Abstract
Platelet-derived growth factor α receptor (PDGFRA)/NG2–expressing glia are distributed throughout the adult CNS. They are descended from oligodendrocyte precursors (OLPs) in the perinatal CNS, but it is not clear whether they continue to generate myelinating oligodendrocytes or other differentiated cells during normal adult life. We followed the fates of adult OLPs in Pdgfra-creERT2/Rosa26-YFP double-transgenic mice and found that they generated many myelinating oligodendrocytes during adulthood; >20% of all oligodendrocytes in the adult mouse corpus callosum were generated after 7 weeks of age, raising questions about the function of the late-myelinating axons. OLPs also produced some myelinating cells in the cortex, but the majority of adult-born cortical cells did not appear to myelinate. We found no evidence for astrocyte production in gray or white matter. However, small numbers of projection neurons were generated in the forebrain, especially in the piriform cortex, which is the main target of the olfactory bulb.
Oligodendrocytes, the myelin-forming cells of the CNS, are mostly generated during the first few postnatal weeks in rodents, peaking in the second week (postnatal day 7–14, P7–P14). They differentiate from proliferative, migratory OLPs that originate in the ventricular zones of the developing spinal cord and brain. OLPs express a characteristic set of markers, including PDGFRA and the NG2 proteoglycan, allowing OLP development to be followed in situ. Both PDGFRA and NG2 are rapidly downregulated when OLPs differentiate into oligodendrocytes1–3, unlike other lineage markers (for example, transcription factors SOX10 and OLIG2), which are expressed in both OLPs and oligodendrocytes. By the time of birth, OLPs are more or less evenly distributed throughout the brain, both in gray matter and developing white matter, and remain so during the early postnatal period, when oligodendrocyte production is in full swing. The size of the OLP population remains relatively stable throughout this time, presumably because, although OLPs continue to proliferate, half of the daughter cells either differentiate or die.
A population of glial cells with the characteristics of OLPs persists into adulthood3–9. These adult OLPs are scattered throughout the gray and white matter and comprise ~4% of all cells in the adult rodent CNS6,9. At least some of these are dividing cells and there is some evidence that they can differentiate into oligodendrocytes during adulthood9,10, although it remains to be shown whether or not they myelinate axons. Adult OLPs have a complex morphology and extend processes to synapses11,12 and nodes of Ranvier13, suggesting that they might monitor, and perhaps modulate, electrical activity in neurons. The finding that OLPs in some parts of the adult CNS receive synaptic input from neurons14 also indicates that they are involved in normal CNS physiology. It seems possible that adult OLPs might fulfill a dual role, participating in information processing and acting as a reservoir of new oligodendrocytes throughout life. Indeed, their precursor role might not be restricted to oligodendrocytes, as OLPs (O-2A progenitors) from perinatal rat optic nerves can also give rise to astrocytes and neurons under certain culture conditions15,16. To reflect their potentially diverse functions and the fact that they have multiple cell processes, adult OLPs have also been named polydendrocytes17. Another name, synantocyte, was coined on the basis of their close contacts with neurons8. They are also referred to simply as NG2 cells or NG2 glia.
To determine the behavior and fates of adult OLPs in vivo, we generated a transgenic mouse line that expresses a tamoxifen-inducible form of the Cre recombinase (CreERT2) under PDGFRA transcriptional control in a phage artificial chromosome (PAC). The PAC transgene was faithfully expressed by OLPs in the postnatal CNS. By combining Pdgfra-creERT2 mice with the Rosa26-YFP reporter line, we were able to induce expression of yellow fluorescent protein (YFP) de novo in adult OLPs and identify their differentiated progeny. We found that OLPs generated mature, myelinating oligodendrocytes in adult mice until at least 8 months of age, raising questions about the function of the newly myelinated axons. We were unable to find any evidence for astrocyte production from adult OLPs in either gray or white matter. Notably, we found small numbers of YFP-labeled neurons in the forebrain, particularly in the piriform cortex (primary olfactory cortex). The YFP+ cortical neurons accumulated with time post-tamoxifen treatment, consistent with their being generated continuously from PDGFRA-expressing precursors. They did not express interneuron markers, but resembled projection neurons. We did not find any YFP-labeled interneurons in the olfactory bulb, reflecting the fact that our Pdgfra-creERT2 transgene is not active in multipotent stem cells of the forebrain subventricular zone (SVZ). We also did not observe substantial numbers of piriform neurons in fate-mapping experiments with Fgfr3-icreERT2, which specifically targets SVZ stem/progenitor cells and astrocytes. We conclude that the new neurons that we observed are probably generated by PDGFRA-expressing adult OLPs/NG2 cells in the parenchyma.
RESULTS
Cycling and noncycling subpopulations of adult OLPs
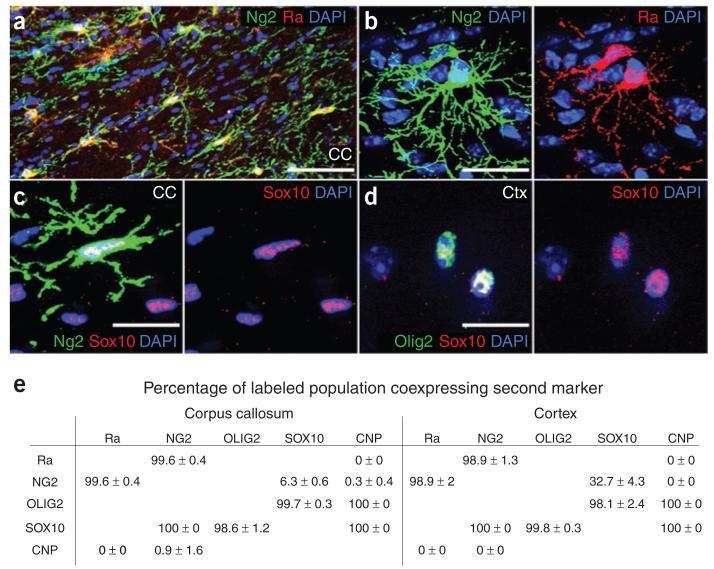
Adult OLPs are usually characterized as NG2+, process-bearing cells in the gray and white matter. We confirmed by immunolabeling that practically all nonvascular NG2-expressing cells in the adult brain coexpress PDGFRA and vice versa (Fig. 1). For example, 99.6% ± 0.4% of NG2+ OLPs in the corpus callosum coexpressed PDGFRA (mean ± s.d., >1,000 cells total from three mice; Fig. 1a,b,e). These two OLP markers could therefore be used interchangeably; this was useful because our antibody to PDGFRA worked only in short-fixed tissue, whereas antibody to NG2 could be used in short- or long-fixed material. We also labeled cells with antibodies to NG2 and SOX10, which works only in long-fixed tissue (see Methods), and found that all nonvascular NG2+ cells coexpressed SOX10 (no SOX10− cells out of >1,000 NG2+ cells scored in corpus callosum or cortex, three sections from each of three mice; Fig. 1c,e). There were, however, many SOX10+ NG2− cells, especially in white matter, that were presumably differentiated oligodendrocytes (Fig. 1c,e). All SOX10+ cells were also OLIG2+ in corpus callosum and cortex, which allowed us to use OLIG2 interchangeably with SOX10 as a general oligodendrocyte lineage marker in long-fixed material (Fig. 1d,e). Antibody to OLIG2 labeled only a subset of PDGFRA+ cells in short-fixed tissue (data not shown), however, and so we did not use our antibody to OLIG2 in short-fixed material.
Figure 1.
Antigenic properties of adult OLPs/NG2 cells. (a–e) Cryosections of young adult (P45) brain were double-immunolabeled for different combinations of oligodendrocyte lineage markers PDGFRA (Ra), NG2, OLIG2, SOX10 and CNP. Representative images are shown in a–d, and cell counts (means ± s.d.) in e. Column headers refer to the labeled populations and row headers refer to the coexpressed second marker. Certain antibody combinations were not possible because of incompatibilities between the required fixation conditions or the species origin of the antibodies (see Methods). Other combinations were not possible because the available antibodies were incompatible. Excluding vascular cells, essentially all of the PDGFRA+ OLPs were colabeled for NG2 and vice-versa, both in corpus callosum (CC) and cortex (Ctx). NG2+ OLPs all colabeled for SOX10, but not all SOX10+ cells were NG2+, as SOX10 continued to be expressed in differentiated oligodendrocytes, whereas NG2 and PDGFRA were downregulated rapidly during differentiation. Scale bars represent 50 μm in a and 10 μm in b–d.
