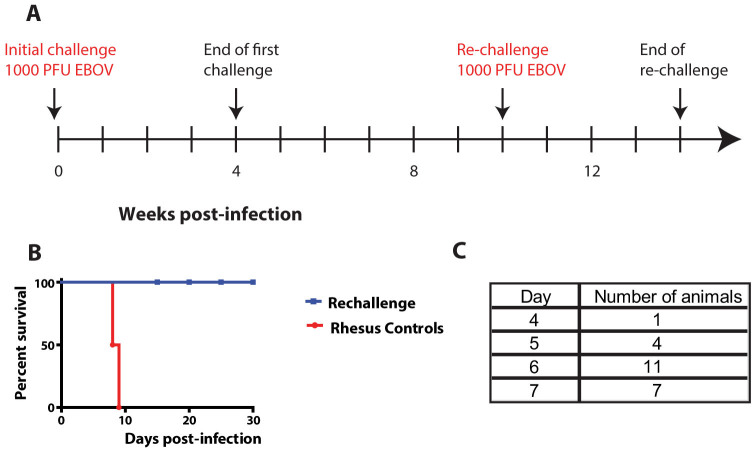
In the first experiment, 6 cynomolgus macaques which survived a previous EBOV challenge by receiving a mouse MAb combination (ZMAb) beginning either 24 hours (A1–A4) or 48 hours (B1, B4) post-infection22, were rechallenged 10 weeks after the initial challenge (Figure 1A) to evaluate whether the immune response developed during treatment was protective without further intervention. The 6 treated survivors were rechallenged intramuscularly (IM) with 1000 PFU of EBOV and monitored daily for survival and clinical signs of disease over 28 days. All ZMAb-treated survivors survived the rechallenge (Figure 1B). Since this was a pilot study, the controls used were from a parallel but different study using rhesus macaques and are only included here to show that the virus dose used to infect the cynomolgus macaques was indeed lethal. The normal time to death for cynomolgus macaques is generally 2–3 days earlier compared to rhesus macaques (Figure 1C).
Figure 1. Experimental design and survival for the first rechallenge experiment.
(A) Six nonhuman primates which survived a first challenge (at week 0) were kept for 6 weeks after the end of that challenge (week 4) and were rechallenged with the same virus (at week 10); (B) survival curve showing complete survival in cynomolgus macaques rechallenged with EBOV, the rhesus controls were part of an unrelated experiment conducted. (C) Table showing the time to death of historical cynomolgus macaque controls.
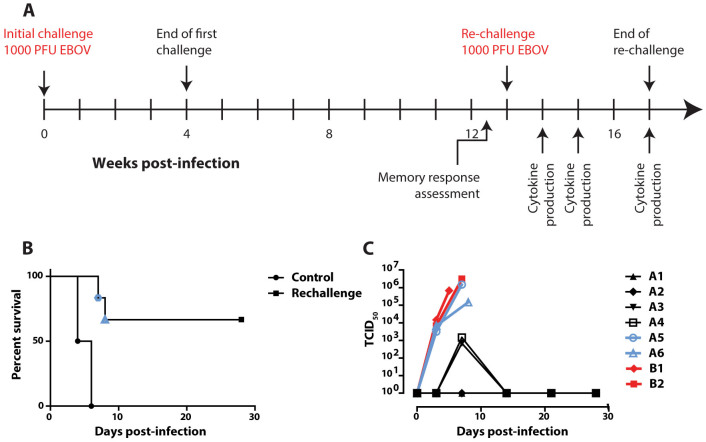
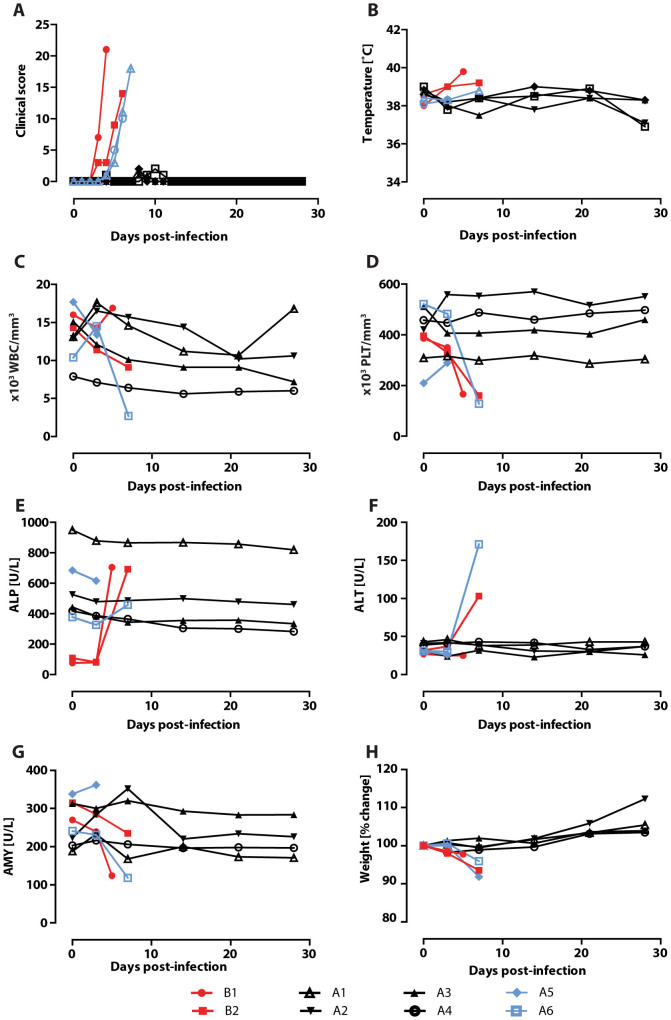
In a subsequent experiment24, 8 cynomolgus macaques were challenged with EBOV and treated using Ad-IFN and ZMAb starting at 48 or 72 hours post-infection, followed by two administrations of ZMAb 72 hours apart and were monitored for 28 days. Six animals survived, one animal in each treatment group died along with two control animals, one receiving a PBS treatment and one receiving normal mouse IgG. The survivors were rechallenged 13 weeks after the initial infection along with two naïve cynomolgus macaque controls (Figure 2A). Four of the 6 animals survived the rechallenge (Figure 2B; p = 0.0039) with low to undetectable viremia (Figure 2C, Supplementary Table 1). The surviving animals experienced no clinical signs of disease (Figure 3A, summarised in Table 1) or fever (Figure 3B). Platelet and white blood cell counts were stable throughout the experiment (Figures 3C and 3D, respectively) and there were no changes in liver enzyme activity (Figures 3E and 3F) or levels of amylase (Figure 3G). In contrast, both controls and animal A6 exhibited decreased platelet counts, white blood cell counts (for B2 and A6), and amylase activity as well as increased liver enzyme activity. Animal A5 was found dead on day 7, therefore blood chemistry and a complete blood count could not be evaluated. All the survivors gained weight throughout the experiment while the animals that succumbed to the infection experienced weight loss (Figure 3H).
