Abstract
High-Resolution Magic-Angle Spinning (HR-MAS) NMR spectroscopy has become an extremely versatile analytical tool to study heterogeneous systems endowed with liquid-like dynamics. Spinning frequencies of several kHz are however required to obtain NMR spectra, devoid of spinning sidebands, with a resolution approaching that of purely isotropic liquid samples. An important limitation of the method is the large centrifugal forces that can damage the structure of the sample. In this communication, we show that optimizing the sample preparation, particularly avoiding air bubbles, and the geometry of the sample chamber of the HR-MAS rotor leads to high-quality low-sideband NMR spectra even at very moderate spinning frequencies, thus allowing the use of well-established solution-state NMR procedures for the characterization of small and highly dynamic molecules in the most fragile samples, such as live cells and intact tissues.
High-Resolution Magic Angle Spinning (HR-MAS) NMR is commonly used in many analysis of high impact, perhaps the most important ones being the study of biopsies or of living organisms. Indeed, while heterogeneous samples do not typically produce high quality NMR spectra, the association of sample spinning and fast dynamics of some of the sample constituents allows to obtain highly resolved spectra in these samples. HRMAS is particularly suited for samples in which the dominant broadening mechanism is due to magnetic susceptibility effects. On the other hand, the technique is routinely applied at relatively high rotation rates (of the order of 4 kHz) since experimentally spectra acquired at lower spinning speeds are plagued with spinning sidebands (SSB). These are spurious signals that are often attributed to an incomplete averaging by the magic angle spinning process of either anisotropic internal interactions (chemical shift anisotropy, dipolar couplings) or distributions of magnetic susceptibilities associated with any heterogeneous material. Other mechanisms for the presence of sidebands have been proposed, but the size of the phenomenon in HRMAS is largely exceeding the expected values. The use of large rotational rates is not preferred for biological samples, as the associated centrifugal forces may disrupt the biological activity, even when the morphology of the sample is preserved. For this reason, HRMAS has been mostly performed at moderate magnetic fields (typically 400 to 600 MHz), as higher spinning speeds are required at higher magnetic fields.
Herein, we explore experimentally the different factors that lead to the appearance of intense SSB in slow HR-MAS NMR spectra and we present a protocol to obtain high-quality multidimensional HR-MAS NMR spectra of heterogeneous systems such as intact tissues, at very moderate spinning speeds. HR-MAS NMR spectroscopy has grown to become a reference analytical technique within a large range of disciplines, including material sciences, combinatorial chemistry, food sciences and biomedical research for investigating partially immobilized molecules in non-solid and heterogeneous environments1. While these molecules generally have sufficient mobility to greatly average nuclear anisotropic interactions, magic angle spinning (MAS) is used as a line-narrowing technique that reduces the effects of distributions of bulk magnetic susceptibilities present in inhomogeneous compounds. In general, the MAS frequency must be large compared to the line broadening in order to avoid the occurrence of SSB in the spectra2,3,4. For heterogeneous systems not containing strong magnetic centers, the line broadening should be about a few hundreds of Hz. In practice, spinning frequencies of several kHz are required to obtain high-resolution SSB-free 1H HR-MAS NMR (about 2.5–4 kHz when using a 400 MHz spectrometer)5,6,7, including signals from the liquid part of the sample. However, many specimens demand more delicate experimental conditions and cannot endure the centrifugal forces induced during HR-MAS experiments8,9,10. In this context, the ability to generate high-quality multidimensional NMR spectra at low MAS frequencies (i.e. less than 1 kHz10) is of prime importance. Indeed, a sensitive aspect of HR-MAS NMR applications is the capability of precise characterization of biochemical compounds and metabolic profiles in live cells, intact tissues or biopsies11,12. This realization has prompted considerable efforts to develop slow HR-MAS NMR experiments incorporating efficient SSB suppression schemes13,14,15. However the transposition of these latter to classical multidimensional solution-state NMR experiments (e.g. TOCSY, HSQC) in the case of tissues remains far for straightforward and has not been demonstrated16,17,18,19,20,21. In order to achieve a simplified slow-spinning HRMAS protocol, we have investigated the use of smaller sample volumes and the impact of complete filling of the sample chamber (“absence of air bubbles”). This is anticipated to reduce susceptibility-induced SSB intensity at low spinning rates. Note that although the possibility of using smaller chambers for HRMAS of biopsies has been suggested previously using commercially available material11,22, no effect on the intensity of the SSB has been described, only on the linewidth of the isotropic peaks.