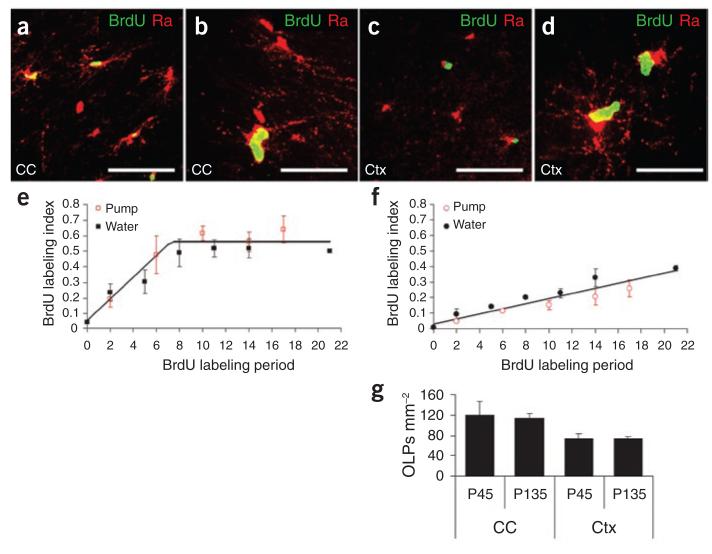
Adult OLPs are the major dividing cell population in the adult rat CNS9. However, neither the proportion of cells in cycle (growth fraction) nor their cell division times have been determined to date. To gain a more detailed understanding of the cell cycle dynamics of adult OLPs, we carried out cumulative BrdU-labeling experiments18,19. BrdU was administered to young adult mice continuously for up to 21 d, either in the drinking water or via osmotic mini-pumps inserted under the skin, starting on P60. Coronal forebrain sections were analyzed after various BrdU-labeling periods by immunohistochemistry for BrdU and PDGFRA (Fig. 2a–d). We calculated the labeling index (fraction of PDGFRA+ cells that incorporated BrdU) separately for cells in the corpus callosum and the medial (mainly motor) cortex. Both routes of BrdU delivery gave similar data (plotted together in Fig. 2e,f). In the corpus callosum, the labeling index increased with time for 7–10 d, but then reached a plateau at labeling index ≈ 0.55 (Fig. 2e). This implies that the cell cycle time is on the order of 1 week and that only ~50% of the PDGFRA+ cells are actively engaged in the cell cycle; that is, there are separate dividing and nondividing subpopulations of OLPs in the adult corpus callosum.
Figure 2.
Cumulative BrdU labeling of adult OLPs/NG2 cells in vivo. BrdU was administered to young adult (P60) mice for up to 17 consecutive days via osmotic mini-pumps or by including BrdU in the drinking water for up to 21 d (see Methods). (a–d) Double-immunolabeling brain sections for BrdU and PDGFRA revealed double-positive (replicating) OLPs in both corpus callosum and cerebral cortex (Ctx). Both routes of BrdU delivery gave comparable data. (e) In corpus callosum, the BrdU labeling index increased to ~0.55 in 7–10 d, but did not increase further. (f) In the cortex, the labeling index increased linearly for 21 d until ~40% of OLPs were BrdU+. At each time point, the labeling index was determined from cell counts (means ± s.d.) on at least three sections from each of three animals. The 2-h time point is from a single intra-peritoneal injection of BrdU (see Methods). (g) The numbers of PDGFRA+ OLPs per unit area were counted in corpus callosum and cortex at P45 and P135 (14-μm sections). There was no significant change (P > 0.7) in OLP cell number or density between these ages. Scale bars represent 45 μm in a, 15 μm in b and d and 40 μm in c.
In the cortex, the labeling index increased steadily over the labeling period (Fig. 2f). At 21 d, nearly 40% of PDGFRA+ cells in the cortex had incorporated BrdU. If the labeling index continues to increase at the same rate until all cortical OLPS are labeled, then that would imply that the whole population divides with an average cycle of ~50 d. However, with the data available, we can conclude only that 40% or more of cortical adult OLPs are dividing with an average cell cycle time of at least 21 d.
We counted PDGFRA+ adult OLPs in the cortex and corpus callosum in 14-μm sections as a function of age. Between P45 and P135 the numbers of cells per unit area did not change significantly (P > 0.7; Fig. 2g). As a proportion of adult PDGFRA+ OLPs divides every 7–10 d on average in the corpus callosum, but the population does not increase, it follows that half of the daughters of OLP divisions must either die or differentiate and downregulate PDGFRA.
Adult OLPs differentiate in gray and white matter
To follow the fates of PDGFRA+ adult OLPs, we generated a transgenic line that expresses a tamoxifen-inducible version of Cre recombinase (CreERT2) under Pdgfra transcriptional control (Supplementary Fig. 1 online). In situ hybridization revealed that creERT2 RNA transcripts were expressed in scattered cells throughout the postnatal brain, similar to endogenous Pdgfra transcripts (Supplementary Fig. 1). Double-label in situ hybridization showed that there was near-complete overlap between Pdgfra and cre expression in the neocortex, piriform cortex, hippocampus and striatum (>99% of cre-expressing cells coexpressed Pdgfra in these locations). In the corpus callosum, where in situ hybridization was less sensitive, >95% of cre-expressing cells expressed detectable Pdgfra (Supplementary Fig. 1).
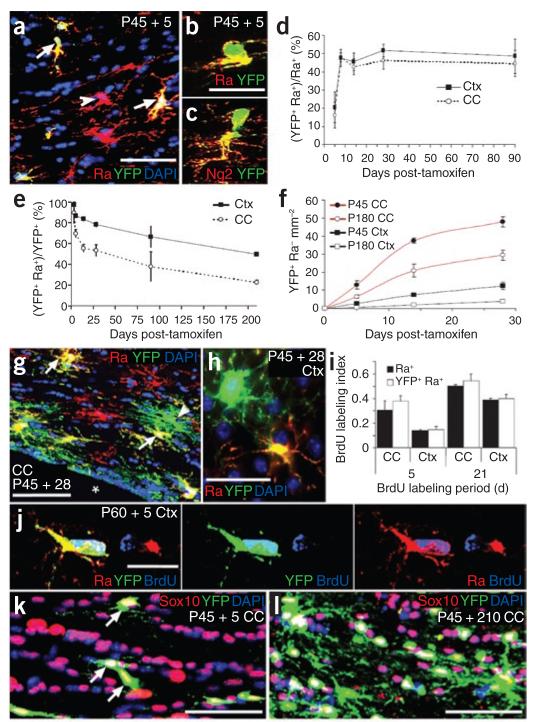
We administered tamoxifen to P45 Pdgfra-creERT2/Rosa26-YFP double-transgenic offspring and immunolabeled for YFP and PDGFRA at various times post-tamoxifen (Fig. 3). At the earliest time examined (3 d after the first dose of tamoxifen, P45 + 3), 89% ± 10% of YFP-expressing cells in the corpus callosum colabeled for PDGFRA and NG2 (mean ± s.d., >400 cells total, nine sections from three mice; Fig. 3a–c,e). This proportion fell at longer times post-tamoxifen as more YFP-labeled cells differentiated and lost PDGFRA expression (Fig. 3e). Only 45–50% of PDGFRA+ cells became YFP-labeled (Fig. 3d), presumably because of the inefficiency of the tamoxifen-induction protocol. We tested whether Cre recombination might have occurred preferentially in the dividing or nondividing subpopulations of OLPs by administering BrdU to tamoxifen-induced mice (P60) via their drinking water before counting YFP+ PDGFRA+ BrdU+ triple-labeled cells. The fraction of YFP+ PDGFRA+ cells that became BrdU-labeled was comparable with that of the PDGFRA+ population as a whole in both corpus callosum and cortex (Fig. 3i,j).
Figure 3.
PDGFRA+ adult OLPs generate differentiated oligodendrocyte lineage cells. (a–c) Sections of P45 tamoxifen-induced Pdgfra-creERT2/Rosa26-YFP mice were immunolabeled for YFP and PDGFRA or NG2 at P45 + 5 (arrows in a indicate YFP+ PDGFRA+ OLPs (yellow), arrowhead indicates a PDGFRA+ YFP− OLP (red)). (d) The fraction of PDGFRA+ cells that coexpressed YFP increased between P45 + 5 and P45 + 8, and then remained constant until at least P45 + 90, indicating that Cre recombination continues for 1–4 d after the final dose of tamoxifen. With our protocol, ~45% of OLPs labeled for YFP after P45 + 8. The fraction of YFP+ cells that coexpressed PDGFRA and NG2 was high at P45 + 3, but declined with time post-tamoxifen (e), as increasing numbers of YFP+, PDGFRA− cells appeared (f–h) (arrows in g indicate YFP+ PDGFRA+ OLPs, yellow; arrowhead indicates a YFP+ PDGFRA− cell, green). This suggests that PDGFRA+ OLPs differentiate and downregulate PDGFRA. (h) YFP+ PDGFRA+ and YFP+ PDGFRA− cells in the cortex. The rate of addition of PDGFRA− (differentiated) cells was higher at P45 than at P180 and was higher in corpus callosum than in cortex at either age (f). (i,j) The subpopulation of OLPs that were labeled for YFP was not biased toward either dividing or nondividing OLPs, as the BrdU labeling index of YFP+ PDGFRA+ cells matched that of the PDGFRA+ population overall. Between P45 + 5 and P45 + 210, all YFP-labeled cells were SOX10+ oligodendrocyte lineage cells, both in the corpus callosum (k,l) and motor cortex (data not shown). Scale bars represent 35 μm in a, g, k and l, and 10 μm in b, c, h and j.