Figure 2. Design, survival, and viremia of the second rechallenge experiment.
(A) Six NHPs were rechallenged 9 weeks after the end of their first challenge: first challenge at week 0; first challenge ends at week 4; rechallenge begins at week 13. (B) survival of the second set of rechallenge cynomolgus macaques along with two controls of the same species. (C) Viremia during the second rechallenge, as measured by TCID50.
Figure 3. Clinical parameters.
Changes in various clinical parameters over the course of the second experiment, Group A is the rechallenged group while group B is the naïve control group. (A) Daily clinical score for each individual animal; (B) temperature; (C) white blood cell count (WBC); (D) platelet count (PLT); (E) alkaline phosphatase levels (ALP); (F) alanine aminotransferase levels (ALT); (G) amylase levels (AMY); and (H) % change in weight.
Table 1. Clinical findings on days 1–28 after EBOV challenge.
| Animal ID | Findings | Status |
|---|---|---|
| A1 | --- | Survived |
| A2 | --- | Survived |
| A3 | Leukocytopenia (7, 21 and 28dpi) | Survived |
| A4* | Moderate rash (7dpi), Thrombocytosis (7 dpi) | Died - 7 dpi |
| A5 | --- | Survived |
| A6 | Moderate rash (7dpi), Leukocytopenia (7 dpi), Thrombocytopenia (7 dpi), ALT↑↑↑ (7 dpi), AMY↓↓ (7 dpi), CRE↑ (7 dpi) | Died - 8 dpi |
| B1 | Fever(5 dpi), Severe rash (5 dpi), Thrombocytopenia (5 dpi), ALP↑↑↑ (5 dpi), TBIL↑ (5 dpi), AMY↓(5 dpi), PHOS↑(5 dpi) | Died - 5 dpi |
| B2 | Severe rash (7 dpi), Leukocytopenia (7dpi), Thrombocytopenia (7 dpi), ALP↑↑↑ (7 dpi), ALT↑ (7 dpi), BUN↑ (7 dpi), CRE↑ (7 dpi), | Died - 7dpi |
Hypothermia was defined as below 35°C. Fever was defined as ≥1.0°C higher than baseline. Mild rash was defined as focal areas of petechiae covering <10% of the skin, moderate rash as areas of petechiae covering 10 to 40% of the skin, and severe rash as areas of petechiae and/or ecchymosis covering >40% of the skin. Leukocytopenia and thrombocytopenia were defined as a >30% decrease in numbers of white blood cells (WBCs) and platelets, respectively. Leukocytosis and thrombocytosis were defined as a twofold or greater increase in numbers of WBCs and platelets over baseline, where WBC count >11.000. ↑, two- to threefold increase, ↑↑, four- to fivefold increase, ↑↑↑, greater than fivefold increase. ↓, two- to threefold decrease, ↓↓, four- to fivefold decrease, ↓↓↓, greater than fivefold decrease. ALP, alkaline phosphatase, ALT, alanine aminotransferase, AMY, amylase, TBIL, total bilirubin, BUN, blood urea nitrogen, PHOS, phosphate, CRE, creatinine, ---, no change.
*No serum biochemistry data for this animal.
EBOV-GP-specific immune responses during rechallenge
Antibody response
The humoural and cell mediated immune responses to the EBOV surface glycoprotein (EBOV-GP) were monitored to evaluate the recall response. The humoural response was characterized by measuring the serum levels of EBOV GP-specific IgG by ELISA because these antibodies were reported to be found at high levels in vaccinated macaques surviving lethal EBOV challenge25. The controls B1 and B2 had no detectable levels of EBOV GP-specific IgG throughout the rechallenge experiment (Figure 4). However, on day 0 of the rechallenge all 6 survivors from the initial challenge and treatment had detectable levels of EBOV GP-specific IgG with titres greater than 1 × 106 for A1–4, and 128,000 for A5–6. While the titres in animal A4 remained stable throughout the rechallenge, the IgG levels decreased for A1–3 early in the course of the infection, before returning to 512,000 by day 28. In contrast, the IgG titres for subjects A5 and A6 that died on days 7 and 8 never increased above 128,000. Overall, a strong EBOV GP-specific IgG response over 1 × 106 prior to a second lethal exposure to EBOV correlated with survival.