Results
The first sample analyzed was a 50 mM phenylalanine solution in 100% D2O recorded at 298 K. Fast and isotropic tumbling of the molecules at 298 K guarantees that no anisotropic NMR interactions can survive. Therefore, the presence of SSB for a solution must depend either on a disruption of the liquid isotropic nature due to the presence of air bubbles and/or on the occurrence of inhomogeneities of the coil radiofrequency field23. In addition, the introduction of sidebands due to wobbling of the rotor around the magic angle during spinning has also been suggested24.
To explore the possible impact of the first effect, a series of 1D 1H HRMAS spectra were recorded for two preparations in which the detected volume was either fully or partially occupied by the solution. This setup reproduces the most classical textbook case of an inhomogeneous system with respect to its magnetic susceptibility. This effect has been extensively studied in the context of MRI and can induce a line broadening of the order of a few hundreds of Hz25,26,27. Figure 1A shows that substantial SSB are present in the HRMAS spectrum of the solution recorded at 1 kHz spinning rate when “air bubbles” are present, while no SSB are detected if the volume is fully occupied by the liquid (Figure 1B). This unambiguously shows that the incomplete filling of the detected volume is capable of inducing a large number of SSB in liquids at moderate spinning speeds. In addition, we observed that the magnetic susceptibility broadening is larger than expected since SSB are still present above a speed of 1 kHz. This can be due to the dislocation of the bubbles, particularly at low-spinning speeds28, and/or to the effect of radial B1 field variations23,29. Indeed, imaging experiments performed along the spinning axis confirm the presence of void space in the sample (Figure 1A, insets), but a precise value of the bubbles distribution and dynamics would be necessary to properly predict numerically the corresponding spectrum breadth30,31. A spectrum devoid of sidebands was also achieved for larger chamber volumes (50 μl) in the absence of air bubbles (Supplementary Figure 1), providing further evidence of this factor as a dominant in generating SSB. Having assessed the role of incomplete filling of the rotor in inducing sidebands in isotropic samples, we addressed the HRMAS NMR spectroscopy of biopsies. This is one of the most important analytical problems concerning HRMAS NMR and it is representative of other biological specimens with respect to the structural heterogeneity. Figure 2 presents water-presaturated 1D 1H HR-MAS NMR spectra obtained on liver tissue at 280 K and at MAS frequencies of 150, 500 and 4000 Hz, using a 4 mm rotor fitted with a KelF insert to limit the sample volume, but with two slightly different geometries. In the first of these two independent preparations the chamber is almost spherical with a volume of 12 μl, while in the other case the insert was positioned in a slightly higher position, to obtain a slightly larger volume with a non-spherical shape. For both rotors, the chamber was thoroughly fully filled in order to avoid forming air bubbles. At 4000 Hz, both corresponding HRMAS spectra show an