When Pdgfra-creERT2/Rosa26-YFP mice were analyzed 5–28 d post-tamoxifen, the number of YFP+ cells increased in both corpus callosum and cerebral cortex (Fig. 3f) and an increasing fraction of them were PDGFRA− (Fig. 3e,g,h), as expected if a proportion of OLPs was proliferating and differentiating. We counted YFP+ PDGFRA− cells in medial cortex and corpus callosum from 5 d to 3 months after administering tamoxifen at either P45 or P180. In both brain regions, the number of these cells increased with time, although at different rates (slower rates of increase in P180 versus P45 animals and in medial cortex versus corpus callosum at either age; Fig. 3f). We initially counted male and female mice separately, but combined data from both sexes (Fig. 3d–f), as there were no major differences (Supplementary Fig. 2 online).
Extensive myelin genesis in adult white matter
All of the YFP-labeled cells in the corpus callosum were also SOX10+ when they were examined 210 d post-tamoxifen (no SOX10− cells were found among >1,500 YFP+ cells scored across three mice at P45 + 210; Fig. 3k,l), identifying them as oligodendrocyte lineage cells. We found that 13.0 ± 1.7% of all SOX10+ cells were YFP+ at P45 + 90 (mean ± s.e.m., >2,700 SOX10+ cells scored in three mice). At P45, ~45% of OLPs were YFP-labeled in our mice (Fig. 3d and Methods) and ~60% of YFP+ cells in the P45 + 90 corpus callosum were PDGFRA− oligodendrocytes (Fig. 3e), indicating that ~17% of oligodendrocytes in the P135 corpus callosum were generated between P45 and P135 (that is, ((13.0 ± 1.7%) × 0.6) ÷ 0.45 = 17.3 ± 2.3%). At P45 + 210, 17.1 ± 2% of SOX10+ cells were YFP+ (>3,000 SOX10+ cells scored in four mice). Of these, 77 ± 2% were PDGFRA− oligodendrocytes (Fig. 3e). Therefore, assuming that only 45% of OLPs were YFP-labeled at the start of the experiment, this implies that ~29% of differentiated oligodendrocytes are formed in the corpus callosum between P45 and P255 (that is, ((17.1 ± 2%) × 0.77) ÷ 0.45 = 29.3 ± 3%).
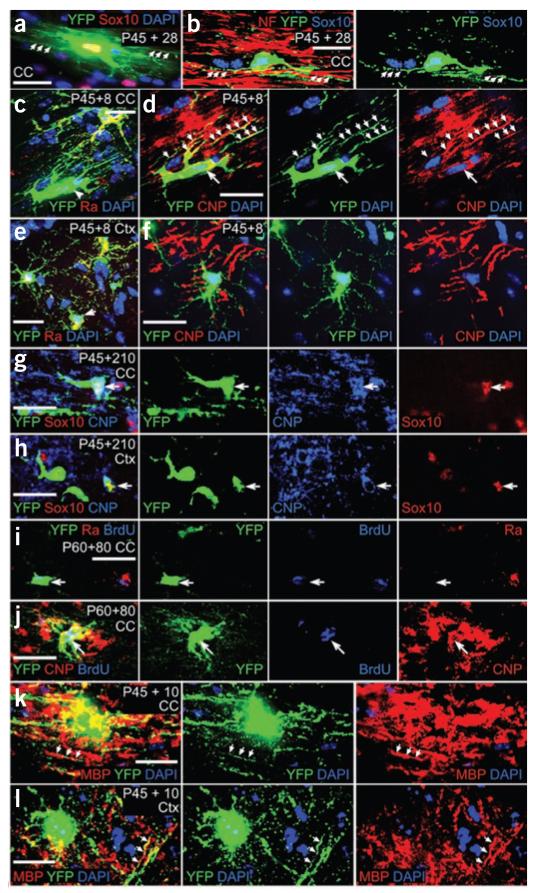
We asked whether the adult-born oligodendrocytes synthesized myelin (Fig. 4). The majority of YFP+ PDGFRA− cells in the corpus callosum had multiple parallel fine processes reminiscent of the cytoplasmic tongue processes of myelinating oligodendrocytes20 (Fig. 4a). These were aligned with neurofilament-positive axons (Fig. 4b). The cell bodies and proximal processes of YFP+ PDGFRA− cells in the corpus callosum at P45 + 8 or P45 + 10 could be immunolabeled for 2′,3′-cyclic nucleotide phosphodiesterase (CNP) or myelin basic protein (MBP), indicating that they were synthesizing myelin (Fig. 4c,d,k). In contrast, PDGFRA+ OLPs did not express detectable amounts of CNP in either the corpus callosum or cortex (Fig. 4e,f and data not shown). The cell bodies of YFP+ SOX10+ CNP+ cells were also found in the corpus callosum at later times post-tamoxifen (for example, P45 + 210; Fig. 4g), but there was very little YFP/MBP colocalization in this material (data not shown), presumably because MBP protein is present mainly in the myelin sheaths, whereas YFP, being a large cytoplasmic protein, is physically excluded from compact myelin.
Figure 4.
Evidence for myelin protein synthesis in adult-born oligodendrocytes. Tamoxifen was administered to P45 Pdgfra-creERT2/Rosa26-YFP mice and forebrain sections were prepared and immunolabeled for YFP and PDGFRA, SOX10, CNP or MBP to identify adult-born (that is, YFP+) oligodendrocyte lineage cells. (a–d) In the corpus callosum at P45 + 28, YFP+ SOX10+ cells possessed many thin parallel cytoplasmic extensions resembling the cytoplasmic tongue processes of myelinating oligodendrocytes (a), which were aligned with neurofilament+ axons (b). In the corpus callosum at P45 + 8, some YFP+ PDGFRA− cells (arrowhead in c) expressed CNP in their cell bodies and proximal processes (arrows in d). (e,f) YFP+ PDGFRA+ OLPs did not colabel for CNP. (g,h) Cell bodies that were triple labeled for YFP, SOX10 and CNP were found at later times (for example, P45 + 210) in the corpus callosum (g) and cortex (h). (i,j) We exposed tamoxifen-induced P60 Pdgfra-creERT2/Rosa26-YFP mice to BrdU via the drinking water for 21 d and analyzed them on P140. Many BrdU+ YFP+ cells in corpus callosum and cortex were PDGFRA− CNP+ (data not shown), confirming that differentiated oligodendrocytes had been generated from dividing PDGFRA+ precursors. (k,l) Some YFP+ cell processes also colabeled for MBP at P45 + 10 (arrows). Scale bars represent 10 μm in a–c, e, k and l), and 20 μm in d, f, and g–j.
If differentiated oligodendrocytes are formed during adulthood from dividing OLPs, as implied above, then we should be able to label the differentiated cells in BrdU pulse-chase experiments. We tamoxifen-treated Pdgfra-creERT2/Rosa26-YFP mice for 4 d starting on P60, added BrdU to their drinking water for 21 d starting on P67, and then immunolabeled them for BrdU, YFP and either PDGFRA or CNP 53 d later (P140). As predicted, many YFP+ PDGFRA− cells and YFP+ CNP+ cells were also BrdU+ (for example, in corpus callosum, 85 ± 4% of YFP+ PDGFRA− cells were BrdU+; in cortex, 68 ± 20%; >100 YFP+ cells counted in three sections from three mice; Fig. 4i,j).
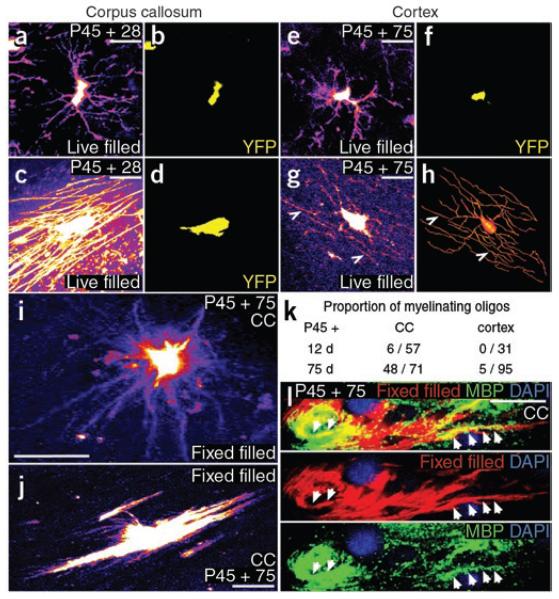
To visualize whole-cell morphology and determine whether YFP-labeled cells could indeed generate myelin internodes, we injected the low molecular-weight fluorescent dye Alexa Fluor 488 into individual YFP-labeled cell bodies in live forebrain slices (300 μm) and observed the dye-filled cells in the two-photon microscope (Fig. 5). Some dye-filled cells in the corpus callosum, presumed to be adult OLPs, had multiple spidery processes (Fig. 5a,b). Other dye-labeled cells had the typical morphology of myelinating oligodendrocytes, elaborating up to ~50 rod-like myelin internodes (Fig. 5c,d).
Figure 5.