Figure 4. A strong EBOV GP-specific humoural immune response during rechallenge.
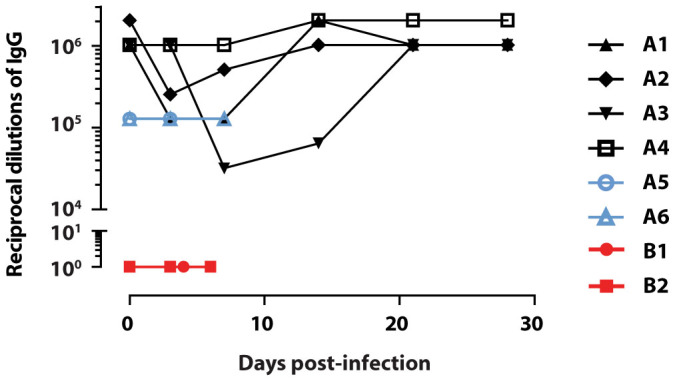
During the EBOV rechallenge, sera collected on the exam dates were assayed for their anti-EBOV GP immunoglobulin G (IgG) levels as measured by eVLP-ELISA. The assay was performed in triplicate, and titres are presented as the average endpoint dilution per subject.
Cytokine production during the rechallenge
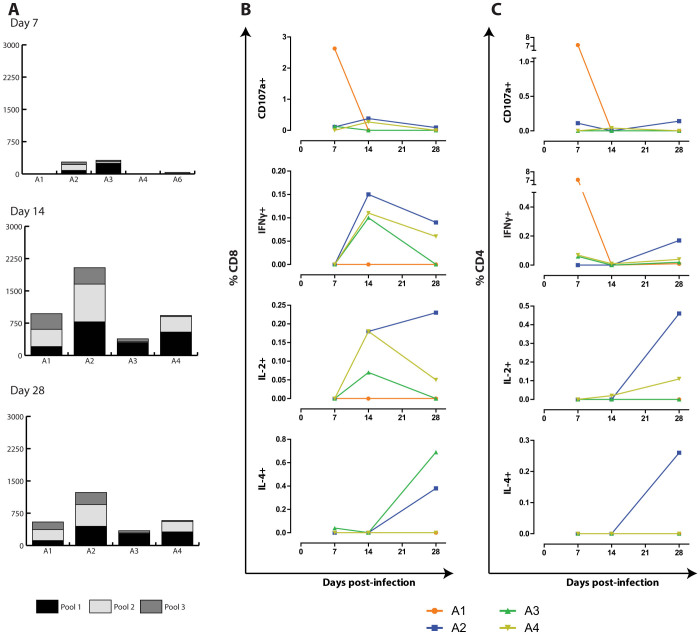
The EBOV GP-specific cell mediated immune response was examined during rechallenge utilizing the standard IFN-γ ELISPOT, and flow cytometric intracellular cytokine staining (ICS) assays. PBMCs isolated on days 7, 14 and 28 after challenge were added to 3 peptide pools spanning the entire region of GP. At 7 dpi in the IFNγ ELISPOT assay A2, A3 and A6 had a combined peptide pool response ranging from 35–319 spot forming units (SFU)/106 PBMCs (Figure 5A). While A2 and A3 responded to all 3 peptide pools A6 responded only to pools 1 and 3. A5 had reached the humane end point prior to conducting the day 7 ELISPOT assay and therefore was not included. At 14 and 28 dpi all 4 survivors had strong responses to all 3 peptide pools, with combined responses ranging from 927–2038 SFU/106 PBMCs and 342–1231 SFU/106 PBMCs, respectively. The highest levels of IFN-γ secreting PBMCs were observed with peptide pool #2, followed by pool #1.
Figure 5. EBOV GP-specific cell mediated immune response during rechallenge.
The cell mediated immune response was examined on days 7, 14 and 28 of the rechallenge utilizing (A) the IFN-γ ELISPOT assay, and the (B) CD8+ and (C) CD4+ flow cytometric intracellular cytokine staining (ICS) assay. PBMCs were added to 3 different peptide pools spanning the entire EBOV GP region, or to media alone. For flow cytometry, a response was considered positive if it was at least twice the media only background, in which case the background value was subtracted. The graphs represent the sum of the three pools.