envelope of broad signals that could arise from macromolecules and components with restricted molecular motions superimposed on well-resolved peaks from small molecules characterized by fast and isotropic mobility. At lower MAS frequencies, figure 2A shows that a significant number of sidebands survived at 500 Hz when using the HRMAS rotor with the larger chamber setup. At 150 Hz, the characteristic pattern of the 1H NMR spectrum becomes unintelligible. Note that, in the presence of tissue, water is not in an isotropic environment and thus its signal can present SSB below a threshold spinning value. Conversely, for the second chamber geometry, both the number and the magnitude of SSB are drastically reduced (Figure 2B). The introduction of air bubbles increases the number of SSB for larger chambers (Supplementary Figure 2), which suggests that the presence of magnetic susceptibility distributions within the sample plays a major role in the SSB observed at very low spinning speeds (starting from around 300–500 Hz). Having assessed this, we examined if high-quality two-dimensional hetero- and homo-nuclear HR-MAS NMR correlation spectra could be obtained at very moderate spinning speed. Figure 3 displays 2D 1H-13C HSQC and 1H-1H TOCSY HR-MAS NMR spectra of liver recorded at 280 K and 500 Hz MAS frequency. Overall, both spectra display a spectral resolution and a sensitivity equivalent to those typically obtained at 4 kHz spinning frequency32,33, but with a reduction of the centrifugal forces of about two order of magnitudes. Unambiguous assignment of 1H and 13C resonances from various metabolites has been obtained by identifying characteristic spin-spin connectivities from amino acids, saccharides and lipids. Upon detailed inspection of the TOCSY spectrum, we found additional correlations involving the water 1H resonance and two atypical cross-peaks of low intensity corresponding to residual SSB from the intense methyl signals of choline and glycerophosphocholine. Although NMR acquisition times of 20 to 30 hours were necessary to obtain a sufficiently high-resolution for our data, the use of non-uniform sampling methods to reduce acquisition times can be now envisioned. These durations are comparable with spectra of similar quality obtained at the faster spinning rates34. In conclusion, these results pave the way towards metabolic characterization of intact cells and tissues close to physiological conditions and under experimental setups that substantially reduce the risks of sample alteration.
Figure 1. 1H HRMAS spectra of a 50 mM aqueous solution of phenylalanine at 298 K and at MAS frequencies ranging from 400 to 4000 Hz.
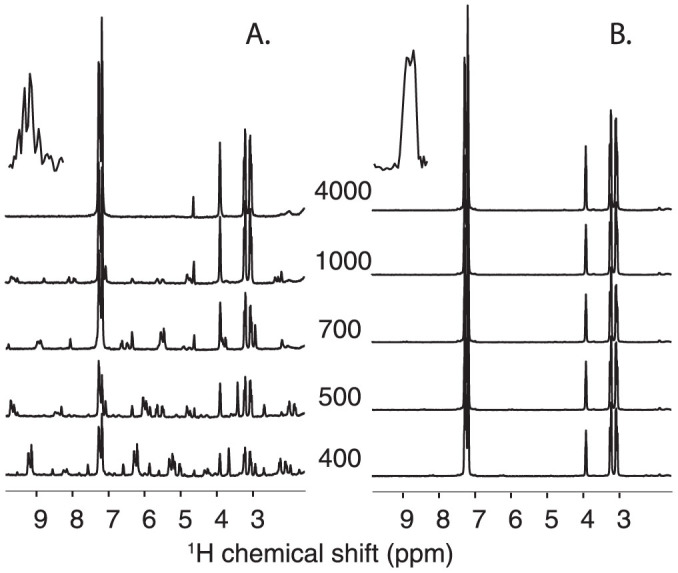
The spectra were acquired using a 4 mm HRMAS rotor with a 12 μl-KelF insert fully (B) or partially (A) filled with the solution. The insets are the images along the spinning direction.
Figure 2. Series of water-presaturated 1H HRMAS spectra of intact liver tissue recorded at 150 (bottom), 500 (middle) and 4000 Hz (top) spinning frequencies using a 4-mm HR-MAS rotor equipped with a 12 μl-KelF insert with minor adjustments of the top insert, resulting in a slightly larger (A) or smaller (B) volume of the sample chamber.