Adult OLPs generate myelinating oligodendrocytes. To reveal full cell morphology, we used YFP in live tissue slices to target cells for Alexa Fluor dye injection via glass patch pipettes. (a–d) Cells with the distinctive morphology of myelinating oligodendrocytes, with up to 50 rod-like myelin internodes, were identified in the corpus callosum (c,d), as well as presumptive OLPs with slender, multiply branching processes (a,b). (e,f) In the cortical gray matter, the large majority of live dye-filled cells resembled OLPs. (g,h) A few filled cells in cortical layers 5/6 did possess some myelin-like structures (arrowheads in g); a digital reconstruction gives a clearer impression of whole cell morphology (h). (i,j) To quantify the number of cells with myelinating morphology in the corpus callosum, we filled YFP+ cells in fixed tissue slices with DiI. This allowed larger numbers of cells to be examined, but the DiI did not perfuse the cells as completely as did Alexa dye in live cells. Nevertheless, we could distinguish cells with progenitor morphology (i) versus myelinating morphology (j). (k) The proportion of myelinating cells increased with time post-tamoxifen in the corpus callosum; at P45 + 75 roughly 70% of YFP+ cells had myelinating morphology. (l) We immunolabeled fix-filled cells with antibody to MBP and showed that dye-filled processes contained MBP, indicating that they were indeed myelin internodes. Scale bars represent 10 μm.
The number of live dye-filled cells that could be analyzed in a given slice was limited because cell morphology deteriorated rapidly in unfixed tissue. To analyze larger numbers of cells, we turned to filling cells in tissue slices that had been previously fixed and immunolabeled for YFP (fixation destroys the intrinsic YFP fluorescence, ‘fix filling’; see Methods). A drawback of this approach was that dye penetration into fine cellular processes (including myelin internodes) was reduced compared with live filling. Nevertheless, we were able to distinguish between cells with progenitor morphology (Fig. 5i) and those with myelinating morphology (Fig. 5j) and determined that the proportion of the latter increased with time post-tamoxifen (Fig. 5k). At P45 + 12, 6 out of 57 (~10%) dye-filled YFP+ cells in the corpus callosum had myelinating morphology, whereas the proportion was 48 out of 71 (~70%) at P45 + 75 (Fig. 5k). This is consistent with our observation that ~62% of YFP+ cells in the corpus callosum were differentiated (PDGFRA−) at P45 + 90 (as ~38% of YFP+ cells were PDGFRA+; Fig. 3e). Finally, to confirm the identity of the myelinating cells, we immunolabeled with antibody to MBP and found that dye-filled processes also contained MBP (Fig. 5l).
All YFP+ cells in the medial (motor) cortex were also SOX10+ up to P45 + 210, indicating that they were of the oligodendrocyte lineage (99.8 ± 0.3% at P45 + 210, mean ± s.d., >1,000 SOX10+ cells scored in nine sections from three mice). The great majority of live dye-filled YFP+ cells in the neocortex had typical OLP morphology (Fig. 5e,f). Cells with many thin processes, some of which resembled rigid myelin internodes, were found in the deeper layers of the cortex (layers 5/6; Fig. 5g,h), but these were infrequent; we found 5 cells with putative internodes among a total of 95 dye-filled YFP+ cells (on the order of 5%; Fig. 5k). We also found YFP+ SOX10+ CNP+ cell bodies in the cortex (Fig. 4h) and occasional YFP+ MBP+ cell processes (Fig. 4l), indicating that myelin-producing cells are formed at low frequency in the cortex during adulthood. The fraction of YFP+ cells that was CNP+ at P45 + 210 was 21 ± 4% (450 YFP+ cells scored in six sections from three mice). NG2+ OLPs in the cortex never expressed detectable CNP (Fig. 1e).
Lack of evidence for astrocyte production from adult OLPs
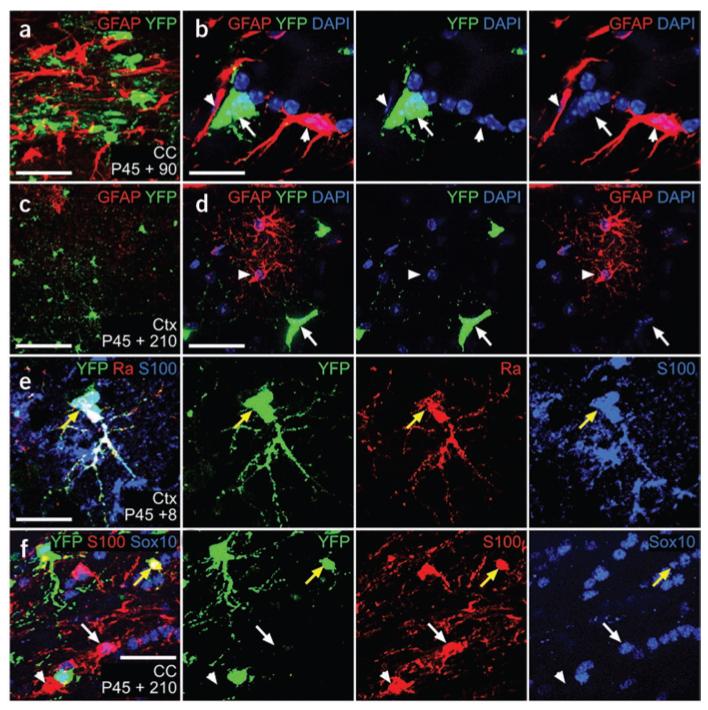
We did not find any cells that co-immunolabeled for YFP and glial fibrillary acidic protein (GFAP), either in gray or white matter (for example, no GFAP+ cells were found among >1,800 YFP+ cells scored in the cortex and >2,200 cells in the corpus callosum, nine sections from three mice; Fig. 6a–d). Because most astrocytes in gray matter do not express detectable GFAP protein, we immunolabeled for YFP and S100β, which is often regarded as a marker of gray matter (protoplasmic) astrocytes, although it is also known to be expressed by some oligodendrocyte lineage cells in the mouse21. We did find some YFP+ S100β+ cells in both the cortex and corpus callosum at all of the times that we examined post-tamoxifen. At P45 + 8, however, the double-positive cells in cortex frequently colabeled for PDGFRA, suggesting that S100β is expressed by a subset of OLPs (Fig. 6e). At later times post-tamoxifen (P45 + 90 or P45 + 210) there were increasing numbers of YFP+ S100β+ cells that were PDGFRA−; however, they all colabeled for SOX10, identifying them as being oligodendrocyte lineage (Fig. 6f). At P45 + 210, YFP+ S100β+ cells accounted for 10.3 ± 1% of all YFP+ cells in the corpus callosum and 12.2 ± 3.7% of YFP+ cells in the medial cortex (mean ± s.d., 1,100 YFP+ cells scored in the corpus callosum and 700 in the cortex from three mice).
Figure 6.
Adult OLPs do not generate astrocytes. Sections of P45 + 90 Pdgfra-creERT2/Rosa26-YFP mice were immunolabeled for YFP and GFAP. (a–d) No double-labeled cells were observed anywhere in the corpus callosum or cortex. Sections were also immunolabeled for YFP and S100β, a marker of protoplasmic astrocytes and some oligodendrocyte lineage cells. (e) In cortex, some YFP+ S100β+ cells were present, but these invariably colabeled for PDGFRA (yellow arrow), identifying them as OLPs. (f) In the corpus callosum, all of the YFP+ S100β+ cells were SOX10+ (yellow arrow in f). The white arrow in f indicates a YFP−,SOX10+ S100β+ oligodendrocyte lineage cell and the white arrowhead indicates a YFP− SOX10−,S100β+ presumptive astrocyte. Scale bars represent 10 μm in b and e, and 25 μm in a, c, d and f.
Even if there were YFP-labeled protoplasmic astrocytes in the cortex that did not express GFAP or S100β, we would almost certainly have been able to identify them by morphology alone. In Fgfr3-icreERT2/Rosa26-YFP mice, many protoplasmic gray matter astrocytes were labeled (Supplementary Fig. 3 online); the latter had a small cell body surrounded by a cloud of extremely fine processes, distinct from the less numerous branched processes of adult OLPs. This also indicates that the Rosa26-YFP reporter is active in differentiated astrocytes; thus, our failure to detect YFP-labeled astrocytes in Pdgfra-creERT2/Rosa26-YFP mice is unlikely to result from downregulation of the reporter.
Few oligodendrocytes are derived from SVZ stem cells
The corpus callosum lies close to the forebrain SVZ, raising the possibility that some or all adult-born oligodendrocytes might be generated directly from stem/progenitor cells in the SVZ, some of which have been reported to express PDGFRA22. However, our Pdgfra-creERT2 mice do not drive recombination in SVZ cells, possibly because our Pdgfra PAC clone is lacking essential regulatory elements. Immunolabeling Pdgfra-creERT2/Rosa26-YFP mice for YFP and GFAP did not reveal any double-labeled subependymal astrocytes (type-B cells) in the SVZ. Similarly, immunolabeling for YFP together with polysialated neural cell adhesion molecule (PSA-NCAM), doublecortin or NeuN did not reveal any migratory neuroblasts or neurons in the SVZ, rostral migratory stream or olfactory bulbs (Supplementary Fig. 3 and data not shown). Moreover, no YFP-labeled neurospheres developed in SVZ cell cultures that were derived from these mice. Note that YFP+ PDGFRA+ and YFP+ PDGFRA− cells were found in the SVZ, but these were invariably SOX10+, marking them out as committed oligodendrocyte lineage cells. Similarly, all YFP+ cells in the olfactory bulbs were SOX10+ (Supplementary Fig. 3)
Analogous experiments with Fgfr3-icreERT2/Rosa26-YFP mice contrasted sharply with the results described above. We found that all SVZ cells, including all GFAP+ type-B cells, were labeled in these mice, many YFP+ neurospheres developed in SVZ cell cultures and many YFP+ NeuN+ neurons appeared in their olfactory bulbs (Supplementary Fig. 3). However, we found only small numbers of YFP+ SOX10+ oligodendrocyte lineage cells in the corpus callosum at P45 + 90. These accounted for only 0.23 ± 0.03% of all SOX10+ cells in the corpus callosum (mean ± s.d., >8,000 SOX10+ cells total, at least three sections from each of three mice), compared with ~20% in Pdgfra-creERT2/Rosa26-YFP mice under similar experimental conditions (see above). We conclude that the great majority of oligodendrocytes formed in the corpus callosum over the 90 d of our experiments were derived from local PDGFRA+ OLPs/NG2 cells that were already resident in the corpus callosum before P45, and not from type-B cells in the SVZ either directly or indirectly through intermediate OLPS.
