Abstract
Patterning of the dorsoventral axis by graded BMP signaling is conserved in the evolution of animals. However, this system has also proven to be highly adaptable, as is now highlighted by its short-range function in the leech Helobdella.
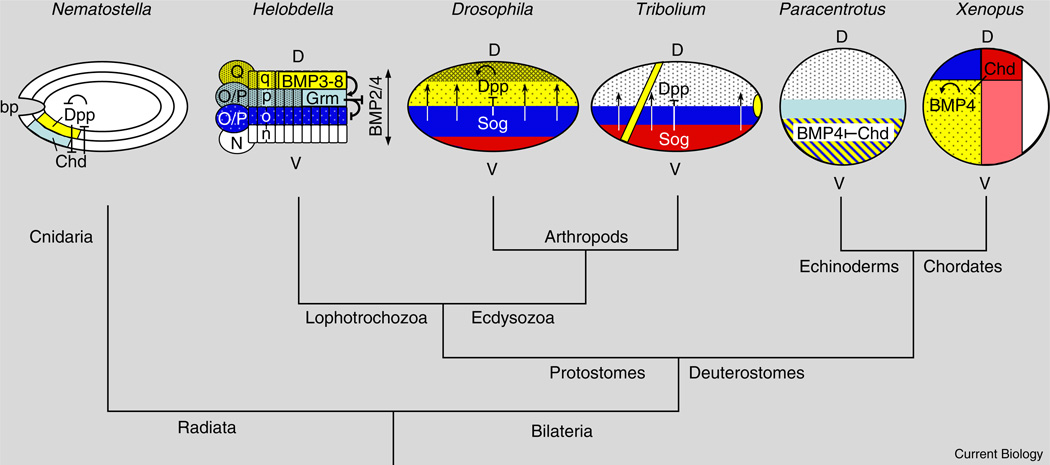
BMPs and their antagonists establish the embryonic dorsoventral axis in many bilaterian groups, including chordates, cephalochordates [1], echinoderms [2], ecdysozoans, such as arthropods [3–5], and lophotrochozoans, such as annelids [6–8] (Figure 1). Indeed, the localized deployment of BMP patterning components predates the emergence of bilaterians, as embryos of several species of the radially symmetric cnidarians display asymmetric expression of these genes [9]. Although this is a matter of ongoing debate, graded BMP signaling appears to have been co-opted during axis formation in a basal bilaterian to determine the relative locations of a neuroectodermal domain giving rise to a condensed central nervous system (CNS) and an ectodermal domain giving rise to the epidermis and peripheral nervous system (PNS). The epidermal and PNS domain is specified by high levels of BMP signaling, whereas inhibition of BMP signaling by antagonists such as Sog/ Chordin, Noggin and Gremlin, defines the location of the CNS. Typically, the protein networks involved in long-range graded BMP signaling are not conserved in species in which cell lineage plays a dominant role in assigning cell fates. However, a new paper by Kuo and Weisblat [10] in this issue of Current Biology provides an intriguing example of ancestral BMP signaling components being adapted for short-range inductive interactions.
Figure 1. Evolution of dorsoventral patterning by BMP signaling.
Key features of BMP signaling in embryos of diverse organisms: the sea anemone Nematostella, the leech Helobdella, studied by Kuo and Weisblat [10], the insects Tribolium and Drosophila, the sea urchin Paracentrotus and the frog Xenopus. Phylogenetic relationships are indicated by the tree, which is not drawn to scale. Note that the dorsoventral (D-V) axis appears to have undergone an inversion in the chordate lineage, which includes vertebrates [1,11]. Red/pink indicates mesoderm; dark blue indicates CNS neuroectoderm; yellow indicates the domain of BMP expression, which corresponds to epidermal ectoderm in vertebrates, Drosophila, and Helobdella; the stippled region indicates region of known BMP activity, darker stippling indicates higher levels of activity; vertical arrows indicate vectorial Sog/Chd-mediated transport of BMPs. D = Dorsal; V = Ventral; Chd = Chordin; Grm = Gremlin; bp = blastopore. For more complete descriptions of the interactions between BMP pathway components in ectoderm patterning, see [11,13,14].
Generating Dorsoventral BMP Activity Gradients
Despite the highly conserved nature of BMP signaling components and their spatial expression along the dorsoventral axis in bilaterian animals, different networks of interactions between these components have been found in different species, revealing that this pathway is at the same time highly conserved and evolutionarily malleable. In the well-studied vertebrate and fruit fly embryos, BMPs and their antagonists are expressed in complementary patterns. Complexes form between BMPs and their inhibitors as well as other extracellular components such as metalloproteases in the BMP1/Tolloid family, which cleave the BMP antagonist Sog/ Chordin, thereby releasing BMPs to signal. Diffusion of such BMP–inhibitor complexes creates broad gradients of BMP activity that can span the entire dorsoventral axis, subdividing the ectoderm into high versus low activity regions, as well as defining distinct cell fates within each of these domains (e.g., at specific thresholds of BMP signaling) [11–14].