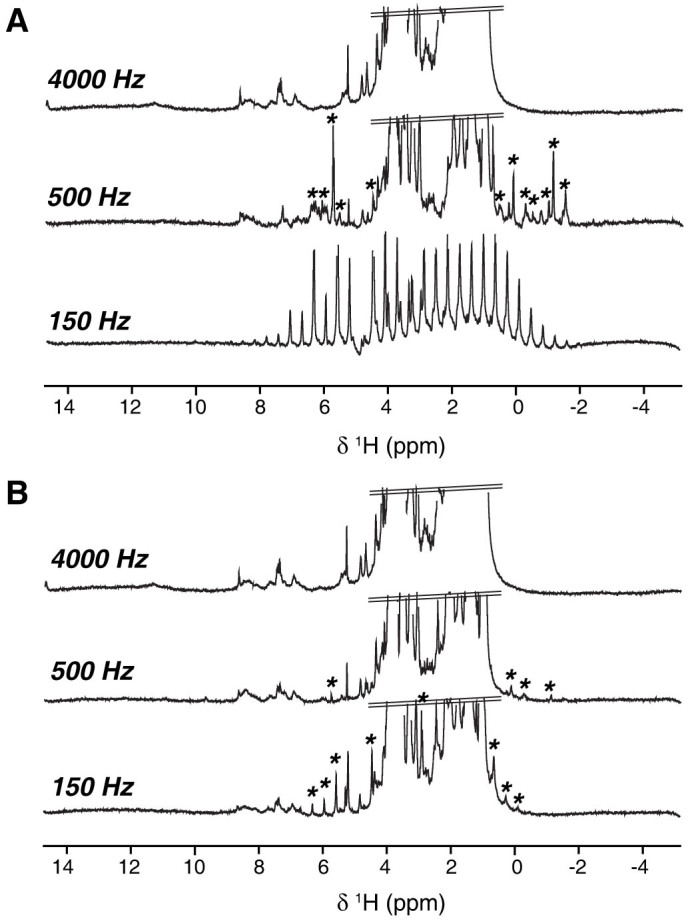
Asterisks indicate the position of SSB from well-characterized isotropic peaks.
Figure 3. Aliphatic regions of the two-dimensional hetero-nuclear (1H-13C) HSQC (top) and homo-nuclear (1H-1H) TOCSY with a rotor-synchronized DIPSI mixing time of 70 ms (bottom) HR-MAS NMR spectra obtained on intact liver tissue at 280 K and 500 Hz spinning frequency.
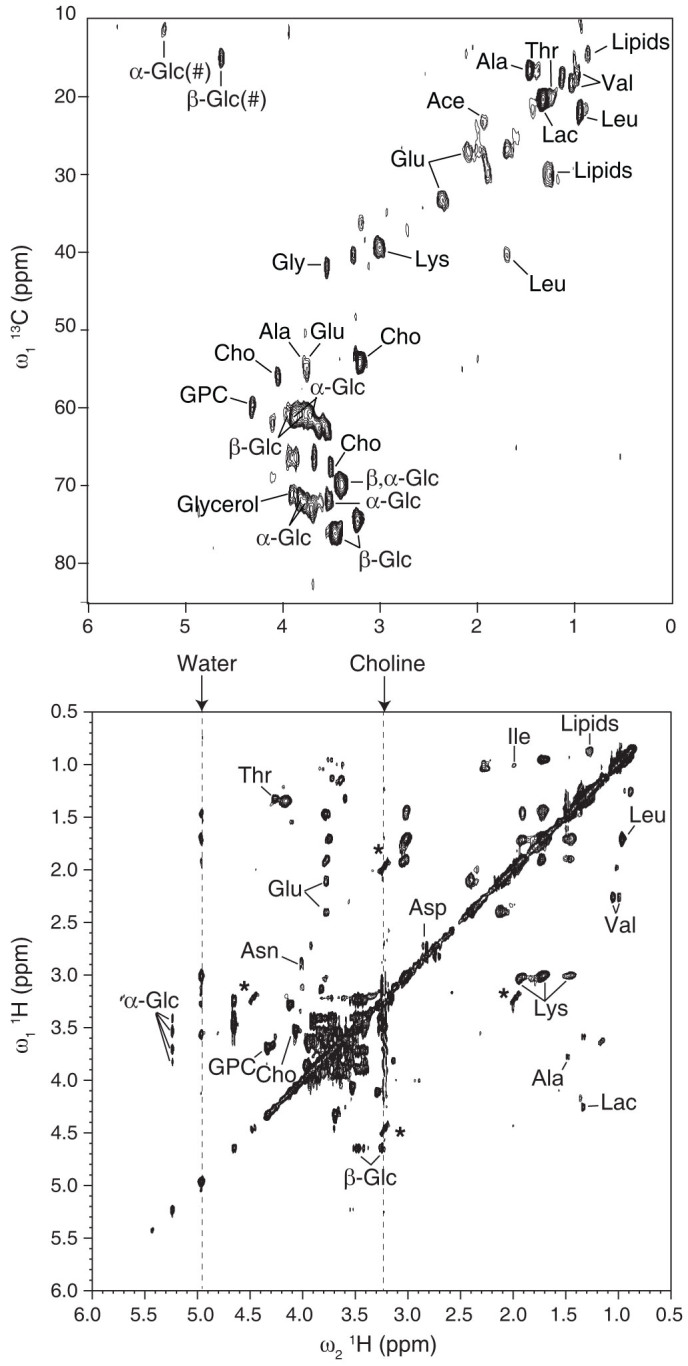
NMR assignments of the most significant metabolites are indicated (Ace, acetate; Ala, alanine; Asn, asparagin; Asp, aspartate, Cho, choline; α/β-Glc, α/β-D-glucose, Glu, glutamate, GPC, glycerophosphocholine; Gly, glycine, Ile, isoleucine; Lac, lactate; Leu, leucine; Lys, lysine; Thr, threonine; Val, valine). 1H frequencies of water and choline resonances are indicated by arrows at the top of the spectrum and materialized by dashed lines in the spectrum. The symbol # designates folded cross-peaks. Asterisks indicate residual SSB originating from of intense choline and GPC signals at 3.21 and 3.23 ppm, respectively. The HSQC and TOCSY spectra were recorded in 26 and 30 hours, respectively.
Discussion
Our results demonstrate that the quality of HR-MAS NMR spectra of highly dynamical molecules in both pure solution or in heterogeneous environments at low MAS frequencies is largely dependent on the rotor configuration. Particularly, the presence and intensity of SSB is shown to be highly dependent on factors linked to the sample preparation (position and shape of the sample, presence of air bubbles). Such observations call for further investigations and detailed analysis of the sample chamber geometry to design optimal HR-MAS rotor systems that can efficiently eliminate or compensate radiofrequency field inhomogeneities and magnetic susceptibility effects while keeping a high signal-to-noise ratio. Nevertheless, it is possible to counterbalance substantially these effects by restricting the sample chamber to a small volume at the center of the coil with the insert located at the top of the rotor. Using this procedure, the number and the magnitude of SSB are drastically reduced and high quality NMR spectra can be obtained at moderate MAS frequencies, thus opening new opportunities to characterize biochemical components within fragile samples by well-established procedures. More specifically, we have shown the first classic series of multidimensional NMR spectra (HSQC, TOCSY) under HR-MAS of intact tissue at very moderate spinning frequency. An interesting point is that the TOCSY experiment is notoriously prone to interfere with the MAS averaging when the spinning frequency is close to the spin-lock RF field amplitude35. This condition is bound to relax for slow spinning as the two timescales diverge when MAS frequencies of few hundreds of Hz are used.