Adult neurogenesis in the piriform cortex
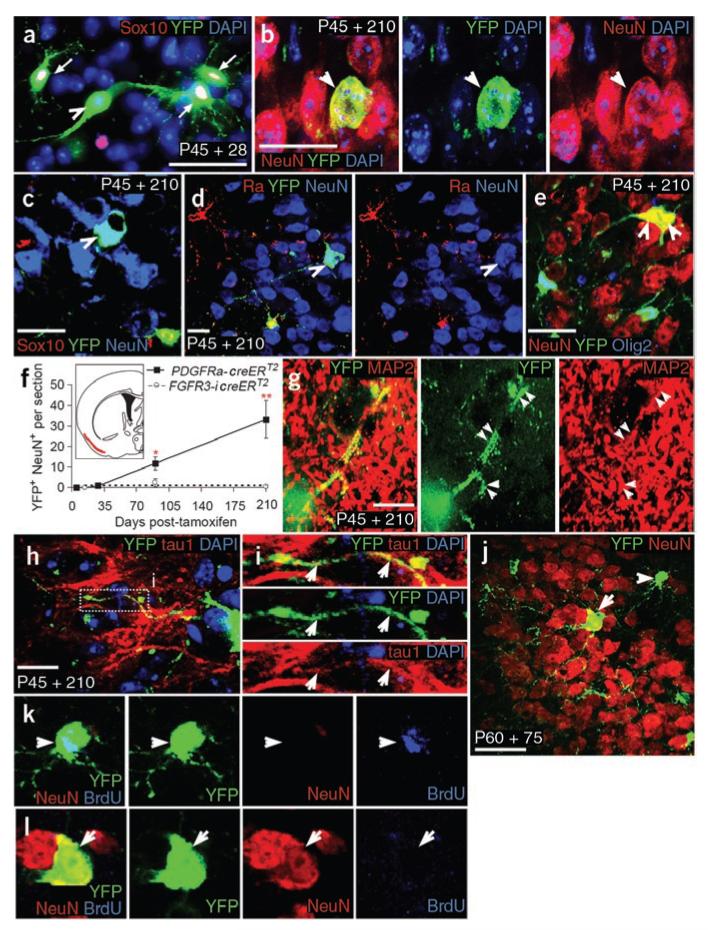
Unexpectedly, we found evidence for neuron generation from OLPs in the adult forebrain (Fig. 7). Starting around a month post-tamoxifen (P45 + 28), small numbers of YFP-labeled cells that did not colabel for SOX10, OLIG2, PDGFRA or NG2 were observed in the piriform cortex and other parts of the ventral forebrain of Pdgfra-creERT2/Rosa26-YFP mice (Fig. 7a,c–e). We focused our analysis on the anterior piriform cortex (aPC), layers 2/3. The YFP+ SOX10− cells expressed the neuronal marker NeuN (Fig. 7b–e,j,l) and they increased in number between 28 and 210 d post-tamoxifen (Fig. 7f). Many of the YFP+ NeuN+ cells had the appearance of projection neurons, with large cell bodies and long axon-like processes (Fig. 7a,h,j and Fig. 8). The long processes could be immunolabeled with antibody to MAP2 or TAU1, which label dendrites and axons, respectively, although it was difficult to distinguish individual processes in the dense mesh of MAP2/TAU1 immunolabeling in the piriform cortex (Fig. 7g–i). Nevertheless, we could determine that the YFP+ TAU1+ processes (putative axons) projected toward the pial surface. The YFP+ neurons could not be co-immunolabeled for neurofilament, reelin or neuronal nitric oxide synthase, nor with any of the interneuron markers calbindin, calretinin, neuropeptide-Y, parvalbumin, somatostatin or tyrosine hydroxylase (Supplementary Fig. 4 online and data not shown).
Figure 7.
OLPs generate cortical projection neurons in vivo. (a–l) Pdgfra-creERT2/Rosa26-YFP mice were tamoxifen-induced at P45 and sections were immunolabeled for YFP and oligodendrocyte and/or neuronal lineage markers. In the ventral forebrain, occasional YFP+ SOX10− cells appeared (arrowheads in a and c; SOX10+ cells indicated by arrows). These cells coexpressed the neuronal marker NeuN (b–e,j,l), but were PDGFRA− and OLIG2− (arrowheads in d and e). The hypothalamus is depicted in a, but all other fields are from layers 2/3 of the aPC (red territory in f, inset). We followed the accumulation of YFP+ NeuN+ cells in the aPC over time (numbers of neurons per 30-μm section, mean ± s.d., n = 3 mice, f). Most YFP+ NeuN+ cells appeared after a delay of 28–90 d and continued to accumulate after that. In parallel, we examined Fgfr3-icreERT2/Rosa26-YFP mice, in which all SVZ stem cells and many protoplasmic astrocytes were labeled for YFP (Supplementary Fig. 3). YFP+ NeuN+ neurons did not accumulate in the aPC of these mice (f), supporting the conclusion that the adult-born neurons are formed from cells outside the SVZ. Some YFP+ cell processes colabeled for MAP2 (g) and TAU1 (h,i). We were unable to label YFP+ NeuN+ neurons with BrdU (arrows in j,l). A BrdU+ YFP+ OLP acts as a control (arrowheads in j and k). Scale bars represent 10 μm in g, 20 μm in b and d, 30 μm in a, c, e and j) and 40 μm in h.
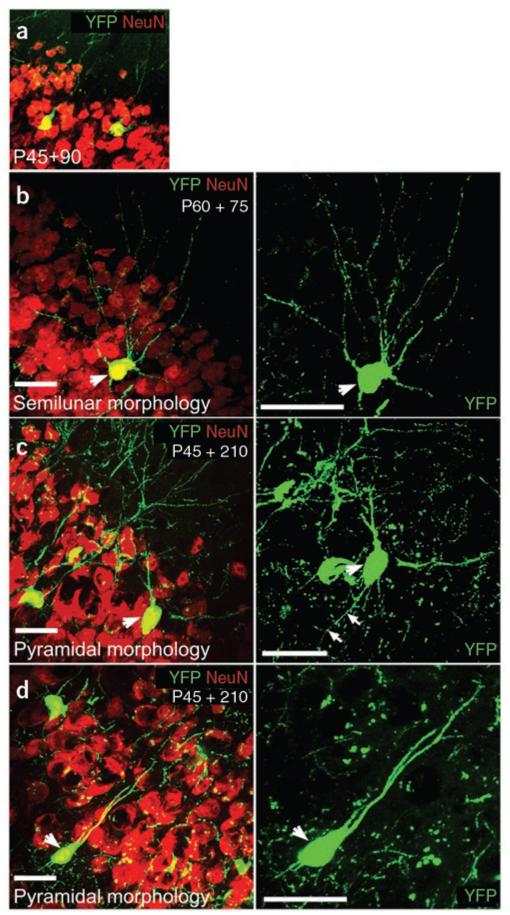
Figure 8.
Morphologies of YFP-labeled neurons in the piriform cortex. (a) NeuN+ neurons in Pdgfra-creERT2/Rosa26-YFP mice were frequently located in layer 2 of the piriform cortex (primary olfactory cortex), with processes projecting into the lateral olfactory tract under the pial surface. In all micrographs the pial surface is top-right. Two types of principal neurons have been described in layer 2; semilunar neurons and superficial pyramidal neurons, which receive inputs from the olfactory bulbs via the lateral olfactory tract40. (b–d) Semilunar neurons are often located more superficially than superficial pyramidal neurons (layer 2a and 2b, respectively). From morphology and their positions in layer 2, we speculate that the YFP+ NeuN+ neurons that we observe correspond to semilunar (b) and superficial pyramidal (c,d) neurons. Scale bars represent 30 μm.