However, in other species, such as echinoderms or corals and sea anemones [9], BMPs and their antagonists are co-expressed on the same side of the embryo; or, only one component is dorsoventrally localized, as in the case of ventrally localized Sog/Chordin in the flour beetle Triboliumcastaneum[4] (Figure 1). Such an overlapping spatial arrangement of BMPs and antagonists can also create BMP activity gradients, as BMPs alone or in complex with antagonists can diffuse to establish domains of relatively higher and lower BMP signaling. Indeed, opposing and overlapping configurations of BMPs and antagonists are coupled in vertebrate embryos to create a robust self-regulating morphogenetic field of cells [15].
The BMP Network as a Short Range Inductive System
The new study by Kuo and Weisblat [10] now reveals just how flexible the BMP patterning system can be. The authors show that BMP patterning is employed in a novel way in an annelid, the leech Helobdella sp. (Austin), to specify ectodermal cell fates along the dorsoventral axis. In contrast to the embryos mentioned above, in which cell fates are not hard-wired by lineage, ectodermal cells in Helobdella derive from one of four possible lineages produced by four stem cells. These stem cells, called teloblasts (labeled Q, O/P, O/P, and N), produce four strings (named q, p, o, n from dorsal to ventral) of adjacent cell progeny (bandlets) organized in a parallel array that give rise to epidermis (q), epidermis and PNS (p), CNS (o), and ventral midline (n) (Figure 1).
Given such defined lineage relationships in Helobdella, one might wonder whether graded BMP signaling would offer any advantage to dorsoventral patterning, as all that would be needed in principle is to confer distinct identities upon the stem cell precursors that generate the different bandlets. Indeed, in other species with lineage-based embryogenesis, such as the nematode Caenorhabditis elegans [16], or ascidians [17], many components of the BMP signaling network have been lost, and this signaling network seems to play little if any role in assigning cell fates along the early dorsoventral axis.
Kuo and Weisblat [10] identified a subset of known BMP signaling components that were candidates for contributing to dorsoventral patterning in Helobdella. RNA interference (RNAi) knock-downs and misexpression experiments revealed reciprocal effects of increasing versus decreasing BMP signaling on the fate of O/P lineages in embryos of Helobdella (Hau) and established the following key facts: First, Hau-bmp2/4a,b and their likely receptor Hau-alk3/6 are expressed broadly throughout the germinal bands, while Haubmp5-8 is expressed only in the dorsal-most q bandlet and Hau-gremlin is expressed only in the p bandlet (adjacent and ventral to the q bandlet). Second, when ectopically expressed, Hau-BMP5-8 can activate expression of Hau-gremlin and other p bandlet markers in both O/P-derived lineages, but in wild-type embryos this ligand acts only in a contact-dependent fashion on cells of the dorso-lateral p bandlet adjacent to the q bandlet. Finally, Hau-Gremlin can block signaling by Hau-BMP2/4a,b, but not by Hau-BMP5-8. The result of this arrangement of BMP pathway components is that Hau-BMP5-8 secreted by the q bandlet induces high level signaling only in the adjacent most dorsal of the two O/P lineages (p), which consequently results in those cells expressing the antagonist Hau-Gremlin. This localized expression of Hau-gremlin in p bandlet cells in turn blocks the response to the ubiquitously distributed Hau-BMP2/4a,b in the ventral most O/P lineage (o).
This sequence of inductive signaling across a single cell diameter leads to high levels of BMP signaling in the dorsal q and dorso-lateral p bandlets, where Hau-BMP5-8 signaling is active, and lower BMP levels in the ventral-lateral o bandlet, in which background Hau-BMP2/4a,b signaling is reduced by Hau-Gremlin, and no response to BMP signaling in the ventralmost n bandlet due to some unknown feature of its lineage determination.
Graded versus Inductive Patterning
The novel use of BMP signaling in Helobdella in a series of contact-dependent inductive events represents a clear departure from its typical role in long-range signaling as a morphogen. However, it is not without precedent that a signaling pathway can be used for both local and longer range signaling. For example, in the case of EGF receptor signaling, cleavage and diffusion of membrane tethered forms of TGF-α or Spitz in Drosophila can lead to long-range signaling over several cell diameters while several mechanisms have been defined that can restrict signaling to neighboring cells [18]. Similarly, signaling by the membrane-tethered ligand Delta to the Notch receptor can be deployed within a field of competent cells or be restricted to signaling between adjacent domains of cells in a for-export-only form of signaling [19] in which one group of cells produces a signal to which they cannot respond. This results in a response only in adjacent cells that are close enough to receive the signal. An extreme illustration of this type of signaling is the activation by Delta of the single-minded gene in a single row of mesectodermal cells abutting the Drosophila mesoderm [20].
An interesting question for future investigation is how the BMP regulatory network, which evolved originally to pattern tissues in a graded, threshold-dependent fashion, was then modified to act in a strictly local, for-export-only form of signaling. It will also be interesting to explore further the role of BMP signaling in very early Helobdella embryos when the primary axes are established. An important lesson from these studies in Helobdella and the explosion of new findings in other alternative model systems is that analysis of embryos with distinct developmental strategies deepens our understanding of both the BMP signaling network itself and how evolution can tinker with this ancient system to generate very different embryonic patterns.
