Abstract
Introduction
Bronchial thermoplasty (BT) is an emerging therapy for patients with severe persistent asthma who remain poorly controlled despite standard maximal medical therapy. Thermoplasty elicits asthma control over time by applying thermal radiofrequency energy to airways to ablate underlying smooth muscle. While this therapy is suggested to eliminate such smooth muscle permanently, no human studies have examined the possibility of treatment failure.
Case report
We present a 62-year-old female with severe, refractory asthma symptoms who underwent BT without apparent complications. However, severe symptoms including multiple clinical exacerbations persisted despite BT treatment. Repeat endobronchial biopsy done six months after BT treatment demonstrated persistent smooth muscle hyperplasia in multiple airways that previously had been treated. The patient continued to have uncontrolled, refractory asthma despite multiple therapies.
Conclusion
This case is the first to describe a failure of BT to reduce or eliminate airway smooth muscle in a patient with severe persistent asthma. It suggests the potential for treatment failure in the management of these patients after BT and highlights the need for further study of potential BT-refractory patients.
Keywords: Bronchial thermoplasty, severe asthma
Introduction
Severe asthma is seen in about 10% of patients with asthma but is responsible for the majority of all morbidity, health care utilization and cost related to asthma [1,2]. Bronchial thermoplasty (BT), approved by the U.S. Food and Drug Administration (FDA) in 2010, is a novel bronchoscopic treatment for patients with severe persistent asthma [3]. In BT, the proximal airways are treated with radiofrequency current (using the Alair catheter, Asthmatx, Inc., Mountain View, CA) that heats the airway wall to 65 °C resulting in a significant reduction of airway smooth muscle (ASM) mass and partial mitigation of bronchial constriction [4]. The procedure is performed in three sequential bronchoscopies approximately 3 weeks apart. Three randomized trials of BT treatment in 260 asthma patients have demonstrated reduced symptoms, fewer exacerbations and improved quality of life [5–9]. Our group has previously reported a case series of severe fixed airflow obstructed patients undergoing successful BT [10]. We now report a case that represents the first known in the literature to discuss a patient with refractory persistent asthma and fixed airflow obstruction with no reduction in ASM on endobronchial biopsy despite BT.
Case report
The patient is a 62-year-old Caucasian female who is a lifetime nonsmoker with a history of poorly controlled, severe persistent asthma diagnosed at age 27. Although she did not report childhood respiratory symptoms, she exhibited bronchodilator reversibility (see below) and identified various asthma triggers such as respiratory infections, perfumes, cigarette smoke, pet dander, and hot or humid weather. The patient had frequent nighttime respiratory awakenings and debilitating shortness of breath, wheezing and cough with minimal activity, all of which limited her ability to complete housework. She had an Asthma Control Questionnaire© (ACQ) score of 3.9 out of 7 consistent with poorly controlled disease. Additional contributing medical problems included a history of gastroesophageal reflux disease and obstructive sleep apnea for which the patient was compliant with proton pump inhibitor and nightly continuous positive airway pressure therapy, respectively.
She had been treated over time with a Step 6 asthma treatment regimen [2], which included a fluticasone/salmeterol 500/50 mcg by dry powder inhaler twice daily, tiotropium 18 mcg daily, zileuton 1200 mg twice daily and prednisone 60 mg daily. For short-term treatment of asthma symptoms the patient used approximately 13–16 inhalations per day of levalbuterol 45 mcg. Despite these therapies, she continued to require monthly hospitalizations and treatment with non-invasive positive pressure ventilation and intravenous corticosteroids for asthma exacerbations. Though she required multiple hospitalizations, the patient never required intubation.