One possibility is that the YFP+ projection neurons are generated from the local population of adult OLPs. Alternatively, they might develop from migratory neuroblasts originating in the SVZ. The latter seems very unlikely, as our Pdgfra-creERT2 transgene is not active in the SVZ (see above); nevertheless, to test the neurogenic properties of the SVZ directly, we examined Fgfr3-icreERT2/Rosa26-YFP mice. There was no accumulation of YFP+ NeuN+ cells in the aPC of these mice (Fig. 7f), supporting the idea that the adult neurogenesis that we describe is driven mainly by precursor cells outside of the SVZ, most likely the local population of OLPs/NG2 cells. The region of aPC that we analyzed spans ~2 mm in the anterior-posterior axis (~67- × 30-μm coronal sections) and ~50% of PDGFRA+ cells in the cortex were labeled in our Pdgfra-creERT2 mice, indicating that ~24 new long-term surviving neurons were added to the aPC each day until at least 8 months of age (YFP+ neurons were added at a rate of 0.18 cells per section per d, (0.18 × 67 ÷ 0.5) = 24.1; Fig. 7f).
We asked whether the YFP+ neurons were derived from proliferating OLPs by tamoxifen-inducing Pdgfra-creERT2/Rosa26-YFP mice starting on P60 and adding BrdU to their drinking water for 21 d starting on P67. The mice were left for a further 53 d (P60 + 80) before immunolabeling for BrdU, YFP and NeuN. None of the 243 YFP+ NeuN+ cells that we examined (n = 3 mice) were BrdU+, suggesting that they were formed from PDGFRA+ cells that had become post-mitotic before being exposed to BrdU (that is, before P67; for example, see Fig. 7j–l).
DISCUSSION
Adult myelinogenesis
There is good evidence that adult OLPs generate new myelinating oligodendrocytes following acute experimental demyelination23,24. There is also evidence that they can generate oligodendrocytes during normal adult life9, although the scale of adult oligodendrogenesis has not been determined, nor has it been established whether they generate mature, myelinating cells. Our experiments with Pdgfra-creERT2 mice confirm that adult OLPs generate myelin-forming oligodendrocytes in the corpus callosum and, to a lesser extent, in cortical gray matter. The number of new oligodendrocytes that are formed in the corpus callosum during the 7-month period after P45 is >20% of the final total and many of these appear to myelinate, judging by dye-filled morphology and the fact that they express myelin proteins. In contrast, only around 5% of adult-born oligodendrocyte lineage cells in the cortex elaborated myelin sheaths, judging by dye-filled morphology. Around 20% of all YFP+ SOX10+ cells in the motor cortex were CNP+, but NG2− at P45 + 210, indicating that a sizeable population of differentiated, apparently nonmyelinating, oligodendrocyte lineage cells is present in the adult cortical gray matter. These cells might have a function unrelated to myelination, or they might form myelin infrequently or at a very low level compared with white-matter oligodendrocytes. Further work is needed to reveal their properties and functions.
The corpus callosum is known to contain a mixture of myelinated and unmyelinated axons. Myelination continues until at least P240, at which time ~70% of axons are still not fully myelinated25. It is possible that the newly formed oligodendrocytes that we observed are engaged in ensheathing these previously naked axons. Alternatively, new oligodendrocytes might be formed to replace some that degenerate during normal life. However, long-term tritium-labeling experiments support the idea that there is a modest accumulation of oligodendrocytes in the mouse corpus callosum during the first year, without detectable turnover26. If, as seems probable, naked axons continue to be myelinated into adulthood, then this would presumably increase their conduction velocity and alter the properties of the neural circuit(s) in which they participate. The functional or behavioral consequence of this is unknown, but it could potentially be involved in neural plasticity, such as learning and/or memory consolidation27. It has recently been shown that some adult OLPs form en passant synapses with axons in white matter and can respond to action potentials in those axons28,29, raising the possibility that OLPs are ‘listening in’ to axonal activity, which might trigger myelination at some threshold.
It has been reported that there is a population of PDGFRA+ GFAP+ multipotent type-B cells in the adult SVZ that contributes to olfactory neurogenesis22. In addition, a small fraction of type-C (transit amplifying) cells originating in the SVZ start to express OLIG2 and generate PDGFRA+ OLPs and mature oligodendrocytes in the corpus callosum, striatum and fimbria fornix of adult mice30. In principle, therefore, the adult-born oligodendrocytes that we observed might all have developed from these cells. However, very few YFP+ oligodendrocytes were formed in control experiments with Fgfr3-icreERT2 mice, in which all SVZ stem/progenitor cells, but not OLPs, are marked. This indicates that the great majority of adult-born oligodendrocytes differentiate from local OLPs, which is consistent with the observation that adult OLPs divide, but their numbers do not increase. Experimental demyelination has been shown to activate cell division and migration from the SVZ, indicating that SVZ-derived precursors might become more important during myelin repair30,31.
We did not detect any astrocyte production from PDGFRA+ OLPs in the adult, either in the gray or white matter. This contrasts with the recent report that some GFP-labeled protoplasmic astrocytes developed in the ventro-lateral forebrain of NG2-cre/CAGG-Z/EG double-transgenic mice32. A constitutively active form of Cre was used in that study, which identified cells that formed mainly in the embryonic and early postnatal period. It is possible, therefore, that NG2+ precursors generate astrocytes during embryonic development, but not in the adult. We cannot test this using our existing Pdgfra-creERT2 mice, as they show ectopic transgene expression in the dorsal neural tube before birth (data not shown). Alternatively, it is conceivable that there are astrocyte precursors among the ~50% of PDGFRA+ OLPs that were not YFP-labeled in our experiments.
An unexpected finding of our study was that there appeared to be two populations of OLPs in the postnatal corpus callosum. At P60, ~50% of OLPs were cycling with an average period of around 1 week while the remaining OLPs were quiescent or dividing much more slowly. There have been previous hints that OLPs are heterogeneous33–35. It will be interesting to discover whether mitotic activity co-segregates with a particular subclass of OLP.
Adult neurogenesis in the pirifom cortex
We have presented evidence that adult OLPs generate small numbers of neurons throughout the ventral forebrain, but particularly so in the piriform cortex (also known as the primary olfactory cortex), the primary projection site of the olfactory bulbs. These neurons did not express known interneuron markers (calbindin, calretinin, neuropeptide-Y, parvalbumin, somatostatin and tyrosine hydroxylase), but had the appearance of projection neurons, with large cell bodies and long axons. Recently, two morphologically and physiologically distinct types of output neurons, semilunar and superficial pyramidal neurons, have been described in the piriform cortex36. The morphologies of the YFP+ neurons that we describe and their location in layers 2a/2b of the aPC are compatible with the idea that they might be a mixture of semilunar and superficial pyramidal neurons. However, detailed electrophysiology would be required to test this.
SOX10− NeuN-positive cells first appeared in small numbers about a month after tamoxifen induction, increased around tenfold over the following 3 months and continued to accumulate after that. No YFP-labeled cells of any kind were ever observed in the absence of tamoxifen. The data therefore strongly suggest that the new neurons were formed over an extended period from progenitor cells that became YFP-labeled at the time of tamoxifen induction. We were unable to label any YFP+ cells for doublecortin or PSA-NCAM, markers of migratory neuroblasts in the SVZ and rostral migratory stream. We were also unable to find BrdU+ NeuN+ YFP+ neurons, even after 21 d of continuous BrdU labeling followed by a long (53 d) chase, suggesting that they might be formed by trans-differentiation of post-mitotic cells.
In vitro studies have shown that OLPs from perinatal optic nerve are capable of generating neurons and astrocytes as well as oligodendrocytes in vitro16. SVZ cells that expressed a Cnp-GFP transgene (and that colabeled for NG2) were reported to generate GABAergic hippocampal neurons in vitro and in vivo37. In addition, there have been several reports of adult neurogenesis outside of the olfactory bulb and hippocampus, both in rodents34,38–40 and primates41,42. The concept of adult neurogenesis outside the accepted neurogenic regions has remained controversial43. We believe that our study avoids some of the pitfalls inherent in previous studies, although it might introduce new problems specific to cre-loxP technology. For example, piriform neurons might express the Pdgfra-creERT2 transgene in their own right. This is unlikely, as we did not observe either PDGFRA or CreER expression in NeuN+ neurons in the piriform cortex or anywhere else. Moreover, if cortical neurons did express the transgene, then YFP-labeled neurons would be expected to appear soon after tamoxifen treatment, and not to accumulate slowly over several months, as we observed. Another possibility is that YFP+ neurons might be formed by the fusion of unlabeled neurons with YFP+ non-neuronal cells, but this is also difficult to reconcile with the slow appearance of YFP+ neurons. Moreover, the putative cell fusion event would need to be highly cell-type specific because we only ever observed YFP+ projection neurons, and not cortical interneurons, and YFP+ projection neurons were not observed in Fgfr3-icreERT2/Rosa26-YFP mice, despite the fact that they contained many YFP+ protoplasmic astrocytes as potential fusion partners.
In summary, we cannot absolutely rule out that the YFP+ neurons were formed by cell fusion, nor can we rule out that they were generated from PDGFRA+ precursors that are distinct from adult OLPs, either a previously unrecognized population in the CNS or from circulating blood. Indeed, there might be other unforeseen artifacts connected with the cre-loxP methodology. However, the simplest scenario is that the YFP+ neurons are formed by differentiation of local, PDGFRA+ OLPs.