Methods
Sample preparation
A solution of 50 mM phenylalanine, prepared by dissolving 4.2 mg of the amino acid powder (Sigma Aldrich) in 500 μl of D2O, was introduced into a 4-mm zirconia HR-MAS rotor (Bruker BioSpin) equipped with either 12-μl or 50-μl KelF inserts. Approximately 23 milligrams of tissue were excised from heifer's liver, washed several times with D2O to remove residual blood and to provide the lock frequency, and then transferred in a 4-mm zirconia HR-MAS rotor equipped with 12 μl KelF inserts. Samples without air bubbles were prepared by using an excess of solvent, which was removed slowly and carefully when placing the top insert. The rotor was then inserted into the HR-MAS probe and immediately subjected to 1H and 13C HR-MAS spectroscopy.
Data acquisition and analysis
HR-MAS spectra were recorded on a Bruker Avance III 400 spectrometer operating at a proton Larmor frequency of 400.36 MHz and equipped with a 4-mm double resonance (1H, 13C) gradient HR-MAS probe. All NMR experiments were conducted between 150 and 4000 Hz MAS frequencies. The spinning rate was controlled using a commercial automatic slow-MAS II speed controller with a frequency stability better than +/− 3 Hz. NMR experiments performed on the phenylalanine solution were conducted at room temperature whereas for the liver tissue, the sample temperature was regulated at 7°C by cooling down the bearing air flowing with a Bruker Cooling Unit II (Xtreme). For each sample, one-dimensional water-presaturated proton spectra were performed using a spectral width of 20 ppm, 16 K data points, 32 scans, a relaxation delay of 2 sec and an acquisition time of 1 sec. The FID was multiplied by an exponential weighing function corresponding to a line broadening of 3 Hz prior to Fourier transformation. All 1D spectra were processed using automated baseline correction routines. For two-dimensional homonuclear and heteronuclear HR-MAS correlation experiments, we assessed the signal stability over time by recording interleaved water-presaturated 1D proton acquisitions using the above-described parameters. The 2D 1H-1H TOCSY spectrum was acquired using a DIPSI2 pulse sequence from Bruker/Topspin 3.0 library and the following acquisition parameters: a 232 msec acquisition time, a 70 msec DIPSI2 mixing time, a 11.0 ppm 1H spectral width and a 3.0 sec relaxation delay. Two identical experiments were sequentially recorded using 64 scans for each of the 256 increments during t1, corresponding to a total acquisition time of 15 h for each experiment. The two datasets were perfectly identical and thus added, zero filled to a 2 k × 1 k matrix and weighted with a shifted square sine bell function prior to Fourier transformation. Similarly, a set of two identical 2D 1H-13C HSQC experiments employing echo-antiecho gradient selection for phase-sensitive detection were acquired using a 96 msec acquisition time with GARP 13C decoupling of 3.3 kHz power level and a 2.0 sec relaxation delay. Two 1 msec sine-shaped gradient pulses of strength 42.8 G/cm and 10.75 G/cm were used. A total of 512 scans were averaged for each of the 42 t1 increments, corresponding to a total acquisition time of 13 h for each experiment. The two datasets were subsequently added, zero-filled to a 2 k × 512 matrix and weighted with a shifted square sine bell function before Fourier transformation. All spectra obtained on liver tissue were referenced on the methyl 1H and 13C resonances of the choline, set at 3.20 and 54.1 ppm, respectively. All spectra were processed and analysed using the Topspin 3.0 software (Bruker BioSpin).
Author Contributions
The project was conceived by S.C. and developed by L.S. and M.P.; M.R. and L.S. performed the experiments, with the assistance of M.P.; M.R. wrote the article with contributions from all other authors.
Supplementary Material
Acknowledgments
This research has been funded by ANR (ANR-08-BLAN-273, ANR-2011- JS08-014-01) and Region PACA (APO-G 2009).
HRMAS spectra in a larger volume rotor of the phenylalanine solution (50 μl) and the liver tissue (about 16 μl), this latter with and without an air bubble.