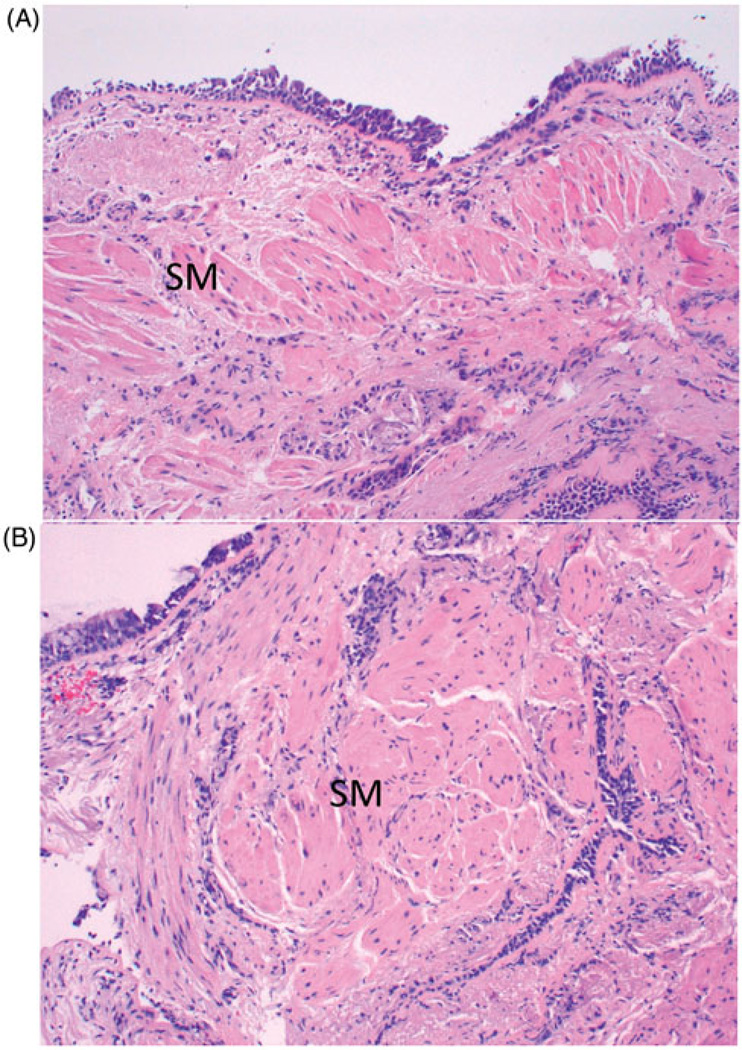
Spirometry demonstrated partially fixed airflow obstruction with forced expiratory volume in 1 second (FEV1) of 0.72 l (26% predicted), a forced vital capacity (FVC) of 1.62 l (45% predicted), and a FEV1 to FVC ratio was 0.44. FEV1 improved to 1.15 l after administration of inhaled albuterol. Endobronchial biopsy of the right lower lobe demonstrated goblet cell hyperplasia, basement membrane thickening, prominent smooth muscle and rare submucosal inflammatory cells consistent with paucicellular asthma (Figure). There was no evidence of granulomatous disease or vasculitis. Computed tomography (CT) imaging of the chest showed mild peribronchial thickening, and the patient had a serum total immunoglobulin E level of 21 U/ml.
Figure 1.
Endobronchial biopsies. (A) Pre-bronchial thermoplasty biopsy shows sub-basement membrane fibrosis, smooth muscle hyperplasia (SM) and minimal inflammation. (B) Post-bronchial thermoplasty biopsy is very similar with sub-basement membrane fibrosis, smooth muscle hyperplasia and no inflammation (H&E stains, original magnification 100×).
A decision was made to pursue BT. The patient’s first treatment with BT was performed under conscious sedation on the right lower lobe per standard clinical practice and required 38 total tissue activations based on the anatomy of that lobe. The patient was hospitalized overnight in the general medicine ward due to need for frequent treatments with nebulized albuterol and discharged the next day without complications. Her second BT procedure was performed 4 weeks later and involved 32 tissue activations of the left lower lobe. This procedure was also performed without any immediate complications, and the patient was discharged the same day. However, 2 days following the second BT procedure, the patient suffered an exacerbation of her asthma requiring general medicine admission for 2 days, which improved after treatment with intravenous corticosteroids and nebulized albuterol. The final BT procedure was performed 3 weeks later and required a total of 23 tissue activations in the right upper lobe and 20 activations in the left upper lobe. There were no immediate complications but the patient was hospitalized overnight for observation in the general medicine ward due to need for frequent treatments with nebulized albuterol. She was discharged the next day.