The scale of neurogenesis that we observed was small. We estimate that ~1.4% of the projection neurons present in layers 2/3 of the piriform cortex at 8 months (P45 + 210) are generated after P45 (~250 surviving YFP+ NeuN+ neurons found in 18 sections containing an estimated 4 × 104 NeuN+ projection neurons). The physiological importance of this small amount of adult neurogenesis is unknown, even if the neurons in question manage to extend axons to their normal targets and form active connections, which we have not yet established. It is possible that the amount of cortical neurogenesis might be increased by exercise or environmental enrichment, which have been shown to stimulate neurogenesis in the adult olfactory bulb and hippocampus. Moreover, small numbers of neurons, even single neurons, are capable of eliciting observable behavior44,45. It has been reported that defined cortical neurons can be regenerated by endogenous precursors after experimental ablation and the replacement neurons can extend long-range axons toward their original targets46. Our results raise the possibility that those replacement neurons might be derived from adult OLPs/NG2 cells; if so, the latter could be a potentially useful resource for neuronal replacement during neurodegenerative disease.
METHODS
Pdgfra-creERT2 and Fgfr3-icreERT2 transgenic mice
The generation and characterization of BAC transgenic mice is described in Supplementary Figures 1 (Pdgfra-creERT2) and 3 (Fgfr3-icreERT2).
Tamoxifen induction
Homozygous creERT2 mouse lines were maintained on the Rosa26-YFP reporter background47. Cre recombination was induced by administering tamoxifen (Sigma, 40 mg ml−1), dissolved in corn oil by sonication for 45 min at 30 °C. Adult mice were given 300 mg per kg of body weight by oral gavage on each of 4 consecutive days starting at P45 or P180. In the corpus callosum of Pdgfra-creERT2/Rosa-YFP mice, 45 ± 1% of PDGFRA+ OLPs were labeled for YFP; in the medial cortex, 49 ± 2% were labeled (means ± s.d., combining data from mice induced at P45 and analyzed 8, 14, 28 or 90 d post-tamoxifen; Fig. 3d). In Fgfr3-icreERT2/Rosa26-YFP mice, practically all of the cells in the forebrain SVZ (including GFAP+ type-B cells) and a high proportion of Fgfr3-expressing astrocytes in the parenchyma labeled for YFP (Supplementary Fig. 3).
BrdU labeling
For the 2-h time point, BrdU (20 mg ml−1 in phosphatebuffered saline, PBS) was administered as a single 2 mg intraperitoneal injection 2 h before perfusion. For cumulative labeling, we loaded mini-pumps (Alzet) with 50 mg ml−1 BrdU (Sigma) in 1% ammonium solution (vol/vol) or vehicle alone and implanted them subcutaneously into adult mice18. Mini-pumps were weighed before and after filling to confirm successful loading, and were then primed at 37 °C in 0.9% saline (wt/vol) for at least 1 h before implantation. They were replaced after 9 d. Alternatively, we added BrdU to the drinking water (1 mg ml−1). Both modes of delivery gave similar data (Fig. 2e,f).
Tissue preparation
Mice were anaesthetized with pentobarbitone and killed by perfusion through the ascending aorta, first with 0.9% saline (wt/vol), and then with 4% paraformaldehyde (wt/vol, PFA, Sigma) in PBS. Brains were removed and immersed in 4% PFA in PBS for 45 min at 20–25 °C (required for the antibody to PDGFRA) or else overnight at 4 °C (required for the antibodies to SOX10 or OLIG2). Other antibodies, including those to GFP and NG2, tolerated short or long fixation. Tissue was cryo-protected overnight at 4 °C in diethylpyrocarbonate-treated 20% sucrose (wt/vol) in PBS, embedded in Tissue-Tek OCT compound, frozen on dry ice and stored at −80 °C. Cryo-sections (14, 20 or 30 μm) were floated onto the surface of PBS or collected onto gelatin-coated slides and dried overnight at 20–25 °C.
Immunohistochemistry
Sections were pre-treated with blocking solution (10% sheep serum (vol/vol), 0.1% Triton X-100 (vol/vol BDH) in PBS) for 1 h at 20–25 °C, and then incubated sequentially in primary antibody and secondary antibody containing Hoechst 33258 dye (Sigma, 104 dilution), all in blocking solution. After washing in PBS, floating sections were transferred to Superfrost Plus slides and dried. All slides were coverslipped in mounting medium (Dako) and examined under the fluorescence microscope. We used primary antibodies to PDGFRA (rat, BD Sciences, 1:500 dilution), GFP (rabbit, AbCam, 1:6,000; rat, Fine Chemical Products, 1:2,000), SOX10 (guinea pig, a gift from M. Wegner, University of Erlangen, 1:4,000), GFAP (mouse, Sigma, 1:2,000), NeuN (mouse, Chemicon, 1:1,000), S100β (mouse, Sigma, 1:400), doublecortin (guinea pig, Chemicon, 1:3,000), PSA-NCAM (mouse IgM, Chemicon, 1:1,000), CNPase (mouse, Chemicon, 1:2,000), NG2 (rabbit, Chemicon, 1:500), MBP (rat, AbD Serotec, 1:100), neuronal nitric oxide synthase (rabbit, Zymed, 1:500), reelin (mouse, a gift from A. Goffinet, University of Louvain, 1:2,000), MAP2a2b (mouse, Sigma, 1:500), Tau1 (mouse, Chemicon, 1:200), neurofilament (mouse, Chemicon, 1:500), OLIG2 (rabbit, Chemicon, 1:700) and BrdU (mouse, American Type Culture Collection, 1:10; rat, AbD Serotec, 1:500). All mouse or rat antibodies were monoclonal. Antibodies that we used to calbindin, calretinin, somatostatin, parvalbumin, tyrosine hydroxylase and neuropeptide-Y have been described previously48,49. Secondary antibodies were Alexa Fluor 488–, 568– or 647–conjugated goat antibody to mouse IgG1, goat antibody to mouse IgM, goat antibody to rabbit IgG (Invitrogen, 1:1,000), goat antibody to rat IgG (Invitrogen, 1:500), Cy3-conjugated goat antibody to guinea pig IgG (Chemicon, 1:500) or antibody to mouse TRITC (Jackson Labs, 1:200). BrdU immunolabeling was carried out as described previously49.
Dye-filling live or fixed cells
To dye-fill live cells (live filling), we prepared 300-μm live brain slices of tamoxifen-induced Pdgfra-creERT2/Rosa26-YFP mice using a vibratome. YFP+ cell bodies (not processes) were visible in the twophoton laser scanning microscope at a 930-nm excitation wavelength. Positive cells were filled with 20 μM Alexa Fluor 488 dye through a 9–12 MΩ glass pipette. Alternatively, for fix filling, perfusion-fixed tissue was post-fixed by immersion overnight at 4 °C in 4% PFA and 90-μm vibra-slices were collected on to the surface of PBS and immunolabeled for YFP as described above, but in the absence of Triton X-100. We delivered 0.1% DiI (wt/vol) into YFP+ cells by iontophoresis using a 10–15 MΩ pipette and a 10–30 V pulse. All YFP+ cells in a given microscopic field were DiI filled. Images of cells before and after filling were collected using custom-made ScanImage software and analyzed with ImageJ (http://rsbweb.nih.gov/ij/).
Microscopy and quantification
Confocal images were captured with an UltraView microscope (Perkin Elmer) using standard excitation and emission filters for visualizing DAPI, FITC (Alexa Fluor 488), CY3, TRITC (Alexa Fluor 568) and Far Red (Alexa Fluor 647). Confocal z stacks were captured for each section (0.5–1-μm increments). We reconstructed xz and yz views using Volocity software (Perkin Elmer). For cell counting, micrographs were made using a 10× objective on a Zeiss Axioplan microscope with a Hamamatsu digital camera or a 20× objective in the Ultraview confocal. Composite images were assembled using Adobe Photoshop and defined areas were measured using Carl Zeiss AxioVision Release 4.5. Quantification of YFP labeled cells was concentrated in the anterior forebrain (neocortex, corpus callosum and anterior piriform cortex at Bregma levels 0–0.26 mm)50. Sections from at least three mice were analyzed for each data point.
Supplementary Material
ACKNOWLEDGMENTS
We thank our colleagues in the Wolfson Institute for Biomedical Research for discussions, help and encouragement. Special thanks to M. Grist and U. Dennehy for expert technical assistance and to T. Mitsumori for helping to construct the Fgfr3-icreERT2 transgene. Thanks also to D. Attwell for critically reading the manuscript and L. Dimou and M. Götz for exchanging data before publication. L.E.R. was supported by a collaborative studentship from the Biotechnology and Biological Sciences Research Council and Eisai London Research Laboratories at University College London. F.J. held a Marie Curie Fellowship from the European Union. K.M.Y. is an Alzheimer’s Society Fellow in Stem Cell Research. M.R. and A.W. hold studentships from The Wellcome Trust and K.P. has a studentship from the Medical Research Council. We are grateful to the Gatsby Foundation for the purchase of a two-photon microscope and to M. Häusser for providing access to it. This work was also supported by Programme grants from the Medical Research Council and The Wellcome Trust.