References
- 1.Yu JK, Satou Y, Holland ND, Shin IT, Kohara Y, Satoh N, Bronner-Fraser M, Holland LZ. Axial patterning in cephalochordates and the evolution of the organizer. Nature. 2007;445:613–617. doi: 10.1038/nature05472. [DOI] [PubMed] [Google Scholar]
- 2.Lapraz F, Besnardeau L, Lepage T. Patterning of the dorsal-ventral axis in echinoderms: insights into the evolution of the BMP-chordin signaling network. PLoS Biol. 2009;7:e1000248. doi: 10.1371/journal.pbio.1000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goltsev Y, Fuse N, Frasch M, Zinzen RP, Lanzaro G, Levine M. Evolution of the dorsal-ventral patterning network in the mosquito, Anopheles gambiae. Development. 2007;134:2415–2424. doi: 10.1242/dev.02863. [DOI] [PubMed] [Google Scholar]
- 4.van der Zee M, Stockhammer O, von Levetzow C, Nunes da Fonseca R, Roth S. Sog/Chordin is required for ventral-to-dorsal Dpp/BMP transport and head formation in a short germ insect. Proc. Natl. Acad. Sci. USA. 2006;103:16307–16312. doi: 10.1073/pnas.0605154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akiyama-Oda Y, Oda H. Axis specification in the spider embryo: dpp is required for radial-to-axial symmetry transformation and sog for ventral patterning. Development. 2006;133:2347–2357. doi: 10.1242/dev.02400. [DOI] [PubMed] [Google Scholar]
- 6.Denes AS, Jekely G, Steinmetz PR, Raible F, Snyman H, Prud’homme B, Ferrier DE, Balavoine G, Arendt D. Molecular architecture of annelid nerve cord supports common origin of nervous system centralization in bilateria. Cell. 2007;129:277–288. doi: 10.1016/j.cell.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 7.Molina MD, Neto A, Maeso I, Gomez-Skarmeta JL, Salo E, Cebria F. Noggin and noggin-like genes control dorsoventral axis regeneration in planarians. Curr. Biol. 2011;21:300–305. doi: 10.1016/j.cub.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Molina MD, Salo E, Cebria F. The BMP pathway is essential for re-specification and maintenance of the dorsoventral axis in regenerating and intact planarians. Dev. Biol. 2007;311:79–94. doi: 10.1016/j.ydbio.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Technau U, Steele RE. Evolutionary crossroads in developmental biology: Cnidaria. Development. 2011;138:1447–1458. doi: 10.1242/dev.048959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuo D-H, Weisblat DA. A new molecular logic for BMP-mediated dorsoventral patterning in the leech Helobdella. Curr. Biol. 2011;21:1282–1288. doi: 10.1016/j.cub.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizutani CM, Bier E. EvoD/Vo: the origins of BMP signalling in the neuroectoderm. Nat. Rev. Genet. 2008;9:663–677. doi: 10.1038/nrg2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niehrs C. On growth and form: a Cartesian coordinate system of Wnt and BMP signaling specifies bilaterian body axes. Development. 2010;137:845–857. doi: 10.1242/dev.039651. [DOI] [PubMed] [Google Scholar]
- 13.O’Connor MB, Umulis D, Othmer HG, Blair SS. Shaping BMP morphogen gradients in the Drosophila embryo and pupal wing. Development. 2006;133:183–193. doi: 10.1242/dev.02214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zakin L, De Robertis EM. Extracellular regulation of BMP signaling. Curr. Biol. 2010;20:R89–R92. doi: 10.1016/j.cub.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reversade B, De Robertis EM. Regulation of ADMP and BMP2/4/7 at opposite embryonic poles generates a self-regulating morphogenetic field. Cell. 2005;123:1147–1160. doi: 10.1016/j.cell.2005.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patterson GI, Padgett RW. TGF beta-related pathways. Roles in Caenorhabditis elegans development. Trends Genet. 2000;16:27–33. doi: 10.1016/s0168-9525(99)01916-2. [DOI] [PubMed] [Google Scholar]
- 17.Lemaire P. Unfolding a chordate developmental program, one cell at a time: invariant cell lineages, short-range inductions and evolutionary plasticity in ascidians. Dev. Biol. 2009;332:48–60. doi: 10.1016/j.ydbio.2009.05.540. [DOI] [PubMed] [Google Scholar]
- 18.Shilo BZ. Regulating the dynamics of EGF receptor signaling in space and time. Development. 2005;132:4017–4027. doi: 10.1242/dev.02006. [DOI] [PubMed] [Google Scholar]
- 19.Bier E. Drawing lines in the Drosophila wing: initiation of wing vein development. Curr. Opin. Genet. Dev. 2000;10:393–398. doi: 10.1016/s0959-437x(00)00102-7. [DOI] [PubMed] [Google Scholar]
- 20.Chitnis AB. Keeping single minded expression on the straight and narrow. Mol. Cell. 2006;21:450–452. doi: 10.1016/j.molcel.2006.02.004. [DOI] [PubMed] [Google Scholar]