For 6 months following completion of BT, the patient noted no improvement in asthma symptoms. Her ACQ score remained significantly elevated at 4.1. She continued to have monthly exacerbations requiring hospitalization. Spirometry performed 6 months after the last BT showed no significant change with an FEV1 of 0.65 l and FEV1 to FVC ratio of 0.55. A chest CT scan performed at that time showed no evidence of pulmonary emboli and no change in peribronchial thickening compared to the study done prior to BT. The patient underwent follow-up bronchoscopy with bronchoalveolar lavage and endobronchial biopsy of the right lower, right upper and left upper lobes. Cultures for acid-fast bacilli, fungi and bacteria were negative. However, airway biopsies from each lobe and segment showed persistent, prominent ASM (Figure 1).
Discussion
BT is indicated for patients with severe persistent asthma that is refractory to standard medical treatment [11]. It has been demonstrated safe and effective at reducing asthma exacerbations and symptoms in clinical trials done to date [5–9]. However, the clinical studies performed in patients with asthma did not address the mechanism by which BT improves asthma control. An early study of BT in dogs showed ablation of ASM which correlated with reduced bronchial reactivity up to 3 years after treatment [12]. Another study in humans without asthma also showed reductions in ASM in as little as 2 weeks following treatment [13]. It is well recognized that ASM plays a critical role in bronchoconstriction in asthma [14,15]. Therefore, it is through reducing ASM that BT is presumed to reduce asthma symptoms, although, significantly, histologic sampling in patients with asthma after BT has not yet been reported.
Our case presents a patient with severe persistent asthma who did not have clinical improvement or histologic reduction in ASM after BT. One potential explanation is that our patient had more severe asthma than patients included in previous clinical trials. The Asthma Intervention Research (AIR2) trial excluded patients with a prebronchodilator FEV1 of less than 60% predicted or with more than three hospitalizations in the previous year [5]. Further, 21% of patients treated with BT in the AIR2 trial did not achieve significant improvement in quality of life scores, demonstrating that BT is not effective in all patients [5]. No airway samples were collected in these subjects, and it is not known whether these non-responders also failed to show reductions in ASM as is the case presented here. Our patient underwent very few tissue activations due to her severe fixed airflow obstruction, which limited the scope and catheter advancement peripherally during bronchoscopy. While this may explain the failure of BT in this case, we note that total activations did not correlate to outcomes in AIR2. In addition, while the mean total number of activations in the BT group of AIR2 was 151 with a standard deviation of 50, the range was quite variable from 73 to 369 total activations per subject (Mario Castro, personal communication, 18 January 2013). The total number of activations performed in our patient was well within the reported standard deviation for AIR2 subjects. We have also previously reported a patient with similar, severe fixed airflow obstruction who had a significant clinical response [16].
Another concern is that in standard BT, all patients are treated at a temperature of 65 °C as this was shown to be the most effective in reducing ASM without causing excessive damage to other airway structures [12,13]. However, as histologic sampling of patients with asthma after BT has not yet been performed, it is unclear whether this temperature is adequate to reduce or eliminate ASM in all patients. It is possible that patients with more severe airway thickening require higher temperatures to ablate ASM successfully. Alternatively, patients with persistent prominent ASM may require additional BTtreatments. To date, no study has reported performing further ablation on previously ablated airways.
Conclusion
BT is a novel procedure that has great promise for the treatment of severe asthma. However, much is still unknown about the mechanism by which it achieves symptom control and improved quality of life in patients with asthma. Our report is the first to describe a failure of BT to reduce ASM in a patient with severe persistent asthma. It highlights the need for further study of the effect of BT on airway smooth muscle ablation and symptom control following treatment in asthma.
Acknowledgments
Supported by NIH grant T32 HL07605.