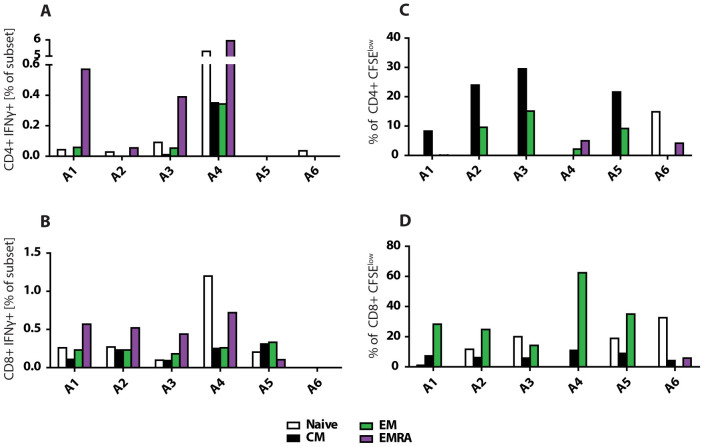
In addition to the ELISPOT assays, an ICS assay was employed in order to examine the cytokine response in more detail. CD8+ (Figure 5B) and CD4+ (Figure 5C) cells were co-stained with antibodies for the cytotoxic degranulation marker CD107a, and intracellular cytokines IL-2, IL-4 and IFN-γ. On 7 dpi, there was no CD4+IL-2 or IL4 response. A1 had a higher percentage of CD4+CD107+ cells and CD4+IFN-γ+ cells, whereas there were lower levels of CD4+CD107a+ in A2, and CD4+IFN-γ+ in A3–4. By 14 dpi, the percentage of CD4+ cells for any of the 4 markers was negligible. At 28 dpi, A2 had the strongest CD4+ percentage for all 4 markers, while A4 had low levels of CD4+IFN-γ+ and CD4+IL-2+, whereas all other responses were undetectable. For the CD8+ cells, there was a negligible response for all markers in all 4 subjects at 7 dpi, except for a strong response in CD8+CD107a+ cells in A1. By 14 dpi, low to moderate levels of cells were seen in the CD8+CD107a+ (A2 and A4), the CD8+IFN-γ+ (A2–4) and CD8+IL-2+ (A2–4) populations. At 28 dpi, there was a negligible CD8+CD107a+ response for all 4 subjects, but there was a very low CD8+IFN-γ+ response (A2–4), and a low CD8+IL-2+ response (A2 and A4), with a high CD8+IL-4+ (A2–3) response. PBMCs double positive for the various cytokines were also evaluated for CD8 (Supplemental figure S1) and CD4 (Supplemental figure S2). For the majority of the animals, the highest levels of CD8+ double positives were found at 14 dpi, whereas for CD4+ they were highest on 7 or 28 dpi. Overall, the ELISPOT and ICS assays demonstrated a robust EBOV GP-specific cell mediated response in all 4 survivors.
EBOV-GP-specific T cell memory responses prior to rechallenge
Cytokine production
In order to determine whether a long term immune response was established in the ZMAb treated survivors of the first challenge, the EBOV-GP-specific cellular immune response was evaluated using flow cytometry. PBMCs were isolated 5 days before rechallenge and included in a memory panel in the EBOV GP-specific proliferation, and IFN-γ ICS assays (Figure 6). The T lymphocyte markers CD45RA and CCR7 were used to determine the proportion of CD4+ and CD8+ EBOV GP-specific central memory (CM = CD45RA − CCR7+), effector memory (EM = CD45RA − CCR7-), or differentiated effector cell (EMRA = CD45RA + CCR7−) populations. The majority of IFN-γ producing CD4+ and CD8+ T cells were found in the EMRA population for subjects A1–4 (Figure 6A and 6B). A1, 3, 4 had CD4+ IFN-γ+ EM populations, with only A4 also having a CM population. In addition to the EMRA, the CD8+ cells in A1–4 also had an EM, CM and naïve population that was half as strong as the EMRA. In contrast, for the subjects who died at 6 dpi, A5 and A6 lacked a CD4+ EBOV GP-specific memory response, and A6 also lacked a CD8+ response. While A5 exhibited a comparable CD8+ IFN-γ response to A1–4, the EMRA was not the dominant population as it was approximately half as strong as the EM and CM populations.
Figure 6. CD4+ and CD8+ EBOV GP-specific memory T cell response prior to rechallenge.
Flow cytometry was utilized to determine the EBOV GP-specific T cell memory immune response in the survivors of the first challenge. PBMCs isolated 5 days prior to rechallenge were added to 3 peptide pools spanning the entire EBOV GP in either an IFN-γ ICS assay, or a 6 day CFSE proliferation assay. The results displayed are the average of the response to the 3 pools combined after the media only background was subtracted for each pool. (A) IFNγ production in CD4+ T cells. (B) IFNγ production in CD8+ T cells. (C) Proliferation of CD4+ T cells. (D) Proliferation of CD8+ T cells.
Proliferation
Upon examining the CD4+ and CD8+ EBOV GP-specific proliferation response, it was the CD4+ CM population that expanded the most in subjects A1, 2, 4, and 5 (Figure 6C and 6D). CD4+ EM populations expanded about half as much in A2, 3, and 5, and somewhat in A4. A4 and A6 had the only CD4+ EMRA populations. In contrast, the most expanded CD8+ memory cell was the EM population in A1, 2, 4–6 with CM expansion at 25% of the EM expansion. The nonsurvivor A6 had a small CD4+ EM population but no CM or EMRA population, along with a CD8 CM and EMRA, but no EM population. Although A5 had proliferative responses similar to the survivors, for the CD4+ panel the CM population of the survivors proliferated more dominantly to either peptide pool 1 or 3 whereas A5 proliferated predominantly to pool 2 (data not shown). A5's CD8+ EM panel responded primarily to pool 1 versus pool 2 for A1–4. Overall the survivors have a much stronger proliferative response to the peptides than A5 and A6. However, when A5 and A6 did respond, they had either a different memory subset responding or they responded predominantly to a different peptide pool.