References
- Power W. P. in Annu. Rep. NMR Spectrosc. Vol. 72 [Webb G. A. ed.] [111–156] (Academic Press, Paris, 2010). [Google Scholar]
- Andrew E. R. & Eades R. G. Removal of dipolar broadening of NMR spectra of solids by specimen rotation. Nature 183, 1802 (1959). [Google Scholar]
- Lippens G. et al. Study of compounds attached to solid supports using high resolution magic angle spinning NMR. Curr. Org. Chem. 3, 147–169 (1999). [Google Scholar]
- Lowe I. J. Free induction decays of rotating solids. Phys. Rev. Lett. 2, 285–287 (1959). [Google Scholar]
- Barbara T. M. Cylindrical demagnetization fields and microprobe design in high-resolution NMR. J. Magn. Reson. A 109, 265–269 (1994). [Google Scholar]
- Elbayed K. et al. Origin of the residual NMR linewidth of a peptide bound to a resin under magic angle spinning. J Magn Reson 136, 127–129 (1999). [DOI] [PubMed] [Google Scholar]
- Garroway A. N. Magic-angle sample spinning of liquids. J. Magn. Reson 49, 168–171 (1982). [Google Scholar]
- Bruno E. et al. Water exchange across the erythrocyte plasma membrane studied by HR-MAS NMR spectroscopy. Magn. Reson. Med. 56, 978–985 (2006). [DOI] [PubMed] [Google Scholar]
- Santos C. F. et al. Metabolic, pathologic, and genetic analysis of prostate tissues: quantitative evaluation of histopathologic and mRNA integrity after HR-MAS spectroscopy. Nmr in Biomed. 23, 391–398 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunescu A. et al. In vivo proton HR-MAS NMR metabolic profile of the freshwater cladoceran Daphnia magna. Molecular BioSystems 6, 121–125 (2010). [DOI] [PubMed] [Google Scholar]
- Beckonert O. et al. High-resolution magic-angle-spinning NMR spectroscopy for metabolic profiling of intact tissues. Nat Protoc 5, 1019–1032 (2010). [DOI] [PubMed] [Google Scholar]
- Lindon J. C., Beckonert O. P., Holmes E. & Nicholson J. K. High-resolution magic angle spinning NMR spectroscopy: Application to biomedical studies. Prog. Nucl. Magn. Reson. Spectrosc. 55, 79–100 (2009). [Google Scholar]
- Antzutkin O. N., Shekar S. C. & Levitt M. H. Two-dimensional sideband separation in magic-angle-spinning NMR. J. Magn. Reson. A 115, 7–19 (1995). [Google Scholar]
- Dixon W. T. Spinning-sideband-free and spinning-sideband-only NMR-spectra in spinning samples. J. Chem. Phys. 77, 1800–1809 (1982). [Google Scholar]
- Hu J. Z., Alderman D. W., Ye C. H., Pugmire R. J. & Grant D. M. An isotropic chemical shift-chemical shift anisotropy magic-angle slow-spinning 2D NMR Experiment. J. Magn. Reson. 105, 82–87, 1252 (1993). [Google Scholar]
- Hu J. Z., Rommereim D. N. & Wind R. A. High-resolution H-1 NMR spectroscopy in rat liver using magic angle turning at a 1 Hz spinning rate. Magn. Reson. Med. 47, 829–836 (2002). [DOI] [PubMed] [Google Scholar]
- Taylor J. L. et al. High-resolution magic angle spinning proton NMR analysis of human prostate tissue with slow spinning rates. Magn. Reson. Med. 50, 627–632 (2003). [DOI] [PubMed] [Google Scholar]
- Wind R. A. & Hu J. Z. In vivo and ex vivo high-resolution H-1 NMR in biological systems using low-speed magic angle spinning. Prog. Nucl. Magn. Reson. Spectrosc. 49, 207–259 (2006). [Google Scholar]
- Wind R. A., Hu J. Z. & Rommereim D. N. High-resolution H-1 NMR spectroscopy in organs and tissues using slow magic angle spinning. Magn. Reson. Med. 46, 213–218 (2001). [DOI] [PubMed] [Google Scholar]