Dr. Hogarth has given 5 industry-funded lectures on Bronchial Thermoplasty and has received unrestricted educational grants from BSCI to teach courses he designed on BT. Our group is currently recruiting for the BSCI-sponsored study of BT called PAS-2.
Footnotes
Declaration of interest
The remaining authors report no conflicts of interest.
References
- 1.Moore WC, Bleecker ER, Curran-Everett D, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–413. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007 Nov;120:S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 3.FDA (U.S. Food and Drug Administration) Asthmatx, Inc.; [last accessed May 2012]. Alair Bronchial Thermoplasty System - P080032. Available from: http://www.fda.gov/medicaldevices/productsandmedicalprocedures/devic eapprovalsandclearances/recently-approveddevices/ucm212594.htm. [Google Scholar]
- 4.Cox PG, Miller J, Mitzner W, Leff AR. Radiofrequency ablation of airway smooth muscle for sustained treatment of asthma: preliminary investigations. Eur Respir J. 2004;24:659–663. doi: 10.1183/09031936.04.00054604. [DOI] [PubMed] [Google Scholar]
- 5.Pavord ID, Cox G, Thomson NC, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176:1185–1191. doi: 10.1164/rccm.200704-571OC. [DOI] [PubMed] [Google Scholar]
- 6.Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. N Eng J Med. 2007;356:1327–1337. doi: 10.1056/NEJMoa064707. [DOI] [PubMed] [Google Scholar]
- 7.Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181:116–124. doi: 10.1164/rccm.200903-0354OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castro M, Rubin A, Laviolette M, et al. Persistence of effectiveness of bronchial thermoplasty in patients with severe asthma. Ann Allergy Asthma Immunol. 2011;107:65–70. doi: 10.1016/j.anai.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Thomson NC, Rubin AS, Niven RM, et al. Long-term (5 year) safety of bronchial thermoplasty: asthma Intervention Research (AIR) trial. BMC Pulm Med. 2011;11:8. doi: 10.1186/1471-2466-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doeing DC, Mahajan AK, White SR, et al. Safety and feasibility of bronchial thermoplasty in asthma patients with very severe fixed airflow obstruction: a case series. J Asthma. 2012 doi: 10.3109/02770903.2012.751997. Epub 2012/12/21. PubMed PMID: 23252954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wahidi MM, Kraft M. Bronchial thermoplasty for severe asthma. Am J Respir Crit Care Med. 2012;185:709–714. doi: 10.1164/rccm.201105-0883CI. [DOI] [PubMed] [Google Scholar]
- 12.Danek CJ, Lombard CM, Dungworth DL, et al. Reduction in airway hyperresponsiveness to methacholine by the application of RF energy in dogs. J Appl Physiol. 2004;97:1946–1953. doi: 10.1152/japplphysiol.01282.2003. [DOI] [PubMed] [Google Scholar]
- 13.Miller JD, Cox G, Vincic L, et al. A prospective feasibility study of bronchial thermoplasty in the human airway. Chest. 2005;127:1999–2006. doi: 10.1378/chest.127.6.1999. [DOI] [PubMed] [Google Scholar]
- 14.James AL, Pare PD, Hogg JC. The mechanics of airway narrowing in asthma. Am Rev Respir Dis. 1989;139:242–246. doi: 10.1164/ajrccm/139.1.242. [DOI] [PubMed] [Google Scholar]
- 15.Woodruff PG, Dolganov GM, Ferrando RE, et al. Hyperplasia of smooth muscle in mild to moderate asthma without changes in cell size or gene expression. Am J Respir Crit Care Med. 2004;169:1001–1006. doi: 10.1164/rccm.200311-1529OC. [DOI] [PubMed] [Google Scholar]
- 16.Mahajan AK, Hogarth DK. Bronchial thermoplasty: therapeutic success in severe asthma associated with persistent airflow obstruction. J Asthma. 2012;49:527–529. doi: 10.3109/02770903.2012.676124. [DOI] [PubMed] [Google Scholar]