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
References
- 1.Hall A, Giese NA, Richardson WD. Spinal cord oligodendrocytes develop from ventrally derived progenitor cells that express PDGF alpha-receptors. Development. 1996;122:4085–4094. doi: 10.1242/dev.122.12.4085. [DOI] [PubMed] [Google Scholar]
- 2.Butt AM, et al. PDGF-alpha receptor and myelin basic protein mRNAs are not coexpressed by oligodendrocytes in vivo: a double in situ hybridization study in the anterior medullary velum of the neonatal rat. Mol. Cell. Neurosci. 1997;8:311–322. doi: 10.1006/mcne.1996.0590. [DOI] [PubMed] [Google Scholar]
- 3.Nishiyama A, Chang A, Trapp BD. NG2+ glial cells: a novel glial cell population in the adult brain. J. Neuropathol. Exp. Neurol. 1999;58:1113–1124. doi: 10.1097/00005072-199911000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Ffrench-Constant C, Raff MC. Proliferating bipotential glial progenitor cells in adult rat optic nerve. Nature. 1986;319:499–502. doi: 10.1038/319499a0. [DOI] [PubMed] [Google Scholar]
- 5.Wolswijk G, Noble M. Identification of an adult-specific glial progenitor cell. Development. 1989;105:387–400. doi: 10.1242/dev.105.2.387. [DOI] [PubMed] [Google Scholar]
- 6.Pringle NP, Mudhar HS, Collarini EJ, Richardson WD. PDGF receptors in the rat CNS: during late neurogenesis, PDGF alpha receptor expression appears to be restricted to glial cells of the oligodendrocyte lineage. Development. 1992;115:535–551. doi: 10.1242/dev.115.2.535. [DOI] [PubMed] [Google Scholar]
- 7.Shi J, Marinovich A, Barres BA. Purification and characterization of adult oligodendrocyte precursor cells from the rat optic nerve. J. Neurosci. 1998;18:4627–4636. doi: 10.1523/JNEUROSCI.18-12-04627.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butt AM, Kiff J, Hubbard P, Berry M. Synantocytes: new functions for novel NG2 expressing glia. J. Neurocytol. 2002;31:551–565. doi: 10.1023/a:1025751900356. [DOI] [PubMed] [Google Scholar]
- 9.Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol. Cell. Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 10.Polito A, Reynolds R. NG2-expressing cells as oligodendrocyte progenitors in the normal and demyelinated adult central nervous system. J. Anat. 2005;207:707–716. doi: 10.1111/j.1469-7580.2005.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ong WY, Levine JM. A light and electron microscopic study of NG2 chondroitin sulfate proteoglycan–positive oligodendrocyte precursor cells in the normal and kainate-lesioned rat hippocampus. Neuroscience. 1999;92:83–95. doi: 10.1016/s0306-4522(98)00751-9. [DOI] [PubMed] [Google Scholar]
- 12.Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligo-dendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- 13.Butt AM, et al. Cells expressing the NG2 antigen contact nodes of Ranvier in adult CNS white matter. Glia. 1999;26:84–91. [PubMed] [Google Scholar]
- 14.Lin SC, et al. Climbing fiber innervation of NG2-expressing glia in the mammalian cerebellum. Neuron. 2005;46:773–785. doi: 10.1016/j.neuron.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 15.Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- 16.Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- 17.Nishiyama A, Watanabe M, Yang Z, Bu J. Identity, distribution and development of polydendrocytes: NG2-expressing glial cells. J. Neurocytol. 2002;31:437–455. doi: 10.1023/a:1025783412651. [DOI] [PubMed] [Google Scholar]
- 18.van Heyningen P, Calver AR, Richardson WD. Control of progenitor cell number by mitogen supply and demand. Curr. Biol. 2001;11:232–241. doi: 10.1016/s0960-9822(01)00075-6. [DOI] [PubMed] [Google Scholar]
- 19.Nowakowski RS, Lewin S, Miller M. Bromodeoxyuridine immunohistochemical determination of the lengths of the cell cycle and the DNA-synthetic phase for an anatomically defined population. J. Neurocytol. 1989;18:311–318. doi: 10.1007/BF01190834. [DOI] [PubMed] [Google Scholar]
- 20.Butt AM, Ransom BR. Visualization of oligodendrocytes and astrocytes in the intact rat optic nerve by intracellular injection of lucifer yellow and horseradish peroxidase. Glia. 1989;2:470–475. doi: 10.1002/glia.440020609. [DOI] [PubMed] [Google Scholar]
- 21.Hachem S, et al. Spatial and temporal expression of S100B in cells of oligodendrocyte lineage. Glia. 2005;51:81–97. doi: 10.1002/glia.20184. [DOI] [PubMed] [Google Scholar]
- 22.Jackson EL, et al. PDGFR alpha–positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron. 2006;51:187–199. doi: 10.1016/j.neuron.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19:197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe M, Toyama Y, Nishiyama A. Differentiation of proliferated NG2-positive glial progenitor cells in a remyelinating lesion. J. Neurosci. Res. 2002;69:826–836. doi: 10.1002/jnr.10338. [DOI] [PubMed] [Google Scholar]
- 25.Sturrock RR. Myelination of the mouse corpus callosum. Neuropathol. Appl. Neurobiol. 1980;6:415–420. doi: 10.1111/j.1365-2990.1980.tb00219.x. [DOI] [PubMed] [Google Scholar]
- 26.McCarthy GF, Leblond CP. Radioautographic evidence for slow astrocyte turnover and modest oligodendrocyte production in the corpus callosum of adult mice infused with 3H-thymidine. J. Comp. Neurol. 1988;271:589–603. doi: 10.1002/cne.902710409. [DOI] [PubMed] [Google Scholar]
- 27.Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kukley M, Capetillo-Zarate E, Dietrich D. Vesicular glutamate release from axons in white matter. Nat. Neurosci. 2007;10:311–320. doi: 10.1038/nn1850. [DOI] [PubMed] [Google Scholar]
- 29.Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat. Neurosci. 2007;10:321–330. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menn B, et al. Origin of oligodendrocytes in the subventricular zone of the adult brain. J. Neurosci. 2006;26:7907–7918. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nait-Oumesmar B, et al. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur. J. Neurosci. 1999;11:4357–4366. doi: 10.1046/j.1460-9568.1999.00873.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- 33.Keirstead HS, Levine JM, Blakemore WF. Response of the oligodendrocyte progenitor cell population (defined by NG2 labeling) to demyelination of the adult spinal cord. Glia. 1998;22:161–170. [PubMed] [Google Scholar]
- 34.Tamura Y, et al. Multi-directional differentiation of doublecortin- and NG2-immunopositive progenitor cells in the adult rat neocortex in vivo. Eur. J. Neurosci. 2007;25:3489–3498. doi: 10.1111/j.1460-9568.2007.05617.x. [DOI] [PubMed] [Google Scholar]
- 35.Karadottir R, Hamilton NB, Bakiri Y, Attwell D. Spiking and nonspiking classes of oligodendrocyte precursor cell in CNS white matter. Nat. Neurosci. 2008;11:450–456. doi: 10.1038/nn2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki N, Bekkers JM. Neural coding by two classes of principal cells in the mouse piriform cortex. J. Neurosci. 2006;26:11938–11947. doi: 10.1523/JNEUROSCI.3473-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aguirre AA, Chittajallu R, Belachew S, Gallo V. NG2-expressing cells in the subventricular zone are type C-like cells and contribute to interneuron generation in the postnatal hippocampus. J. Cell Biol. 2004;165:575–589. doi: 10.1083/jcb.200311141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dayer AG, Cleaver KM, Abouantoun T, Cameron HA. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J. Cell Biol. 2005;168:415–427. doi: 10.1083/jcb.200407053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science. 2005;310:679–683. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- 40.Shapiro LA, et al. Origin, migration and fate of newly generated neurons in the adult rodent piriform cortex. Brain Struct. Funct. 2007;212:133–148. doi: 10.1007/s00429-007-0151-3. [DOI] [PubMed] [Google Scholar]
- 41.Gould E, Reeves AJ, Graziano MS, Gross CG. Neurogenesis in the neocortex of adult primates. Science. 1999;286:548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- 42.Zhao M, et al. Evidence for neurogenesis in the adult mammalian substantia nigra. Proc. Natl. Acad. Sci. USA. 2003;100:7925–7930. doi: 10.1073/pnas.1131955100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rakic P. Adult neurogenesis in mammals: an identity crisis. J. Neurosci. 2002;22:614–618. doi: 10.1523/JNEUROSCI.22-03-00614.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Houweling AR, Brecht M. Behavioural report of single neuron stimulation in somato-sensory cortex. Nature. 2008;451:65–68. doi: 10.1038/nature06447. [DOI] [PubMed] [Google Scholar]
- 45.Huber D, et al. Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice. Nature. 2008;451:61–64. doi: 10.1038/nature06445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- 47.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fogarty M, et al. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J. Neurosci. 2007;27:10935–10946. doi: 10.1523/JNEUROSCI.1629-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young KM, Fogarty M, Kessaris N, Richardson WD. Subventricular zone stem cells are heterogeneous with respect to their embryonic origins and neurogenic fates in the adult olfactory bulb. J. Neurosci. 2007;27:8286–8296. doi: 10.1523/JNEUROSCI.0476-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Academic Press; New York: 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.