Discussion
The high fatality rate of EBOV, coupled with the unavailability of approved vaccines or treatments, make outbreaks or lab exposures a major challenge1. Post-exposure treatments are being developed to fill the gap and reduce mortality in those who could not receive a protective vaccine in time6,7,8,16,17,18,19. We previously demonstrated that the administration of ZMAb within 24 hours after exposure completely protected all treated NHPs22. The current study aims to evaluate the potentially protective role of an anti-EBOV GP-specific immune response that was generated in the presence of ZMAb during the first EBOV infection. This scenario was explored in experiment 1, a pilot study in which 6 ZMAb treated animals that survived a first challenge with EBOV were rechallenged with EBOV 6 weeks after the end of the initial study. All of the rechallenged animals survived and showed no signs of disease. A more in-depth study was then carried out on 6 animals which survived an initial challenge by receiving a combination of adenovirus-vectored IFNα and ZMAb followed by two ZMAb administrations. This second set of animals was rechallenged 9 weeks after the end of their first challenge along with two naïve controls. Four of the 6 rechallenged animals survived with no signs of illness. High antibody titres were observed at the beginning of the experiment. The production of IFN-γ as detected by ELISpot increased between days 7 and 14, suggesting that there is an expansion of specific T cells. Flow cytometric analysis of cytokine production in CD8+ T cells also show the same pattern for IFN-γ and IL-2 production. Cytokine production in CD4+ T cells is distinguishable when the signal is decomposed between single and double positives and shows a temporal pattern similar to the ELISpot and CD8+ T cells. The two animals that did not survive died 1–2 days later than the controls, suggesting that their immune response may not have been sufficient to protect them against an EBOV infection.
The overall survival for both rechallenge studies is 83% (10 out of 12 animals); with all 6 animals in experiment 1 surviving as well as 4 out of 6 animals in experiment 2. The differences in survival between these two experiments may be accounted for by a number of factors. First, the rechallenge occurred 3 weeks later in experiment 2 which may have allowed the immune response to diminish more than in experiment 1. Second, the treatments administered during each experiment were different in nature and in schedule. In experiment 1, the treatment consisted only of ZMAb with the administration starting on days 1 or 2 post-infection, whereas the treatment in experiment 2 included Ad-IFNα in the first dose administered on days 2 or 3 post-infection. The difference in the initiation of ZMAb may have modified the immune response by allowing more cell death in the immune compartments to occur with treatment being initiated later26. It is also possible that the IFNα may have polarized the Th1/Th2 balance towards a stronger cell mediated Th1 response. There is evidence that a strong antibody response is needed to increase survival rates25,27.
Immune responses were evaluated during the second study. The levels of anti-EBOV-GP IgG were found to be generally very high at the beginning of the rechallenge (titres ≥ 1 024 000 for animals A1–A4) with the exception of the two animals that succumbed to the infection (A5 and A6, titers of 128 000). This observation is again congruent with previously published data suggesting a crucial role for the antibody response, as VSV-EBOVGP vaccinated NHPs that survived had titres of 64,000 versus 125 for the non-survivors25. However, the titres observed in the rechallenge experiment were higher25,27. This may be due to the use of different capture antigens and cut-off values for the ELISA. Nevertheless, there consistently is a measurable difference between the antibody levels of survivors and non-survivors, and these values may differ depending on the immunizing antigen or treatment used. Furthermore, the VSV-EBOVGP vaccinated animals were only exposed to EBOV GP before challenge, whereas the ZMAb treated animals had been exposed to all the EBOV proteins before rechallenge. Additionally, the humoural response generated during post-exposure treatments may suffer from the presence of sGP produced by the virus28, which could increase in vitro titres that do not contribute to protection in vivo. Mohan et al suggest that when sGP is present during immunization against GP, it biases the antibody response towards epitopes shared by the two proteins. The affinity of those antibodies for sGP may mean that they will be produced in large quantities, increasing the titres detected in an anti-GP ELISA. However, those antibodies would be “mopped up” by sGP during the rechallenge. Thus the actual absolute value of the titres might be less relevant when comparing post-exposure treatment-induced immunity with vaccine-induced immunity. In regards to correlates of protection, these result suggest that the optimal protective titres may need to be determined on a per intervention basis, with an emphasis on the difference between prophylactic and post-exposure interventions because of the different antigens that are present during immunization.
The non-surviving animals in experiment 2 also differed from the survivors in their cell-mediated immune responses. The memory T cell response was assessed 5 days before the rechallenge. Animals A5 and A6 showed low to non-existing IFNγ-producing CD4 T cells. Even animal A2, which had the lowest CD4+ IFNγ production, had EMRA cells producing IFNγ. Animal A5 did have some IFNγ production in its CD8+ cells, but unlike the others its EMRA subset was under-represented. Animals A5 and A6 also had very different proliferative responses compared to each other. A5 had a CD4+ proliferation profile very similar to the survivors A1–A3, except that it responded to different peptide pools. On the other hand, A6 had proliferation of naïve cells which are absent from all the other animals, and no proliferation of its CM and EM subsets. The CD8+ proliferation profile of A5 was again very similar to that of the survivors but A6 had more of a “reversed” profile when compared to the survivors, i.e. high levels of naïve cells proliferating but low to no proliferation of the memory subsets. These data suggest that animals A5 and A6 likely developed a non-protective memory response during the initial challenge, in both the T- and B- cell compartments. Although the NHPs are outbred and genetic diversity could account for the differing immune response, it is not possible to distinguish between treatment effects and the genetic variation between individuals in this study. These data support the hypothesis that ZMAb treatment does not impair the establishment of an immune response. It is not possible to compare the immune responses produced during the first challenge to that produced by vaccines as vaccination studies challenge the animals 4 weeks after the last vaccination and no information has been published to date on their long-term protective responses before a challenge. However, the data presented here, along with previously published data25, suggest that the antibody levels of survivors may be a good indicator of when the immunity becomes too low to protect the individual without further intervention.
The current study demonstrates a protective memory response that lasts at least 9 weeks after the initial infection. This could be of benefit in outbreak situations where infected people receiving the ZMAb treatment could return to their community without risk of serious disease if re-infected. Additionally, first responders would also be able to resume outbreak response functions if needed in a large outbreak. This data further supports the development of ZMAb therapy for outbreak responses, and for use in combination with other treatments that could possibly extend the post-exposure treatment window. This study also suggests that anti-EBOV-GP antibody levels could potentially be used as a rapid and simple readout of the quality of the immune response induced by EBOV infection.