- Wind R. A., Hu J. Z. & Rommereim D. N. High-resolution H-1 NMR spectroscopy in a live mouse subjected to 1.5 Hz magic angle spinning. Magn. Reson. Med. 50, 1113–1119 (2003). [DOI] [PubMed] [Google Scholar]
- Wong A., Aguiar P. M. & Sakellariou D. Slow magic-angle coil spinning: a high-sensitivity and high-resolution NMR strategy for microscopic biological specimens. Magn. Reson. Med. 63, 269–274 (2010). [DOI] [PubMed] [Google Scholar]
- Waters N. J. et al. High-resolution magic angle spinning 1H NMR spectroscopy of intact liver and kidney: optimization of sample preparation procedures and biochemical stability of tissue during spectral acquisition. Anal. Biochem. 282, 16–23 (2000). [DOI] [PubMed] [Google Scholar]
- Elbayed K., Dillmann B., Raya J., Piotto M. & Engelke F. Field modulation effects induced by sample spinning: application to high-resolution magic angle spinning NMR. J. Magn. Reson 174, 2–26 (2005). [DOI] [PubMed] [Google Scholar]
- Avni R., Mangoubi O., Bhattacharyya R., Degani H. & Frydman L. Magnetization transfer magic angle spinning z-spectroscopy of excised tissues. J. Magn. Reson 199, 1–9 (2009). [DOI] [PubMed] [Google Scholar]
- Bertolina J. A. et al. Experimental verification of inhomogeneous line-broadening calculations in lung models and other inhomogeneous structures. J. Magn. Reson. 99, 161–169 (1992). [Google Scholar]
- Case T. A., Durney C. H., Ailion D. C., Cutillo A. G. & Morris A. H. A mathematical model of diamagnetic line broadening in lung tissue and similar heterogeneous systems: Calculations and measurements. J. Magn. Reson. 73, 304–314 (1987). [Google Scholar]
- Durney C. H. et al. Calculation and interpretation of inhomogeneous line broadening in models of lungs and other heterogeneous structures. J. Magn. Reson. 85, 554–570 (1989). [Google Scholar]
- Doty F. D., Entzminger G. & Yang Y. A. Magnetism in high-resolution NMR probe design. II: HR-MAS. Concepts Magnetic Res. 10, 239–260 (1998). [Google Scholar]
- Goldman M. & Tekely P. Effect of radial RF field on MAS spectra. C. R. l'Academie. Sci., Ser. IIC - Chem. 4, 795–800 (2001). [Google Scholar]
- Holland D. J., Blake A., Tayler A. B., Sederman A. J. & Gladden L. F. A Bayesian approach to characterising multi-phase flows using magnetic resonance: Application to bubble flows. J. Magn. Reson 209, 83–87 (2011). [DOI] [PubMed] [Google Scholar]
- Holland D. J., Blake A., Tayler A. B., Sederman A. J. & Gladden L. F. Bubble size measurement using Bayesian magnetic resonance. Chem. Eng. Sci. 84, 735–745 (2012). [Google Scholar]
- Bollard M. E. et al. High-resolution (1)H and (1)H-(13)C magic angle spinning NMR spectroscopy of rat liver. Magn. Reson. Med. 44, 201–207 (2000). [DOI] [PubMed] [Google Scholar]
- Martinez-Granados B. et al. Metabolite identification in human liver needle biopsies by high-resolution magic angle spinning 1H NMR spectroscopy. NMR Biomed. 19, 90–100 (2006). [DOI] [PubMed] [Google Scholar]
- Martínez-Bisbal M. C. et al. 1H and 13C HR-MAS spectroscopy of intact biopsy samples ex vivo and in vivo1H MRS study of human high grade gliomas. NMR Biomed. 17, 191–205 (2004). [DOI] [PubMed] [Google Scholar]
- Piotto M. et al. Destruction of magnetization during TOCSY experiments performed under magic angle spinning: effect of radial B1 inhomogeneities. J. Magn. Reson 149, 114–118 (2001). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