Methods
Ethics statement
Animal studies were performed under CL4 conditions and approved by the CSCHAH Animal Care Committee following the guidelines of the Canadian Council on Animal Care. The animal use document (AUD) numbers associated with these experiments were H-11-002 for the pilot experiment and H-12-007 for the second, complete, experiment.
Viruses and peptides
The challenge virus consisted of Ebola virus H.sapiens-tc/COD/1995/Kikwit-9510621 (EBOV) (order Mononegavirales, family Filoviridae, species Zaire ebolavirus; GenBank accession no. AY354458) obtained from the Center for Disease Control and Prevention, Atlanta, Georgia, USA, passaged twice on Vero E6 cells cultured in complete minimal essential medium (cMEM). Peptides spanning the GP of EBOV were 15 amino acids (aa) long with 11 aa overlaps (167 peptides total). The peptides were combined in three pools as follows: pool 1 has 56 peptides (amino acids 1 to 235), pool 2 has 56 peptides (amino acids 236–459), and pool 3 has 55 peptides (amino acids 460 to 676).
Animal experiments
In the first experiment, six healthy male and female cynomolgus macaques (Macaca fascicularis; 2.5 to 4.9 kg; Animal Use Document (AUD) # H-11-002) which previously survived an EBOV challenge after receiving a mouse mAb cocktail (ZMAb)22, and two previously untreated and unchallenged male rhesus macaques (Macaca mulatta; 2.5 to 4.9 kg) used as controls of infection received were infected 10 weeks after the original challenge on the cynomolgus macaques. In the second experiment, eight cynomolgus macaques (weight: 2.8–7.6 kg), consisting of six animals that survived an initial challenge and two naïve controls, were infected 13 weeks after the initial challenge. The diet was composed of commercial monkey chow, treats, vegetables and fruits; enrichment consisted of commercial toys and visual enrichment. For sampling and examinations, the NHPs were first sedated with ketamine (at 6–8 mg/kg) and the anaesthesia was maintained using isoflurane at 2.5–3.5% carried by oxygen. Animals that reached the humane endpoint or the end of the experiment were first anaesthetised and were euthanized by terminal bleeding after the induction of deep anaesthesia with ketamine (25–50 mg/kg) or by injection of 100 mg/kg pentobarbital after ketamine (10 mg/kg)-induced anaesthesia. Husbandry enrichment consisted of commercial toys and visual stimulation. All macaques received 1000 PFU (two intramuscular injections of 1 ml) of EBOV in Dulbecco's modified Eagle's medium (DMEM). The subjects were monitored daily and scored for disease progression with an internal filovirus scoring protocol approved by the Canadian Science Centre for Human and Animal Health (CSCHAH) Animal Care Committee. The scoring rates changes from normal, including: the subject's posture/activity level, attitude, feces/urine output, food and water intake, weight, temperature, respiration, and scored disease manifestations such as visible rash, hemorrhage, cyanosis, or flushed skin. The animals were examined and sampled (blood; oropharyngeal, nasal, and rectal swabs into 1 ml of DMEM) on the specified dates. Blood analyses included: hematological analyses (Animal Blood Counter, scil Vet abc); blood biochemistry for albumin, alkaline phosphatase, alanine aminotransferase, amylase, blood urea nitrogen, carbohydrate antigen, creatinine, globulin, glucose, K+, Na+, phosphate, total bilirubin, and protein (VetScan vs2, Abaxis); blood samples for virus titration and ELISpot and FACS analysis; and, serum samples for ELISAs and neutralization assays. Surviving animals were kept until day 28. Animal studies were performed under CL4 conditions and approved by the CSCHAH Animal Care Committee following the guidelines of the Canadian Council on Animal Care.
EBOV titration by TCID50
Viremia was assessed on specified days by evaluating the TCID50 of blood samples. The samples were assayed in triplicates on Vero E6 cells as previously described22.
EBOV titration by RT-qPCR
Total RNA was extracted from 140 ul of blood or swab resuspension using the QIAmp Viral RNA Mini Kit (Qiagen). EBOV genome copies (genome equivalents; GEQ) were detected by reverse-transcription qPCR (RT-qPCR) using the LightCycler 480 RNA Master Hydrolysis Probes kit (Roche) with probes targeting the RNA polymerase (nucleotides 16472 to 16538, AF086833). Reaction conditions were: 63°C for 3 min; 95°C for 30 s; and cycling of 95°C for 15 s and 60°C for 30 s for 45 cycles on a StepOne Plus cycler (Applied Biosystems). The lower detection limit for this assay is 1 GEQ per reaction well which is equivalent to 86 GEQ/ml of blood; negative results were given a value of half that of the detection limit (0.5 GEQ) for graphing purposes. The primer sequences were: EBOVLF2 (CAGCCAGCAATTTCTTCCAT), EBOVLR2 (TTTCGGTTGCTGTTTCTGTG), and EBOVLP2-FAM (FAM-ATCATTGGCGTACTGGAGGAGCAG-BHQ1).
EBOV GP-specific Enzyme-linked immunosorbent assay (ELISA)
ELISAs for NHP EBOV GP-specific IgG were performed using recombinant Ebola GPΔTM (IBT Services) as the antigen29. Briefly, 96 well half-well plates were coated with 1 μg/ml of protein, and then blocked with PBS 5% skim milk. The sera dilutions were incubated at 37°C for 1 hour. A goat anti-human IgG-HRP was added for 1 hour at 37°C. The plates were read using ABTS Peroxidase substrate (KPL). Assays were performed with each sample assayed in triplicate. A titre was considered positive if the average value was higher than the day 0 average plus two standard deviations.
EBOV GP-specific ELISPOT assay
PBMCs were isolated from whole blood diluted 1:1 with PBS and layered on Ficoll. The samples were centrifuged at 750 g for 45 min, the buffy coat was harvested and washed twice. PBMCs were resuspended in complete RPMI 1640 (cRPMI). ELISPOT assays for IFNγ secretion (BD Biosciences) were performed in triplicates according to the manufacturer's protocol using 5 × 105 PBMCs/well in cRPMI. Three peptide pools spanning the EBOV GP were used for stimulation (final concentration of 2.5 μg/ml) and incubated for 18 hours. The spots were visualized using the AEC substrate (BD Biosciences) and quantified with the ELISpot Plate Reader (AID Cell Technology). The average number of spots from the media-only wells were subtracted from the average spot count of the peptide-stimulated wells.
Intracellular cytokine staining (ICS) assays
PBMCs were isolated using the same protocol as the ELISPOT assay. IFN-γ, IL-2, IL-4 production and CD107a surface expression in CD4+ and CD8+ lymphocytes was assessed by flow cytometry, as described previously24. The data were analysed using FlowJo vX.0.6 (TreeStar). A response was considered positive when the peptide stimulated frequencies were at least 2 times the media-only frequencies, in which case the media-only frequencies were subtracted from the peptide stimulated frequencies.
Flow cytometry memory assays
For the memory response assays, PBMCs were isolated as for the ELISPOT assays. For the ICS assay the 5 × 105 PBMCs per well were rested overnight before adding 2 ug/ml EBOVGP peptides and GolgiPlug plus GolgiStop mixture and then incubating for 5 hours. The cells were then washed once with PBS, 2% HI FBS, then blocked with 10 μl 1 mg/ml human γ-globulin for 10 minutes at room temperature before adding 100 μl of the following mastermix of antibodies from BD Biosciences CD45RA FITC (clone 5H9), CCR7 PE (R&D Systems, FAB197P), CD3 Alexa Fluor 700 (clone SP34-2), CD4 PerCP-Cy5.5 (clone L200), and CD8 APC-Cy7 (clone RPA-T8) for 30 minutes, 4°C. Amplification of CCR7 using Miltenyi's PE FASER kit was conducted according to manufacturer's instructions, one cycle was used. Cells were then washed and the pellet resuspended in 200 μl Cytofix/Cytoperm, spun, and resuspended in fresh Cytofix/Cytoperm for removal from CL4. Cells were subsequently stained for IFN-γ using the intracellular staining protocol of BD's Cytofix/Cytoperm kit. Samples were acquired (at least 350 000 single lymphocytes) on the LSRII (Becton Dickinson) using the FACS Diva software v6, and analyzed using FlowJo vX.0.6. The positivity threshold for IFNγ secretion was set at 2 times the media-only frequency; for positive samples, the media-only frequency was subtracted from the sample frequency.
Cell proliferation on day −5 was monitored by a decrease in carboxyfluorsecein diacetate succinimidyl ester (CFDA-SE) staining. On day 0 of the assay, 107 PBMCs/ml PBS were stained with 100 ul of 3 mM CFDA-SE (Invitrogen) for 8 minutes, 37°C. After adding an equal volume of cold FBS for 1 minute, cells were washed with 10 ml of PBS twice and resuspended at 5 × 105 cells/ml in cRPMI plus 2 ug/ml ZEBOV GP1,2 peptide pools. On the sixth day samples were subjected to the same blocking and surface staining as the ICS samples (antibodies from BD Biosciences: CD8 APC, CD4 PerCP-Cy5.5, CD45RA APC-H7, and CD3 Pacific Blue; antibodies from R&D Systems: CCR7 PE; all clones are the same as for the memory ICS) before washing the cells and fixing. Samples were acquired (70 000 to 250 000 single lymphocytes) on the LSRII and analyzed as above. If the average frequency of a subpopulation (Naïve, CM, EM, or EMRA) of CFDA-SElow cells across all three peptide pools was greater than the media-only background, the difference was taken and graphed.
Statistics
The Log-Rank test for survival was used to compare the survival of the second set of animals with their controls. For the ELISA, a dilution was called positive if its average OD405 was at least 2 SD above the average of the day 0 sample (day 0 of the first challenge).
Author Contributions
X.Q. generated the antibodies, designed and conducted the experiments, wrote the paper and performed animal husbandry. J.A. conducted the experiments, wrote the paper. G.W., S.P., A.B., L.F. conducted the experiments and/or performed animal husbandry. J.B.A. designed/performed experiments, performed animal husbandry, and wrote the paper. G.P.K. designed and conducted the experiments, wrote the paper and performed animal husbandry.
Supplementary Material
Supplementary materials
Acknowledgments
We thank Shane Jones and Jason Gren for technical assistance with the animals and sample processing.
Footnotes
Her Majesty the Queen in right of Canada holds a patent on the monoclonal antibodies PCT/CA2009/000070, “Monoclonal antibodies for Ebola and Marburg viruses”. The authors declare that they have no other competing interests.
References
- Sanchez A., Geisbert T. W. & Feldmann H. in Fields Virology (eds Knipe, D.M., Howley, P. M.,Griffin, D. E., Lamb, M. A. et al.) 1409–1448 (Lippincott, Williams. & Wilkins, 2007). [Google Scholar]
- Kobinger G. P. et al. Replication, pathogenicity, shedding, and transmission of Zaire ebolavirus in pigs. J. Infect. Dis. 204, 200–208 (2011). [DOI] [PubMed] [Google Scholar]
- Falzarano D., Geisbert T. W. & Feldmann H. Progress in filovirus vaccine development: evaluating the potential for clinical use. Expert Rev. Vaccines 10, 63–77 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich B. M. et al. Potential Vaccines and Post-Exposure Treatments for Filovirus Infections. Viruses 4, 1619–1650 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. M. et al. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat. Med. 11, 786–790 (2005). [DOI] [PubMed] [Google Scholar]
- Feldmann H. et al. Effective post-exposure treatment of Ebola infection. PLoS Pathog. 3, e2 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisbert T. W. et al. Postexposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: a proof-of-concept study. Lancet 375, 1896–1905 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren T. K. et al. Advanced antisense therapies for postexposure protection against lethal filovirus infections. Nat. Med. 16, 991–994 (2010). [DOI] [PubMed] [Google Scholar]
- Macias W. L. & Nelson D. R. Severe protein C deficiency predicts early death in severe sepsis. Crit. Care Med. 32, S223–8 (2004). [DOI] [PubMed] [Google Scholar]
- Hensley L. E. et al. Recombinant human activated protein C for the postexposure treatment of Ebola hemorrhagic fever. J. Infect. Dis. 196 Suppl 2, S390–9 (2007). [DOI] [PubMed] [Google Scholar]
- Geisbert T. W. et al. Mechanisms underlying coagulation abnormalities in ebola hemorrhagic fever: overexpression of tissue factor in primate monocytes/macrophages is a key event. J. Infect. Dis. 188, 1618–1629 (2003). [DOI] [PubMed] [Google Scholar]
- Bernard G. R. et al. Safety and dose relationship of recombinant human activated protein C for coagulopathy in severe sepsis. Crit. Care Med. 29, 2051–2059 (2001). [DOI] [PubMed] [Google Scholar]
- Geisbert T. W. et al. Treatment of Ebola virus infection with a recombinant inhibitor of factor VIIa/tissue factor: a study in rhesus monkeys. Lancet 362, 1953–1958 (2003). [DOI] [PubMed] [Google Scholar]
- Emond R. T., Evans B., Bowen E. T. & Lloyd G. A case of Ebola virus infection. Br. Med. J. 2, 541–544 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mupapa K. et al. Treatment of Ebola hemorrhagic fever with blood transfusions from convalescent patients. International Scientific and Technical Committee. J. Infect. Dis. 179 Suppl 1, S18–23 (1999). [DOI] [PubMed] [Google Scholar]
- Jahrling P. B. et al. Passive immunization of Ebola virus-infected cynomolgus monkeys with immunoglobulin from hyperimmune horses. Arch. Virol. Suppl. 11, 135–140 (1996). [DOI] [PubMed] [Google Scholar]
- Jahrling P. B. et al. Evaluation of immune globulin and recombinant interferon-alpha2b for treatment of experimental Ebola virus infections. J. Infect. Dis. 179 Suppl 1, S224–34 (1999). [DOI] [PubMed] [Google Scholar]
- Oswald W. B. et al. Neutralizing antibody fails to impact the course of Ebola virus infection in monkeys. PLoS Pathog. 3, e9 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye J. M. et al. Postexposure antibody prophylaxis protects nonhuman primates from filovirus disease. Proc. Natl. Acad. Sci. U. S. A. 109, 5034–5039 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzi A. et al. Protective efficacy of neutralizing monoclonal antibodies in a nonhuman primate model of Ebola hemorrhagic fever. PLoS One 7, e36192 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olinger G. G. Jr et al. Delayed treatment of Ebola virus infection with plant-derived monoclonal antibodies provides protection in rhesus macaques. Proc. Natl. Acad. Sci. U. S. A. 109, 18030–18035 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X. et al. Successful treatment of ebola virus-infected cynomolgus macaques with monoclonal antibodies. Sci. Transl. Med. 4, 138ra81 (2012). [DOI] [PubMed] [Google Scholar]
- Wu J. Q. et al. Pre- and post-exposure protection against Western equine encephalitis virus after single inoculation with adenovirus vector expressing interferon alpha. Virology 369, 206–213 (2007). [DOI] [PubMed] [Google Scholar]
- Qiu X. et al. mAbs and Ad-Vectored IFN-alpha Therapy Rescue Ebola-Infected Nonhuman Primates When Administered After the Detection of Viremia and Symptoms. Sci. Transl. Med. 5, 207ra143 (2013). [DOI] [PubMed] [Google Scholar]
- Wong G. et al. Immune parameters correlate with protection against ebola virus infection in rodents and nonhuman primates. Sci. Transl. Med. 4, 158ra146 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wauquier N., Becquart P., Padilla C., Baize S. & Leroy E. M. Human fatal zaire ebola virus infection is associated with an aberrant innate immunity and with massive lymphocyte apoptosis. PLoS Negl Trop. Dis. 4, e837 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan N. J., Martin J. E., Graham B. S. & Nabel G. J. Correlates of protective immunity for Ebola vaccines: implications for regulatory approval by the animal rule. Nat. Rev. Microbiol. 7, 393–400 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan G. S., Li W., Ye L., Compans R. W. & Yang C. Antigenic subversion: a novel mechanism of host immune evasion by Ebola virus. PLoS Pathog. 8, e1003065 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X. et al. Mucosal immunization of cynomolgus macaques with the VSVDeltaG/ZEBOVGP vaccine stimulates strong ebola GP-specific immune responses. PLoS One 4, e5547 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials