Abstract
The use of liposomes for drug delivery began early in the history of pharmaceutical nanocarriers. These nanosized, lipid bilayered vesicles have become popular as drug delivery systems owing to their efficiency, biocompatibility, nonimmunogenicity, enhanced solubility of chemotherapeutic agents and their ability to encapsulate a wide array of drugs. Passive and ligand-mediated active targeting promote tumor specificity with diminished adverse off-target effects. The current field of liposomes focuses on both clinical and diagnostic applications. Recent efforts have concentrated on the development of multifunctional liposomes that target cells and cellular organelles with a single delivery system. This review discusses the recent advances in liposome research in tumor targeting.
Keywords: active targeting, drug delivery, liposome, passive targeting, receptor, tumor vasculature
Over the past three decades, liposomes have gained attention as a carrier system for therapeutically active agents, owing to their unique characteristics, including biocompatibility, bio-degradability, low toxicity, lack of immune system activation, and capability to incorporate both hydrophilic and hydrophobic drugs (Figure 1). Liposomes have shown tremendous therapeutic potential as carriers for payloads and for delivery to targeted sites, which has led to several liposomal formulations designed for the clinic and clinical trials for cancer therapy [1]. Liposomal drug delivery systems improve the pharmacokinetic and pharmacodynamic profiles of the therapeutic payload, promote controlled and sustained release of drugs and exhibit lower systemic toxicity compared with the free drug. The concept of targeting liposomes to tumor sites was derived from Ehrlich's concept of a `magic bullet' coined in 1906 [2]. Liposomes can be surface modified by various strategies to endow them with multiple functionalities, including long systemic circulation, increased accumulation at the target tissue, increased cellular internalization and organelle-specific drug delivery [3].
Figure 1.

Hydrophilic and hydrophobic drugs encapsulated within a PEGylated liposome.
In recent years, research has significantly developed in terms of liposomal systems with an improved drug delivery potential for cancer therapy [1,3–6]. Subsequent work on improving the therapeutic potential of liposomes has focused mainly on developing strategies for actively targeting the liposomes to a tumor site, intracellular delivery followed by organelle-specific targeting and triggered release of therapeutic payloads utilizing pathological differences in the tumor's microenvironment. The current review outlines the recent developments in the strategies employed to improve currently existing liposomal systems.
Strategies for liposomal targeting to tumors
Various strategies have been adopted for targeting liposomes to the tumor sites [1,3–6]. Here, we outline the recent advances in the strategies for liposomal targeting with emphasis on spontaneous/passive targeting and active targeting via liposomal surface functionalization to recognize various extracellularly overexpressed biomarkers, and on local stimuli-triggered release of a therapeutic payload.
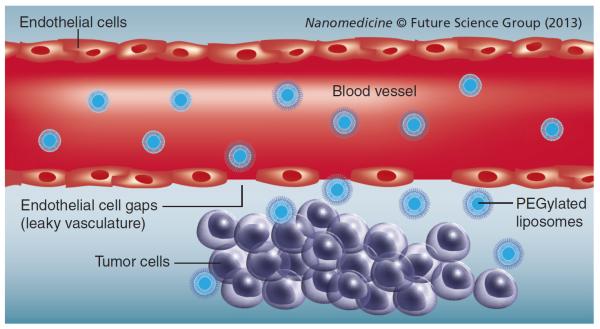
Passive targeting
Owing to the leaky nature of the tumor-associated blood vessels, biomacromolecules and nanosized drug delivery systems readily translocate across the capillary endothelium and enter the interstitial space. The size of the gaps between the endothelial cells lining the tumor capillaries ranges from 100 to 780 nm depending on the cancer type, as opposed to that in a typical normal endothelium of 5–10 nm [7]. In addition, solid tumors lack adequate lymphatic drainage. Therefore, there is limited circulatory recovery of the extravasated molecules, resulting in the accumulation of macromolecules and nanoparticles in the tumor microenvironment. This well-established phenomenon that leads to a nanopreparation's accumulation in the tumor microenvironment has been termed the enhanced (Figure 2) [8–12]. Utilization of the EPR effect is an effective strategy for targeting nanopreparations, such as liposomes, to the site of a tumor. Unlike liposomes and other nanoparticles, low-molecular-weight drugs are not retained in the tumor site for a longer period of time since they re-enter the circulation primarily via diffusion. Targeting of these drugs relies solely on the pathophysiological properties of the tumor tissues, and is referred to as `passive drug targeting' [8,13,14].
Figure 2.
Passive targeting via the enhanced permeability and retention effect.
Prolonged systemic circulation allows longer interaction of liposomes with the target because the higher number of passages of blood through the target enhances the EPR effect [15]. Longevity in blood is achieved by coating the liposomes with polymers, such as PEG, which efficiently hides them from uptake by the reticuloendothelial system (RES) [14]. PEG is usually added on the surface of liposomes to create a `steric stabilization' effect. PEG molecules form a protective hydrophilic layer on the surface of the liposomes that prevents their aggregation and interaction with the blood components [16]. The mechanisms by which PEG prevents opsonization include shielding of the surface charge, enhancing the repulsive interaction between polymer-coated liposomes and blood components, increasing surface hydrophilicity and forming a polymeric layer over the liposome surface to render them impermeable to large molecules or opsonins even at relatively low polymer concentrations [15]. As a result, grafting of PEG-like polymers on the surface of liposomes reduces uptake by the macrophages of the mononuclear phagocytic system and prolongs their circulation in the blood.
Another important parameter that impacts passive targeting through the EPR effect is the size of the liposomes. The accumulation of liposomes in the tumor strongly depends on the size of the endothelial gaps in the capillary vasculature for a particular cancer.
To utilize the EPR effect, the liposomes should usually be smaller than 400 nm in size [17]. The threshold vesicle size of approximately 400 nm has been reported for extravasation into tumors. However, more effective extravasation has been shown to occur with many particles <200 nm [18].
The composition and charge on the surface of liposomes are other factors that influence passive targeting. Anionic or neutral liposomes escape from renal clearance [17]. Although the cationic liposomes have a tendency for localizing in newly formed tumor vessels, their positive surface charge leads to nonspecific interactions with the anionic species in the blood, resulting in rapid clearance from circulation by the RES, which reduces the EPR effect [19]. However, aggregation of liposomes increases with greater amounts of cationic lipids in the liposomal membrane. Still, an optimum surface modification with cationic lipids could significantly enhance tumor penetration.
Active targeting
In general, actively targeted liposomes are designed to minimize off-target effects. Actively targeted liposomal systems are prepared by conjugating targeting moieties, including small-molecule ligands, peptides and monoclonal antibodies, on the liposomal surface [20,21]. For example, certain receptors, such as folate and transferrin (Tf) receptors (TfR), are overexpressed on many cancer cells and have been used to make liposomes tumor cell specific [15,21]. Liposomes that accumulate in the tumor microenvironment can be subsequently endocytosed into the cells by interacting with specific cell surface receptors [15,22]. To efficiently target liposomes to cancer cells, it is necessary to link the targeting moiety in sufficient quantities to have optimum affinity for the cell surface receptors [21]. In the field of active targeting, the number of tumor-specific targeting ligands has greatly expanded [23].
Targeting cancer cell surface receptors
Use of targeting ligands on liposomes, specific for cancer cell surface receptors is crucial considering that their involvement in the cellular uptake mechanisms may amplify the therapeutic response manifold. Attachment of liposomes to vascular cells via a noninternalizing epitope increases the extracellular drug concentration, increasing the amount of the drug delivered to the target cells [20]. The most common strategy to target overexpressed cell surface receptors on cancer cells is the use of receptor-specific ligands or antibodies [24]. Active targeting via cell surface receptor targeting has been explored widely in cancer since many cancer cell types display upregulation of tumor-specific receptors. For example, TfRs and folate receptors (FRs) are greatly overexpressed by many tumor cell types in response to their increased metabolic demand [23].
Targeting FRs
Folic acid has recently been used as a targeting ligand for specialized drug delivery owing to its ease of conjugation to nanocarriers, its high affinity for FRs and the relatively low frequency of FRs, in normal tissues as compared with their overexpression in activated macrophages and cancer cells [25]. FRs are overexpressed in certain ovarian, breast, lung, colon, kidney and brain tumors [25,26].
Owing to the overexpression of FR on macrophages, which is an indication of inflammatory diseases, such as psoriasis, Crohn's disease, rheumatoid arthritis and atherosclerosis, folate-mediated targeting can also be used to treat inflammatory disorders [26]. Folate-linked nanoparticles deliver their cargo intracellularly through receptor-mediated endocytosis [25,26]. Intracellular trafficking can be directed to acidic compartments that facilitate drug release, and, most importantly, release of the drug can be altered or delayed until it reaches the cytoplasm or vicinity of target organelles to overcome the efflux associated with multidrug resistance pumps [26]. Delivery of imaging, diagnostic and therapeutic agents using folate-linked liposomes has proven that they are superior to nontargeted liposomes. The attachment of folate directly to the lipid head groups is unfavorable for intracellular delivery of folate-conjugated liposomes, since they do not efficiently bind to cells expressing FR. By contrast, folate attached to the liposomal surface by a PEG spacer arm enters cancer cells very efficiently [25].
Several folate-conjugated liposomal systems have been reported for the delivery of various diagnostic and therapeutic agents to tumor cells overexpressing FR [25,27–29]. Low et al. explored the activity of doxorubicin-loaded PEGylated liposomes by testing their toxicity against FR+ tumor cells. The targeted liposomes demonstrated a 45-fold higher uptake than the nontargeted liposomes. Doxorubicin-mediated cytotoxicity was 85-times higher in targeted liposomes compared with unmodified plain liposomes [25].
Glutathione (GSH)-conjugated folate has been conjugated to a PEG-distearoylphosphatidylethanolamine (DSPE) polymer and subsequently incorporated into the liposomal lipid bilayer. Folate-GSH-PEG-DSPE-modified liposomes were efficiently transported into FR+ KB cells and exhibited higher cytotoxicity of a therapeutic payload of vincristine, compared with vincristine-loaded unmodified liposomes. The greater hydrophilicity imparted by the GSH moiety was suggested to impart better FR targeting [30].
Watanabe et al. developed a novel conjugate of folate-poly(l-lysine) (PLL) and coated doxorubicin-loaded anionic liposomes composed of hydrogenated soybean phosphatidylcholine/ cholesterol/sodium cholesteryl sulfate with this conjugate [31]. Folate–PLL-coated liposomes had an increased association and an enhanced cytotoxicity against KB cells as compared with PLL-coated liposomes. The effect was abolished by preincubation of cells with free folic acid, suggesting the presence of FR-mediated endocytosis.
Folate-targeted cationic magnetoliposomes have also been prepared with coencapsulated doxorubicin and anionic superparamagnetic iron oxide nanoparticle (SPION) γ-Fe2O3 cores. Comparison of uptake in two different cell lines, HeLa (with high FR expression) and ZR-75-1 (with low FR expression), showed increased uptake and surface binding in HeLa cells and no uptake in ZR-75-1 cells, indicating the significance of the folate moiety attached to these magnetic liposomes. Additionally, a threefold increase in doxorubicin release was achieved over a 2-h period when an alternating current was applied to the SPIONs [32].
The strategy of targeting liposomes to FRs has also been used to improve gene delivery [33]. Duarte et al. have shown the potential of folate-conjugated liposomes in the form of lipoplexes for gene delivery [33]. Their potential usefulness was shown by their successful promotion of cell death and reduction of tumor growth in an animal model of oral cancer [33].
Many other liposomal systems coupled to folate have been developed for the delivery of various drugs, including platinum-based carboplatin in an ovarian cancer model [34], complexes of anionic liposomes and adenovirous vectors modified with folate-conjugated phosphatidyl ethanolamine for enhanced gene transduction [35], folic acid-based liposomal systems coupled with the cell-penetrating peptide (CPP) TAT using paclitaxel as the chemotherapeutic agent [36] and delivery of zoledronic acid via folate-modified liposomes for enhanced uptake by FR-expressing cells [37]. All of these studies indicate the wide-spread usefulness of folate as a targeting moiety in liposomal drug delivery systems.
Targeting TfR
Tf, a monomeric serum glycoprotein of approximately 80 KDa, is involved in the transport of iron throughout the body [38–41]. Tf binds to the TfR and translocates into cells via receptor-mediated endocytosis [38]. The expression of TfR is higher in tumor cells as compared with normal cells and is associated with the increased iron demand in rapidly proliferating cancer cells [38,40].
The overexpression of TfR in tumor tissues has led to the development of TfR-targeted anticancer therapy [42]. Li et al. used TfR-targeted stealth liposomes encapsulating doxorubicin to demonstrate that a TfR-targeted doxorubicin-loaded liposomal system improved the intracellular uptake, pharmacokinetic profile and bio-distribution of doxorubicin, and led to improved therapeutic efficacy against liver cancer [42].
The work of Zhai et al. showed that Tf-targeted liposomes were an effective delivery system for the chemotherapeutic docetaxel, which has been used in the treatment of breast, colon, ovarian, head, neck and non-small-cell lung cancer [43]. In vitro studies indicated a 3.6-fold increase in the cytotoxicity of TfR-targeted liposomes in KB cells when compared with plain liposomes loaded with docetaxel.
TfR-targeted liposomes encapsulating boron compounds have been developed for boron neutron capture therapy for the treatment of malignant oral tumors [44,45]. TfR targeting has also been proven to be effective for gene delivery [46–48]. Transfection of HeLa cells with Tf-N-(1-[2,3-dioleoyloxy]-propyl)-N, N, N-trimethylammonium methyl sulfate:1,2-dioleoyl-sn-glycero-3-phosphoethanolamine lipoplexes resulted in a sixfold enhancement of transgene activity. A similar effect was observed with several other cell lines [48].
A TfR-targeted, proapoptotic factor and a lysosome destabilizing agent (a ceramide-encapsulated liposomal formulation) was developed for delivery to lysosomes for induction of apoptosis via lysosomal membrane permeabilization [49].
Multifunctional liposomes employing more than one targeting moiety such as CPP, along with Tf, have been widely studied [50].
Sharma et al. developed a bifunctional liposomal system containing the combination of Tf and poly-l-arginine [50]. The system proved effective: the Tf-modified liposomes demonstrated tumor targeting and poly-l-arginine promoted cell penetration, leading to drug transport across the endothelium of the blood–brain barrier [50].
Targeting EGFRs
EGFR, is a tyrosine kinase receptor belonging to the ErbB family of receptors that mediates cell growth, differentiation and repair in non-cancerous cells [51]. EGF is overexpressed in many solid tumors, including colorectal, non-small-cell lung cancer, squamous cell carcinoma of the ovary, kidney, head, pancreas, neck and prostate, and especially breast cancer, which makes it an attractive target for therapeutic drug delivery [17,52]. In cancer cells, EGFR mediates several processes, including proliferation, angiogenesis and metastasis.
EGFR-targeted monoclonal antibodies linked to liposomal systems have been extensively studied for signs of improved active tumor targeting [51–55]. Such antibodies, attached to the surface of liposomes as targeting ligands, provide high specificity and have emerged as one of the most promising approaches for drug delivery. In a related study, cetuximab (an antibody against EGFR)-biotin liposomes demonstrated higher cytotoxicity for SKOV-3 cells compared with nontargeted biotin liposomes at a doxorubicin concentration of 10 mM. Targeted liposomes showed 22- to 38-times higher binding than the nontargeted ones on the SKOV-3 cells [51]. These findings indicate the potential of this strategy for the treatment of ovarian cancers.
HER-2 is often overexpressed in patients with breast cancer [17]. HER-2, encoded by the ERBB2 gene, has been one of the most common targets for immunoliposomes. It is over expressed in approximately 20% of breast cancers. HER-2 overexpression has been documented in several other types of cancers, including those of the lung, bladder, prostate, brain and stomach. Upon cellular association, the receptor–antibody complex is internalized by formation of an endosome for delivery to the cytoplasm [56].
Shmeeda et al. studied the binding capability of HER-2-targeted PEGylated liposomal doxorubicin in cells overexpressing HER-2 in a J6456 ascitic lymphoma model. The extravasation of these liposomes into the tumor compartment was quantitatively evaluated. Compared with several other ligands, HER-2-targeted PEGylated liposomal doxorubicin showed greater accumulation in the tumor cells, showing the efficacy of the formulation as an anticancer therapy [57].
Another study conducted by Chiu et al. explains the role of HER-2 targeting in a HER-2-overexpressing cell line and in a breast cancer model [58]. Trastuzumab was conjugated to a maleimide-PEG polymer to target HER-2 in a multivalent fashion. HER-2-targeted modified liposomes significantly downregulated the key survival molecule Akt in cancer cell lines compared with free or bivalent trastuzumab.
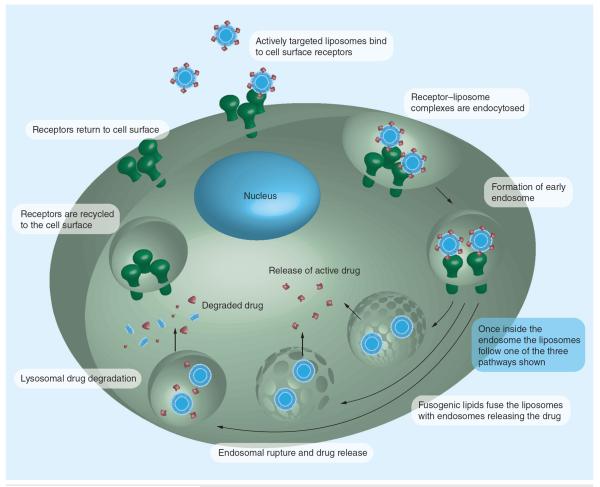
Receptor-mediated targeting faces several challenges, including ligand/target affinity, the quantity of receptors on the cell surface, and PEGylation acting as a barrier against interaction with receptors. The use of immuno liposomes enables the delivery of a large number of drug molecules with a few ligand molecules per liposome producing an extremely high drug:ligand ratio. Multivalent presentation of targeting molecules also helps to increase the uptake and signaling properties of antibody fragments. For immunoliposomes, the issue of ligand density also needs to be addressed [24]. High ligand densities on the liposomes have proven advantageous for increased binding to target cells. However, this has been difficult and expensive to achieve. With the use of whole antibodies as ligands, increased clearance of the immunoliposomes from the circulation has been observed owing to their high densities, resulting in decreased tumor localization. Early elimination of immunoliposomes from the circulation by macrophages has been overcome largely with the use of sterically stabilized liposomes and new technologies for linking ligands to the terminus of molecules such as PEG, which is anchored in the liposome bilayer [24]. It is clear that the active targeting strategies used to improve cellular uptake of liposomes are effected through internalization-prone cell surface receptors and the overexpression of these receptors on cancer cells (Figure 3). Overall, increased intracellular delivery is responsible for the enhanced anti-tumor efficacy of actively targeted liposomes.
Figure 3.
Surface receptor-mediated endocytosis.
Targeting the tumor microenvironment
Targeting the tumor vasculature/microenvironment has several advantages compared with the cell surface receptors for the following reasons: the barrier to the diffusion of liposomes through the tumor can be overcome by directly targeting the tumor vasculature; destruction of the vasculature diminishes or inhibits the growth and metastasis of the tumor; phenotypic variations of the neovascular endothelial cells can be curbed, which can prevent development of resistance; and the tumor vasculature is not specific for any cancer type [20].
Targeting VEGF
Solid tumors induce the growth of new blood vessels that supply nutrients and oxygen. However, these newly formed blood vessels possess specific characteristics not observed in normal tissues, including excessive leakiness, extensive angiogenesis, impaired lymphatic drainage and increased expression of mediators of cell surface permeability. These differences can be exploited to develop antiangiogenic therapies for cancer [59,60]. VEGF and its receptors are well-known proangiogenic molecules and are well-characterized targets for antiangiogenic therapy [61,62]. Bevacizumab (Avastin®; Genentech, CA, USA), an antihuman VEGF monoclonal antibody approved by the US FDA as an anticancer drug, has shown efficient anti-tumor effects. Besides bevacizumab, many small-molecule inhibitors of receptor tyrosine kinases, such as VEGFRs or basic FGFRs, have been developed as anticancer agents [60].
Chang et al. designed a method to select peptides that bind specifically to the tumor vasculature of human cancer xenografts [59]. Coupling these peptides to a liposome loaded with doxorubicin improved its efficacy against several types of human cancers xenografted on SCID mice. The neovasculature-specific phages, IVO-8 and IVO-24, specifically bound both tumor vessels of xenografts in animal models and the blood vessels of six types of human solid tumors. Coupling of the phage IVO peptides to the PEG terminus of stealth liposomes demonstrated that the phage IVO peptide-anchored liposomal doxorubicin improved therapeutic efficacy, increased cancer cell apoptosis and decreased tumor angiogenesis in mice, resulting in a decline in tumor growth [59].
Katanasaka et al. utilized a tumor-homing peptide APRPG for the modification of the liposomal surface to render such liposomes specific for the angiogenic site [60]. SU1498, an inhibitor of VEGFRs, was successfully encapsulated into APRPG-PEG-modified liposomes. SU1498 inhibited VEGF-stimulated endothelial cell proliferation in vitro and significantly decreased tumor microvessel density in Colon26 NL-17 tumor-bearing mice, increasing their survival. These findings suggested that APRPG-PEG-modified liposomes effectively deliver SU1498 to angiogenic endothelial cells in tumors and, thus, inhibit tumor-induced angiogenesis.
Targeting VCAM
The vascular endothelium plays a key role in the pathogenesis of inflammation, thrombosis and atherosclerosis. During an inflammatory stimulus, various cell adhesion molecules (CAMs) expressed by endothelial cells play an important role in the recruitment of leukocytes from the circulat ing blood to the endothelium. Since CAMs are involved in inflammatory disorders including cancer, they represent a logical target for anticancer therapy. The CAMs are of the following types: E- and P-selectins, VCAM-1 and ICAMs. Among the CAMs, VCAM-1 is overexpressed in tumor vessels and is an attractive target for anticancer drug delivery. It is said to promote the binding between leukocytes and endothelial cells by binding to very late antigen-4 (α4β1 integrin) expressed on activated leukocytes [63].
Kang et al. demonstrated the possibility of delivery of anti-inflammatory drugs specifically to the endothelium after activation by an inflammatory stimulus [63]. Liposomes were surface functionalized with the Fab′ fragments against VCAM-1 and loaded with celecoxib, a selective COX-2 inhibitor. The antibody against VCAM-1 greatly enhanced the delivery of celecoxib to endothelial cells upon activation by TNF-α, a well-known proinflammatory cytokine. In addition, the uptake of liposomes into HUVEC cells, whose VCAM-1 expression was significantly induced by a 4-h preincubation with TNF-α, was greatly increased after incubation with anti-VCAM-1 and Fab′-coupled liposomes, but not after incubation with conventional liposomes [63].
Gosk et al. developed PEGylated immunoliposomes targeted to VCAM-1 [64]. These anti-VCAM-1 immunoliposomes bound specifically to activated endothelial cells in vitro and accumulated in tumor vessels in a time-dependent manner in vivo. No differences in the uptake of these immunoliposomes by the RES was observed [64].
Targeting matrix metalloproteases
Matrix metalloproteases (MMPs) belong to the family of zinc-dependent endopeptidases, which are involved in tissue remodeling, tumor invasiveness, resistance to apoptosis and metastasis. The activity of the MMPs is regulated by their association with four MMP inhibitors called TIMP1–4, which determine the balance between tumor growth inhibition and metastasis [65].
One major protein involved in the angiogenesis of tumor vessels is MT1-MMP, expressed on newly formed vessels and tumor tissues. The proteolytic activity of MT1-MMP cleaves proteins, such as fibronectin, elastin, collagen and laminin, at the plasma membrane and activates soluble MMPs, such as MMP-2, which degrades the matrix. An anti-tumor effect has been observed in tumor-bearing mice administered with inhibitors of the MMP family that are associated with suppressed angiogenesis [66]. In another study, Hatakeyama et al. investigated the role of MT1-MMP in the suppression of angiogenesis [66]. Doxorubicin-loaded, sterically modified liposomes were conjugated to a Fab′ fragment derived from antihuman MT1-MMP monoclonal antibody via a PEG spacer. Fab-conjugated liposomes showed enhanced cellular uptake by approximately fivefold, when compared with nontargeted liposomes. In addition, in vivo systemic administration of doxorubicin-loaded anti-MT1-MMP liposomes to tumor-bearing mice produced significant suppression of tumor growth compared with plain liposomes with doxorubicin [66]. In addition to targeting overexpressed MMPs for active tumor targeting, attempts have been undertaken to prepare MMP-sensitive liposomal systems that are degraded in the presence of elevated MMP levels to then release a therapeutic payload [67].
Targeting αβ-integrins
Integrins, a group of transmembrane glycoprotein receptors, mediate attachment between a cell and its surrounding tissues or extracellular matrix. Integrins contain two distinct chains (heterodimers) called α- and β-subunits. Integrins, overexpressed in many tumors, play an important role in invasion and metastasis by facilitating adherance of the tumor cells to the endothelial lining of blood vessels of other organs and tissues. A tripeptide, RGD has a strong binding efficiency for integrins and has demon strated an inhibitory effect on the adhesion and angiogenesis of tumor cells [68]. The tumor tissue-specific expression of integrin receptors has been utilized for targeted delivery of drugs.
Chen et al. developed an integrin-targeted liposomal system for the delivery of doxorubicin. A cyclic RGD was covalently coupled to the liposomes. The RGD-coupled liposomal system had a 2.5-fold higher cellular uptake of doxorubicin compared with the unmodified liposomes in the U87MG cell line. A competitive binding experiment indicated that the liposomes were internalized by an integrin receptor-mediated endocytic pathway [69]. Similar studies by other groups utilized schemes for targeting integrins with RGD peptide-conjugated liposomes for the purpose of developing tumor-targeted delivery systems [68,70,71].
Surface grafting of liposomes with aptamers
Aptamers are ssDNA or RNA oligonucleotides that impart high affinity and specific recognition of the target molecules by electrostatic interactions, hydrogen bonding and hydro phobic interactions as opposed to the Watson–Crick base pairing, which is typical for the bonding interactions of oligonucleotides. Surface functionalization of liposomes with aptamers has advantages over antibody grafting for the following reasons: aptamers demonstrate higher target antigen recognition compared with antibodies; aptamers are more stable and smaller in size compared with antibodies; aptamers can be easily synthesized and chemically modified for molecular conjugation; and aptamers can be changed in sequence for improved selectivity and can be developed to recognize poorly immunogenic targets [72,73].
A sgc8 aptamer-functionalized liposomal system developed for tumor-targeted drug delivery was selected based on its high affinity for leukemia CEM–CCRF cells. The aptamer was covalently linked to the liposomes by a PEG spacer. Within 30 min of cell incubation, aptamer-conjugated liposomes bound specifically with target cells and released a loaded small drug molecule [73]. A few other promising aptamer-based liposomal delivery systems have been developed for cancer-targeted drug delivery systems [74–76].
Therefore, these liposomal systems are able to target various areas of the tumor using specific targeting ligands and circumvent some of the problems associated with multidrug resistance. An additional plus for the use of antibodies is the ability to achieve synergy between the signaling antibodies and chemotherapeutics, since two distinct techniques are being used to target the cells. Many of the ligands employed demonstrate a large overlap in their targets and, thus, could help provide synergistic anti-tumor effects. In addition, by combining properties of cell surface receptor targeting and tumor microenvironment targeting, an ideal liposome system could be built that helps intracellular delivery of the cargo while avoiding tumor cell metastasis.
Utilizing local stimuli strategies for enhanced drug release
A new active targeting strategy that utilizes subtle pathological changes in the tumor microenvironment has been extensively studied for improved efficiency of liposomal drug release [17]. The concept of stimuli sensitivity is based on certain characteristics of the tumor microenvironment, including a lower pH, higher temperature and overexpression of several proteolytic enzymes [15]. The stimuli-sensitive liposomes maintain their structure and physical properties throughout circulation. However, they are designed to undergo rapid changes (aggregation, disruption and permeability) that trigger drug release when exposed to a particular tumor microenvironment [10,17,77]. These stimuli can be either internal, such as at low pH, high temperature and enzymes/antigens at the tumor site, or can be externally applied and controlled for drug release. Here, we discuss stimuli-sensitive liposomes that utilize either internal stimuli that are characteristic for a tumor microenvironment or externally applied stimuli, such as magnetic fields, ultrasound or light, to target tumor tissues.
pH-triggered drug delivery
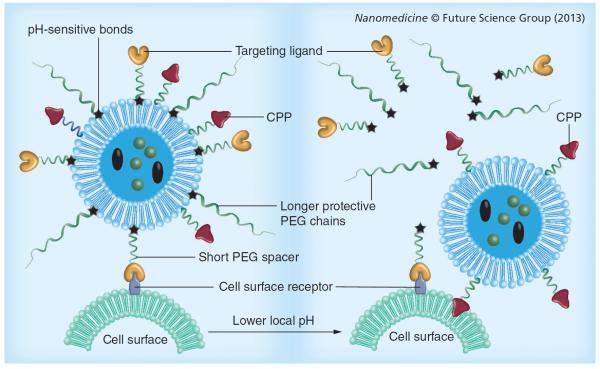
Although PEGylation of liposomes is a desired modification that enhances their longevity in the blood, the PEG chains sometimes act as barriers to intracellular delivery or release of drugs. In the case of endocytosis, the PEG brush may sometimes preclude the escape of liposomes from endosomes, allowing degradation of their contents. To circumvent this problem, labile linkages have been introduced between the hydrophilic PEG and the hydrophobic moiety, such as a lipid, which is cleaved only upon exposure to the relatively acidic conditions characteristic of the endocytic vacuole or the acidotic tumor mass. pH-sensitive copolymers can also be incorporated in the liposomes to provide shielding. Diortho esters, vinyl esters, cysteine-cleavable lipopolymers, double esters and hydrazones are a few examples of pH-sensitive bonds that are quite stable at pH 7.5, but are hydrolyzed relatively rapidly at pH 6 and below [15]. The pH-sensitive degradation of a liposomal carrier releases the entrapped payloads in tissues with a low pH, such as tumors, the cell cytoplasm or endosome (Figure 4) [17]. Liposomes made of pH-sensitive components fuse with the endovacuolar membrane after endocytosis and subsequently release their contents into the cytoplasm under the action of low endosomal pH [15,77].
Figure 4. Stimuli-sensitive multifunctional liposomes with low pH-degradable bonds in the tumor microenvironment.
CPP: Cell-penetrating peptide.
One such example is a long-circulating PEGylated pH-sensitive liposome, which combines features of PEGylation and pH sensitivity in the same system, with a terminally alkylated copolymer of N-isopropylacrylamide and methacrylic acid [15]. This copolymer facilitates liposomal destabilization and drug release in compartments with decreased pH values [15]. The most commonly studied ionic polymers for generation of pH-responsive liposomes include poly(methacrylic acid), poly(diethylaminoethyl methacrylate), poly(acrylamide) and poly(acrylic acid) [77].
In addition to providing long circulation, the pH-sensitive PEG coating keeps specific functions temporarily `hidden' or `shielded', until cleaved in the low-pH environment to expose the previously hidden functionality. The work of Koren et al. involved a system based on the above concept of shielding/deshielding to expose a hidden, nonspecific CPP (TATp) on exposure to the low-pH characteristics of tumors [78].
Temperature-triggered drug delivery
Many pathological areas, such as inflamed tissues and tumors, show a distinctive hyperthermia compared with normal tissues [17]. Utilizing this hyperthermia is an attractive strategy in cancer therapy since hyperthermia is associated with increased tumor permeability and enhanced drug uptake. This technique involves local heating of the tumor site to increase microvascular pore size and blood flow, which, in turn, results in an increased extravasation of drug-loaded nanocarriers.
Temperature-sensitive liposomes can be prepared from thermosensitive lipids or polymers with a low critical solution temperature. Above the low critical solution temperature (e.g., at a tumor site), the polymer precipitates, disrupting the liposomes to release the drug. Lipids with a specific gel-to-liquid phase transition temperature are used to prepare these liposomes [79]. The most commonly used lipid for thermosensitive liposomes is dipalmitoylphosphatidylcholine. Thermosensitive magnetoliposomes formulated with dipalmitoylphosphatidylcholine and cholesterol have been reported to release more than 80% of encapsulated methotrexate within 30 min when the temperature was raised from 37 to 41°C [77]. These liposomes retained approximately 60% of the drug at 37°C for up to 24 h. Formulation of liposomes with thermosensitive polymers can also facilitate the destabilization of liposomes followed by drug release. The most commonly used thermosensitive polymer is poly (N-isopropylacrylamide) [77,79]. Another temperature-triggered liposomal system, lysolipid temperature-sensitive liposomes (ThermoDox®; Celsion, NJ, USA) has also demonstrated improved efficacy for cancer-targeted drug delivery [80,81]. This formulation is in Phase III clinical trials for the treatment of hepatocellular carcinoma and Phase II trials for breast cancer and colorectal liver metastases.
Redox-triggered drug delivery
The difference in redox potential between normal and tumor tissues, and between the intra- and extra-cellular environments has been exploited for drug delivery in cancer [79]. For example, GSH is a reducing agent abundant in cells, especially in the cytosol, mitochondria and nucleus. The GSH concentrations in blood and extracellular matrix are just one out of 100 to one out of 1000 of the intracellular concentration, respectively. This high redox potential difference caused by GSH, cysteine and other reducing agents can break the reducible bonds, destabilize the liposomal system and release a payload. The disulfide bond has been popularly used as the cleavable/reversible linker in liposomes, because it causes sensitivity to redox owing to the disulfide-to-thiol reduction reaction [79].
One such system has been reported by Goldenbogen et al., in which liposomes were made reduction sensitive by grafting two forms of disulfide-conjugated multifunctional lipid on to the surface of liposomes [82]. The cleavage of the disulfide bond was studied in vitro using tris(2-carboxyethyl)phosphine, dithiothreitol, l-cysteine or GSH, which cause removal of the hydrophilic head group of the conjugate and alter the membrane organization leading to the release of encapsulated molecules. Calcein release from reduction-sensitive liposomes containing a disulfide conjugate was reported to be more efficient than reduction-insensitive liposomes composed only of phospholipids [82].
Enzyme-triggered drug delivery
Enzymes present in the tumor vasculature have been utilized as triggers to achieve site-specific drug delivery from stimuli-sensitive liposomes. Enzymes, including MMPs (e.g. MMP2), phospholipase A2, alkaline phosphatase, transglutaminase or phosphatidylinositol-specific phospholipase C, have been found to be overexpressed in tumor tissues. In the presence of these enzymes, specially engineered enzyme-sensitive liposomes are disrupted and release the encapsulated drug at the tumor site [77]. Another approach is the use of a linker cleaved off in the presence of overexpressed enzymes, exposing other hidden functionalities [17,67].
One such example is liposomes prepared by incorporation of a PEG–polyethylene (PE) copolymer containing a MMP2-cleavable octapeptide (Gly-Pro-Leu-Gly-Ile-Ala-Gly-Gln) linker between the lipid and long PEG chain [67]. This MMP-2-sensitive PEG chain provides steric shielding to the cell-penetrating TATp functionality, which is cleaved in the tumor microenvironment in the presence of higher concentrations of MMP-2. In addition, these liposomes were actively targeted to cancer cells using the antinucleosome antibody 2C5. The in vitro study demonstrated that the Gly-Pro-Leu-Gly-Ile-Ala-Gly-Gln octapeptide was cleaved off in the presence of high levels of MMP2, which removed the PEG cloud and exposed the surface-bound CPPs that subsequently enhanced cell penetration of the liposomes [67]. Similarly, a lipopeptide was synthesized by conjugating a MMP-9 substrate peptide to a fatty acid incorporated into the liposomes. These liposomes released their encapsulated contents in response to the elevated levels of MMP-9 [83].
Light-triggered drug delivery
Use of light for activation/inactivation of specific biochemical processes has been recognized as a promising tool for several biomedical applications. Activated light, made by adjustment of parameters such as wavelength, intensity, pulse duration and cycle, has been used extensively in biomedical research. Among visible light, UV and near-infrared light used in the clinic, near-infrared is the most desirable for tumor targeting, since it penetrates deep into tissues [79]. Photodynamic therapy has become a well-established tool for the treatment of superficial tumors, where photosensitizing agents, such as porphyrin derivatives, chlorins, phthalocyanines and porphycenes, are employed to sensitize and eradicate malignant cells. These agents generate radical oxygen species, which kill the targeted malignant tumor cells. Various light-sensitive lipids have been applied that facilitate phototriggered structural and conformational changes, which lead to direct interaction of liposomes with the target cells via membrane fusion, photo-isomerism, photofragmentation or photopolymerization [15,79].
Benzoporphyrin photosensitizer-encapsulated PEGylated liposomes, modified with a peptide (Ala-Pro-Arg-Pro-Gly) specific for angiogenic endothelial cells were used for tumor cell-specific targeting and resulted in higher tumor growth inhibition [79].
Ultrasound-triggered drug delivery
Ultrasound-mediated drug delivery allows non-invasive penetration into deep tissues and can produce focused, controlled drug delivery [77]. This concept is based on the enhanced permeability of blood capillaries, generation of thermal energy and the destruction of cell membranes due to microconvection or inertia cavitation [77]. Ultrasound waves can induce both thermal and mechanical changes. Liposomes containing a small quantity of particular gases, including air or perfluorated hydrocarbon, were initially developed as an ultrasound contrast agent, but can be loaded with various drugs that are released after damage by an applied ultrasound treatment [4,84].
Low-frequency ultrasound (LFUS) has been used to trigger drug release from sterically stabilized liposomes without affecting the physicochemical properties of the drug. Mice bearing lymphomas treated with cisplatin-loaded sterically stabilized liposomes in combination with LFUS, showed very significant anti-tumor activity after intraperitoneal injection, as compared with control groups, those with free cisplatin with or without LFUS, LFUS alone, or cisplatin-loaded nano-SSL without LFUS. However, LFUS is only appropriate for superficial tumors. Local heating induced by high-intensity focused ultrasound has been considered ideal for deeper tumors [17,77].
`Magnetic' drug delivery
`Magnetic' colloids can carry chemotherapeutic agents to target sites and maintain them at the site until the drug is completely released [77]. To use an external magnetic field for tumor targeting, liposomes are magnetized by incorporation of magnetites, such as Fe3O4 or γ-Fe2O3, that are less than 10 nm in size [79]. These liposomes have biomedical applications such as magnetic hyperthermia, magnetic transfection, and manipulation of cells and proteins. Owing to their superior magnetic properties and nanoscale size, they are also referred to as SPIONs. One approach for targeted drug delivery by exposure to a magnetic field is the use of liposomes loaded with a drug plus a ferromagnetic material [4].
SPIONs loaded with therapeutics and coated with both hydrophilic and hydrophobic materials can be encapsulated and delivered by liposomes. For example, magnetic nanoparticles coated with palmityl-nitro-l-3,4-dihydroxyphenylalanine have been successfully embedded in liposomal membranes. The magnetic nanoparticles significantly elevated the temperature of liposomal membranes during exposure to a high-frequency alternating magnetic field, which ruptured the liposomes and selectively released the encapsulated drugs [85].
Methotrexate- and glutamic acid-chelated γ-Fe2O3 have been successfully coloaded into the aqueous core of liposomes using a reverse phase evaporation method [79]. The resultant magnetic carrier significantly increased methotrexate accumulation by more than fivefold in the target tissue when exposed to a magnetic field compared with the same formulation without an external magnetic stimulus in a mouse model [79].
In utilizing these strategies, the drug is released at the tissue level and can interact nonspecifically with neighboring cells. Therefore, drug penetration into the tumor cells may be limited due to their acquired resistance mechanisms. By contrast, targeting cell surface receptors may help overcome the resistance by receptor-mediated endocytosis when drug release is intracellular, allowing the drug to reach its subcellular targets. Thus, combinations of cell surface receptor target ing (cellular level) and triggered drug release (tissue level) could promote synergistic action.
Intracellular delivery
Once inside the tumor tissue, liposomes may need to cross the cell membrane barrier to deliver their cargo to the cytoplasm and exert their therapeutic effect through either passive or active targeting strategies. The site of drug action could be organelles and compartments such as the cytoplasm, nuclei, mitochondria, lysosomes or endoplasmic reticulum. Unlike small molecules that translocate through the cell membranes by diffusion, the nanocarriers, including liposomes and other macromolecular therapeutics, require energy-dependent endocytosis for cellular internalization. Since liposomes follow the endocytic pathway, they are entrapped in the endosomes (pH 6.5–6) and subsequently fuse with lysosomes (pH <5), where they undergo degradation that results in a lower therapeutic potential. Thus, overcoming the challenge of lysosomal degradation and effective delivery into the cytoplasm are important considerations that affect intracellular targeting.
Endosomal escape
Many attempts have been made to develop liposomes that promote cytosolic delivery of the payload by endosomal disruption. Although PEGylation increases longevity in the systemic circulation, it may diminish cellular uptake and further promote endosomal degradation of both the liposomes and their cargo [86]. The low endosomal pH has been taken advantage of to escape degradation by use of either fusogenic lipids or peptides, which destabilize the endosomal membrane after the conformational transition/activation at a lowered pH. Amines are protonated at an acidic pH and cause endosomal swelling and rupture by a buffer effect [86]. Unsaturated dioleoylphosphatidylethanolamine (DOPE) readily adopts an inverted hexagonal shape at a low pH, which causes fusion of liposomes to the endosomal membrane. This process destabilizes nanocarriers comprised of DOPE and releases the cargo into the cytoplasm [15]. Sasaki et al. demonstrated that Tf-PEG liposomes modified with two derivatives of the fusogenic lipid GALA, cholesteryl-GALA and PEG-GALA showed a highly efficient endosomal release (68%) of the entrapped sulforhodamine B dye that stained the cytoplasmic space extensively, indicating both GALA derivatives were necessary to induce the efficient release from endosomes [87]. Kullberg et al. attached a pore-forming protein listeriolysin O to HER-2-targeted bleomycin-loaded liposomes, and demonstrated greater cytotoxicity in vitro compared with the targeted bleomycin-loaded listeriolysin O-unmodified liposomes. The concentration of the drug needed to reduce tumor cell growth was approximately 57,000-fold less than if the drug was delivered extracellularly, indicating an endosomal escape mechanism [88]. Similarly, histidine-rich peptides have the ability to fuse with the endosomal membrane, resulting in pore formation, and can buffer the proton pump causing membrane lysis. This property can be used for modification of liposomes to escape endosomal degradation [89].
Cell-penetrating peptides
CPPs facilitate uptake of macromolecules through cellular membranes and, thus, enhance the delivery of CPP-modified molecules inside the cell [15]. CPPs can be split into two classes: amphipathic helical peptides, such as transportan and MAP, where lysine residues are major contributors to the positive charge; and Arg-rich peptides, such as TATp, Antennapedia or penetratin.
TATp is a transcription-activating factor with 86 amino acids that contains a highly basic (two Lys and six Arg among nine residues) protein transduction domain, which brings about nuclear localization and RNA binding [90]. Other CPPs that have been used for the modification of liposomes include the following: the minimal protein transduction domain of Antennapedia, a Drosophilia homeoprotein, called penetratin, which is a 16-mer peptide (residues 43–58) present in the third helix of the homeodomain; a 27-amino acid-long chimeric CPP, containing the peptide sequence from the amino terminus of the neuropeptide galanin bound via the Lys residue, mastoparan, a wasp venom peptide; VP22, a major structural component of HSV-1 facilitating intracellular transport and transportan (18-mer) amphipathic model peptide that translocates plasma membranes of mast cells and endothelial cells by both energy-dependent and -independent mechanisms [91]. When liposomes are modified with these CPPs, their intracellular delivery proceeds via energy-dependent macropinocytosis followed by endosomal escape [15]. Our group demonstrated that attachment of octa-arginine onto the surface of bleomycin- or doxorubicin-loaded liposomes enhanced tumor growth inhibition [92,93].
UV-triggered CPPs involved adding a TATp–lipid conjugate, with the TATp–PEG2000–DSPE conjugate linked to a less stable single-chain hydrophobic group of 12 or 16 carbons, via a UV-cleavable linker. Furthermore, UV-dependent internalization of liposomes (a 15-fold increase in cellular association and internalization after irradiation) was not observed with an uncleavable linker [86].
Organelle-specific targeting
Delivery of drugs or drug-loaded liposomes into the cytoplasm is frequently not enough to achieve a maximum therapeutic response. Cytoplasmic delivery and random interaction of liposomes with organelles results in elevated off-target effects. Delivery of drug-loaded liposomes directly to their `organelle of interest' enhances the therapeutic window and minimizes the degradation of the therapeutic cargo.
Along with being the cell powerhouse, mitochondria also store a suicidal trigger. Many cell death-associated signal transduction pathways are promoted through mitochondrion's action. They regulate apoptosis by trans locating apoptotic proteins from the mitochondrial inter-mediate space to the cytosol. Mitochondrial dysfunction has been linked to many of the hallmarks of cancer cells, including their limitless proliferative potential, impaired apoptosis, insensitivity to antigrowth signals, enhanced anabolism and decreased autophagy [94]. Mitochondria- targeted drug delivery systems have emerged as an extremely potent tool for cancer therapy. Significant efforts are being focused on the development of liposomes modified with mitochondria-targeting molecules [95]. Drug-loaded liposomes surface-functionalized with the triphenylphosphonium (TPP) moiety are effective in the delivery of cargo to mitochondria. TPP modified with a stearyl residue was anchored in the liposome and used to deliver ceramides to the mitochondria. Increased levels of ceramide caused cytochrome C release and initiation of apoptosis [96]. Subsequently, our group developed TPP-modified paclitaxel-loaded liposomes that exhibited a similar effect. However, they were free of cytotoxicity caused by the stearyl group in TPP modified with a stearyl residue, because TPP was attached to the lipid anchor via the PEG spacer group [97]. Another lipophilic cation, rhodamine 123, was used by Biswas et al. to develop paclitaxel-loaded liposomes that exhibited good mitochondrial targeting and enhanced cytotoxicity [98]. A mitochondrial-targeting nanopreparation, MITO-Porter, developed by the Harashima group is a promising system for the delivery of both large and small molecules to mitochondria [99]. MITO-Porter is a liposome-based nanocarrier with a composition of DOPE/sphingomyelin/stearyl-octa-arginine (9:2:1) that delivers macromolecular cargos to the mitochondrial interior via membrane fusion. High-density octa-arginine on liposomes induces cell uptake and cytoplasmic delivery by macropinocytosis. Subsequently, the MITO-Porters translocate into the mitochondrial membrane through electrostatic interactions, inducing fusion with the mitochondrial membrane. The lipid composition of the MITO-Porter facilitates this fusion and release of its cargo within the mitochondria.
Lysosomes act as the digestive organelles in cells and process the breakdown of endocytosed cargo. The idea that lysosomes act largely as waste units has recently changed into the belief that they are a useful tool, with a role in cell-death mechanisms in cancer. The lysosomal cathepsins play a pivotal role in bone remodeling, epidermal homeostasis, prohormone processing, antigen presentation, maintenance of the CNS in mice, angiogenesis, cell death and cancer cell invasion, which makes it an attractive target for cancer therapy [100]. Koshkaryev et al. prepared fluorescein isothiocyanate-dextran-loaded lysosome-targeted liposomes surface modified with a lysosomotropic ligand, octadecyl rhodamine B. The modified liposomes successfully delivered the loaded cargo to lysosomes as observed through their colocalization with a lysosomal marker and a twofold higher fluorescence of the lysosome-enriched fractions treated with modified liposomes compared with unmodified ones was observed in HeLa cells [101]. Ceramides are useful tools in inducing lysosomal membrane permeabilization; futhermore, intracellular delivery of ceramides via Tf-conjugated liposomes enhanced apoptosis in vitro in HeLa cells and in vivo in an A2780 ovarian carcinoma xenograft mouse model [49].
A unique strategy to develop liposomes targeting the nucleus is the nuclisome particles concept comprised of a two-step targeting strategy that aims to deliver short-range Auger-electron-emitting radionuclides to nuclear DNA of tumor cells [102]. Here, PEG-stabilized liposomes loaded with a unique DNA-intercalating compound were targeted to tumor cells to promote the delivery of radionuclides to DNA. This system has proven beneficial in eliminating tumor cells with minimum off-target effects. Similarly, efforts have been undertaken to target other organelles, such as the endoplasmic reticulum, of tumor cells for effective cancer therapy. These include the system designed by Pollock et al. to develop liposomes that could be trafficked directly to the endoplasmic reticulum, fuse with its membrane and deliver their cargo [103].
Multifunctional liposomes for tumor targeting
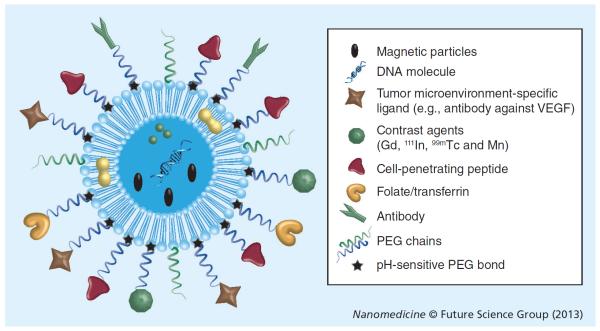
Recent developments in the field of drug delivery emphasize building a system that simultaneously demonstrates more than one useful attribute of targeting in the same system [104]. Attaching more than one functional group to the surface of liposomes enhances their accumulation in pathological sites and promotes organelle-specific delivery [78]. These multifunctional systems are engineered in such a way that they either simultaneously or sequentially exhibit the following properties: longevity in blood; specifically target the tumor; respond to the local stimuli typical of the tumor microenvironment, including elevated temperature, decreased pH values and externally applied stimuli such as a magnetic field, heat or ultrasound; promote intracellular delivery of the cargo; and carry a contrast agent to demonstrate the pharmacokinetic profile of liposomes and their accumulation (Figure 5) [104–106].
Figure 5.
A multifunctional liposome for targeted drug delivery.
Doxorubicin-loaded multifunctional liposomes developed by Koren et al. are described here as a prototype. The monoclonal antinucleosome antibody 2C5-attached PEG3.4K-PE copolymer, TAT-PEG1K-PE, and the pH-sensitive copolymer PEG2K-hydrazone-PE were embedded in the liposomal lipid bilayer. PEG2K-hydrazone-PE shielded TATp's functionality during circulation. Eventual degradation of the hydrazone bond in the lowered pH environment of the tumor cleaved the shielding and exposed the TATp functionality, which promoted intracellular delivery. Incubation of cells with multifunctional pH-sensitive liposomes, pretreated at pH 5.0 or 7.4 for 30 min showed a significant increase in cellular association for pH 5.0 (6.7- and 7.4-fold stronger for B16-F10 and MCF-7, respectively, against plain liposomes) compared with pH 7.4 (2.4- and 2.2-fold stronger binding for B16-F10 and MCF-7, respectively, against plain liposomes), suggesting that the TATp was exposed at pH 5.0. Immuno-Doxil® (Janssen Pharmaceuticals, NJ, USA) was observed to have enhanced cytotoxicity after it was pre-exposed to low pH, which was indicative of the effect of pH on removal of the shielding of TATp allowing exposure of the previously hidden TATp function [78].
Conclusion & future perspective
The field of liposomal drug delivery systems has been growing based on its significant contribution to the improved pharmacokinetics of many poorly soluble drugs in cancer-targeted therapies. Many liposomal candidates have shown great promise in the clinic, indicating broad potential for the therapeutic liposomal systems in the near future (Table 1).
Table 1.
Liposome-based drugs marketed and in clinical development for cancer therapy.
| Product name | Drug | Indications | Status | Ref. |
|---|---|---|---|---|
| Approved drugs | ||||
|
| ||||
| Doxil®/Caelyx® (Janssen Pharmaceuticals, NJ, USA) | Doxorubicin | AIDS-related Kaposi's sarcoma, recurrent ovarian cancer, metastatic breast cancer and multiple myeloma | Approved | [114] |
|
| ||||
| Lipusu® (Luye Pharma Group Ltd, Shanghai, China) | Paclitaxel | Solid tumors | Approved | [115] |
|
| ||||
| DaunoXome® (Galen Ltd, Craigavon, UK) | Daunorubicin | Kaposi's sarcoma | Approved | [116] |
|
| ||||
| Myocet® (Enzon Pharmaceuticals, NJ, USA) | Doxorubicin | Metastatic breast cancer | Approved | [117,118] |
|
| ||||
| Lipo-dox® (Sun Pharma, Mumbai, India) | Doxorubicin | Kaposi's sarcoma, breast and ovarian cancer | Approved | [119,120] |
|
| ||||
| Marqibo® (Talon Therapeutics, CA, USA) | Vincristine | Acute lymphoblastic leukemia | Approved | [121–123] |
|
| ||||
| Products in clinical trials | ||||
|
| ||||
| EndoTAG®-1 (Medigene, Martinsried, Germany) | Paclitaxel | Antiangiogenic properties, breast cancer, pancreatic cancer | Phase II | [124,125] |
|
| ||||
| Atragen™ (Aronex Technologies Inc., KS, USA) | Tretinoin | Acute promyelocytic leukemia, hormone-refractory prostate cancer | Phase II | [124] |
|
| ||||
| Lipoplatin™ (Regulon Inc., CA, USA) | Cisplatin | Pancreatic cancer, head and neck cancer, breast and gastric cancer and non-squamous non-small-cell lung cancer | Phase III | [124,126–128] |
|
| ||||
| SPI-077 | Cisplatin | Head and neck cancer, lung cancer | Phase I/II | [124,129,130] |
|
| ||||
| ThermoDox® (Celsion, NJ, USA) | Doxorubicin | Primary hepatocellular carcinoma | Phase III | [131–133] |
| Colorectal liver metastasis | Phase II | |||
| Refractory chest wall breast cancer | Phase II | |||
|
| ||||
| LE-SN38 | SN-38 active metabolite of irinotecan | Metastatic colorectal carcinoma | Phase I/II | [124,134] |
|
| ||||
| L-Annamycin | Annamycin | Acute lymphocytic leukemia, doxorubicin-resistant blood cancer | Phase I/II | [135,136] |
|
| ||||
| INX-0125 | Vinorelbine | Advanced solid tumors | Phase I | [137] |
|
| ||||
| CPX-1 | Irinotecan HCI: floxuridine | Colorectal cancer | Phase II | [138] |
However, to ensure efficient development of drug-loaded liposomes, it is important to experimentally examine the drug retention and circulation properties of the system. Leakage of either hydrophilic or hydrophobic drugs from the liposomes is a crucial factor that needs special attention.
One method suggested to circumvent this problem is the development of a pH gradient technique to avoid the liposomal leakage of hydrophilic drugs such as adriamycin [107]. A pH gradient is considered to be the driving force for translocating and retaining the amphiphilic weak bases and acids. The presence of various ions (citrates, sulfates, divalent metal ions or others) inside the liposomes causes the drugs to precipitate, and these ions are used to create pH/ion gradients that lead to the formation of soluble precipitates. This helps to restore the osmolarity balance and reduce the apparent drug concentration in the vesicles, increasing its accumulation inside. The ammonium sulfate method is one of the most popular pH gradient methods used for amine encapsulation. The sulfate counter-ion stabilizes the anthracycline accumulation for a long duration and this salting-out effect enhances accumulation, leading to efficient encapsulation [107]. By contrast, hydrophobic drugs do not abide by this technique and other techniques have to be employed for them, indicating that there is no single universal method, and that each drug requires a different strategy to manage all of its properties. Such active loading strategies are extremely crucial in the advancements of liposome production, since a low drug-to-lipid ratio could hamper the liposomal activity.
Along with parameters concerning the encapsulation and stability of drugs, the active targeting strategies need careful consideration for production or scale-up of cancer therapeutics. A ligand used to modify liposomes should bind firmly to antigens that are selectively (over) expressed on tumor cells. Furthermore, these antigens should be present in sufficient quantities to induce an effect. Moreover, the over-modification of the ligand for the attachment to the liposomal surface could hinder its interaction with the target. In the case of PEGylated liposomes modified with various targeting ligands, the quantity of attached antibodies or peptides should not compromise the longevity too much [18]. Another parameter that poses a challenge in the development and testing of targeted liposomes is the complexity of tumor biology, apart from the size and stability of the liposomes. Alternatively, all the systems mentioned throughout the paper are promising yet quite complex, making them difficult to synthesize and scale-up by the pharmaceutical industry, since their manufacturing involves many different synthetic and purification steps [13]. These challenges are accompanied by increases in cost, complexity and batch-to-batch variance; thereby affecting their commercial attractiveness and clinical relevance.
To address the above mentioned issues, many newer systems that hold promise towards the development of liposomes in terms of their scalability and bulk properties are currently underway. One such technique is the combination of liposome- and polymer-based systems for sustained release of bioactive compounds [108]. This system demonstrates attractive properties of liposomal preparations, such as stability, sustained drug release over prolonged periods, improved viscosity and improved half-life for both the drugs and liposome. On the other hand, the polymeric component of the system helps to maintain and preserve drug efficacy and bioactivity. In spite of the tremendous progress with these systems, they still face challenges, including damage to the drug during production due to toxic organic solvents or high heat, insufficient initial release of drug-loaded liposomes due to lack of degradability of the polymer altering the release profiles and high overdose during the rapid degradation period of polymer.
Another example is multistage drug delivery systems called mesoporous silicon nanoparticles (MSNs), which are able to circumvent many of the biological barriers and maximize site-specific delivery and drug release [109]. The vascular journey of the MSNs was mathematically compartmentalized into three main areas: margination dynamics, firm adhesion and control of internalization. This compartmentalization helped to alter the kinetics of injectables along with minimizing RES uptake. Based on the same concept, Roggers et al. developed MSN covalently attached to dipalmitoyl moieties supporting a lipid bilayer with phosphorylated lipids (LB-MSNs). This system demonstrated controlled release of entrapped fluorescein molecules under reducing conditions of disulfide. Fluorescently labeled LB-MSNs demonstrated entry into normal and cancer cells; while the flow cytometry analysis confirmed their biocompatibility. This system has the ability to be functionalized with the desired properties, including a high degree of dispersibility, and the ability for rapid and diverse cell interaction layer functionalization. These particles were innocuous to the cells, and their biocompatibility could be adjusted by changing the composition of the cell interaction layer. Additionally, the system could release a model molecule, fluorescein, from the pores of LB-MSNs via a chemically triggered reduction of disulfide [110]. On similar lines, many other MSN systems have been developed that support the above inferences, such as the system developed by Cauda et al. for the delivery of colchicine, a drug deactivating the microtubule polymerization, into HuH7 liver cancer cells [111]. The multifunctional design of MSN systems offers next-generation nanocarriers combining features such as targetability of ligands and triggered responses that simulate molecular valves, when multicompartmentalized cargos are delivered to target cells [112,113].
Careful analysis of the challenges mentioned above, along with detailed understanding of the exact pathways and mechanisms by which these complicated multifunctional liposomal systems reach their target site and exert drug action at the cellular level, could help achieve a revolution in liposome applications in cancer. In addition, there remains an unmet need to identify additional novel biomarkers for various types of cancers, to be exploited to develop more efficacious tools for tumor targeting. These strategies should open up new possibilities for personalized cancer therapy for patients unresponsive or resistant to the established modes of treatment.
Executive summary.
Liposomes: smart nanocarriers
-
■
The use of nanocarriers in cancer therapy poses several advantages, including increased drug accumulation in desired target tissues, absence of harmful effects on nontarget tissues, improved drug distribution dynamics, simplified administration procedures, increased potency of drugs and reduction in cost of therapy.
-
■
Liposomes, being biocompatible, biodegradable, less toxic, and having the ability to encapsulate both hydrophilic and hydrophobic drugs, are the most popular and well-studied nanocarrier for cancer therapy.
Passive targeting
-
■
Tumor vasculature is abnormal in nature, with a leaky capillary basement membrane. Owing to this leaky nature of capillaries, molecules easily cross the vessel walls and enter the interstitium.
-
■
Since the lymphatic drainage in tumor vasculature is poor, small particles, such as liposomes, can accumulate. The phenomenon is called the enhanced permeability and retention effect.
-
■
The enhanced permeability and retention effect is one of the primary criterions to be considered in the design of liposomal cancer drug delivery systems for passively targeting these systems to tumor cells.
Active targeting
-
■
Actively targeted liposomes are designed with the goal of reducing off-target effects by conjugating targeting moieties, such as small-molecule ligands, peptides or monoclonal antibodies, peripherally to liposomal drug delivery systems.
-
■
Long-circulating actively targeted liposomes that accumulate in the tumor vasculature through passive targeting are endocytosed into the cells through the targeting moiety, thus, enhancing uptake and the therapeutic effect.
-
■
Liposomes can be actively targeted by modifying the liposomal surface with various ligands that interact with overexpressed cancer cell surface receptors, or receptors and antigens present in the tumor microenvironment. The ligands utilized for cancer targeting include folate, transferrin, RGD, antinucleosome antibodies, or antibodies against VEGF, VCAM, matrix metalloproteases and integrins.
Intracellular & organelle targeting
-
■
Intracellular delivery helps overcome problems such as multidrug resistance in cancer therapy.
-
■
Liposomes need to cross the cell membrane barrier in order to deliver their cargo into the cytoplasm to exert their therapeutic effect. Postinternalization, the site of action could be the cytoplasm, nucleus, mitochondria, lysosomes or endoplasmic reticulum.
-
■
Delivery of drug-loaded liposomes directly to their organelle of action enhances the therapeutic window minimizing adverse side effects.
Multifunctional liposomes for tumor targeting
-
■
Recent developments in the field of drug delivery systems emphasize the need to build a system that simultaneously demonstrates more than one useful function for improving drug action.
-
■
These liposomes simultaneously or sequentially exhibit properties such as longevity in blood, specifically targeting the tumor site and responding to the local stimuli of the tumor site, such as elevated temperature or pH, to expose hidden functionalities. Liposomes can be specifically designed with multifunctionalities to respond to an externally applied magnetic field, heat or ultrasound for enhanced intracellular delivery of the loaded therapeutics.
Acknowledgements
The authors wish to thank W Hartner for his help in editing the manuscript.
This work was supported in part by NIH grants RO1 CA121838 and RO1 CA128486 to VP Torchilin.
No writing assistance was utilized in the production of this manuscript.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Voinea M, Simionescu M. Designing of `intelligent' liposomes for efficient delivery of drugs. J. Cell. Mol. Med. 2002;6(4):465–474. doi: 10.1111/j.1582-4934.2002.tb00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strebhardt K, Ullrich A. Paul Ehrlich's magic bullet concept: 100 years of progress. Nat. Rev. Cancer. 2008;8(6):473–480. doi: 10.1038/nrc2394. [DOI] [PubMed] [Google Scholar]
- 3.Huwyler J, Drewe J, Krähenbühl S. Tumor targeting using liposomal antineoplastic drugs. Int. J. Nanomed. 2008;3(1):21–29. [PMC free article] [PubMed] [Google Scholar]
- 4.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug. Discov. 2005;4(2):145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 5.Elbayoumi T, Torchilin V. Current trends in liposome research. In: Weissig V, editor. Liposomes. Humana Press; NJ, USA: 2010. pp. 1–27. [DOI] [PubMed] [Google Scholar]; ■■ Provides a brief overview of various liposomal products currently under development at the experimental and preclinical level.
- 6.Sawant RR, Torchilin VP. Liposomes as `smart' pharmaceutical nanocarriers. Soft Matter. 2010;6(17):4026–4044. [Google Scholar]; ■ Discusses the evolution of liposomes with a focus on stimuli-sensitive liposomes.
- 7.Haley B, Frenkel E. Nanoparticles for drug delivery in cancer treatment. Urol. Oncol. 2008;26(1):57–64. doi: 10.1016/j.urolonc.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug. Deliv. Rev. 2011;63(3):131–135. doi: 10.1016/j.addr.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Torchilin VP. Drug targeting. Eur. J. Pharm. Sci. 2000;11:S81–S91. doi: 10.1016/s0928-0987(00)00166-4. [DOI] [PubMed] [Google Scholar]
- 10.Kale AA, Torchilin VP. Environment-responsive multifunctional liposomes. Methods Mol. Biol. 2010;605:213–242. doi: 10.1007/978-1-60327-360-2_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maeda H. Macromolecular therapeutics in cancer treatment: the EPR effect and beyond. J. Control. Release. 2012;164(2):138–144. doi: 10.1016/j.jconrel.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 12.Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug. Deliv. Rev. 2012;65(1):71–79. doi: 10.1016/j.addr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Lammers T, Kiessling F, Hennink WE, Storm G. Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J. Control. Release. 2012;161(2):175–187. doi: 10.1016/j.jconrel.2011.09.063. [DOI] [PubMed] [Google Scholar]; ■ Summarizes important targeting systems and strategies, and discusses recent advances along with future directions in the development of tumor-targeted nanomedicines.
- 14.Torchilin V. Passive and active drug targeting: drug delivery to tumors as an example. Handb. Exp. Pharmacol. 2010;197:3–53. doi: 10.1007/978-3-642-00477-3_1. [DOI] [PubMed] [Google Scholar]
- 15.Torchilin VP. Targeted pharmaceutical nanocarriers for cancer therapy and imaging. AAPS J. 2007;9(2):128–147. doi: 10.1208/aapsj0902015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang M, Thanou M. Targeting nanoparticles to cancer. Pharmacol. Res. 2010;62(2):90–99. doi: 10.1016/j.phrs.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Danhier F, Feron O, Préat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anticancer drug delivery. J. Control. Release. 2010;148(2):135–146. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]; ■■ Provides a good overview of nanocarriers that exploit the characteristics of the tumor microenvironment for efficient drug delivery.
- 18.Sawant RR, Torchilin VP. Challenges in development of targeted liposomal therapeutics. AAPS J. 2012;14(2):303–315. doi: 10.1208/s12248-012-9330-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ Highlights the evolution of liposomes for both passive and active targeting, and challenges in the development of targeted liposomal therapeutics, in particular antibody-targeted liposomes.
- 19.Zhao W, Zhuang S, Qi XR. Comparative study of the in vitro and in vivo characteristics of cationic and neutral liposomes. Int. J. Nanomed. 2011;6:3087–3098. doi: 10.2147/IJN.S25399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv. Drug Del. Rev. 2008;60(15):1615–1626. doi: 10.1016/j.addr.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Egusquiaguirre SP, Igartua M, Hernández RM, Pedraz JL. Nanoparticle delivery systems for cancer therapy: advances in clinical and preclinical research. Clin. Transl. Oncol. 2012;14(2):83–93. doi: 10.1007/s12094-012-0766-6. [DOI] [PubMed] [Google Scholar]
- 22.Talekar M, Kendall J, Denny W, Garg S. Targeting of nanoparticles in cancer: drug delivery and diagnostics. Anticancer Drugs. 2011;22(10):949. doi: 10.1097/CAD.0b013e32834a4554. [DOI] [PubMed] [Google Scholar]
- 23.Liechty WB, Peppas NA. Expert opinion: responsive polymer nanoparticles in cancer therapy. Eur. J. Pharm. Biopharm. 2012;80(2):241–246. doi: 10.1016/j.ejpb.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nat. Rev. Cancer. 2002;2(10):750–763. doi: 10.1038/nrc903. [DOI] [PubMed] [Google Scholar]
- 25.Low PS, Henne WA, Doorneweerd DD. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc. Chem. Res. 2007;41(1):120–129. doi: 10.1021/ar7000815. [DOI] [PubMed] [Google Scholar]
- 26.Kularatne SA, Low PS. Targeting of nanoparticles: folate receptor. Methods Mol. Biol. 2010;624:249–265. doi: 10.1007/978-1-60761-609-2_17. [DOI] [PubMed] [Google Scholar]
- 27.Ling SSN, Yuen KH, Magosso E, Barker SA. Oral bioavailability enhancement of a hydrophilic drug delivered via folic acid-coupled liposomes in rats. J. Pharm. Pharmacol. 2009;61(4):445–449. doi: 10.1211/jpp/61.04.0005. [DOI] [PubMed] [Google Scholar]
- 28.Xiang G, Wu J, Lu Y, Liu Z, Lee RJ. Synthesis and evaluation of a novel ligand for folate-mediated targeting liposomes. Int. J. Pharm. 2008;356(1):29–36. doi: 10.1016/j.ijpharm.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malhi SS, Budhiraja A, Arora S, et al. Intracellular delivery of redox cyclerdoxorubicin to the mitochondria of cancer cell by folate receptor targeted mitocancerotropic liposomes. Int. J. Pharm. 2012;432(1–2):63–74. doi: 10.1016/j.ijpharm.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Xu S, Teng L, et al. Synthesis and evaluation of a novel lipophilic folate receptor targeting ligand. Anticancer Res. 2011;31(5):1521–1525. [PubMed] [Google Scholar]
- 31.Watanabe K, Kaneko M, Maitani Y. Functional coating of liposomes using a folate–polymer conjugate to target folate receptors. Int. J. Nanomed. 2012;7:3679–3688. doi: 10.2147/IJN.S32853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bothun GD, Lelis A, Chen Y, Scully K, Anderson LE, Stoner MA. Multicomponent folate-targeted magnetoliposomes: design, characterization, and cellular uptake. Nanomed. Nanotechnol. Biol. Med. 2011;7(6):797–805. doi: 10.1016/j.nano.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duarte S, Faneca H, Lima MC. Folate-associated lipoplexes mediate efficient gene delivery and potent anti-tumoral activity in vitro and in vivo. Int. J. Pharm. 2011;423(2):365–377. doi: 10.1016/j.ijpharm.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 34.Chaudhury A, Das S, Bunte RM, Chiu GNC. Potent therapeutic activity of folate receptor-targeted liposomal carboplatin in the localized treatment of intraperitoneally grown human ovarian tumor xenograft. Int. J. Nanomed. 2012;7:739–751. doi: 10.2147/IJN.S26172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong Z, Wan Y, Han J, Shi S, Zhang Z, Sun X. Improvement of adenoviral vector-mediated gene transfer to airway epithelia by folate-modified anionic liposomes. Int. J. Nanomed. 2011;6:1083–1093. doi: 10.2147/IJN.S19745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niu R, Zhao P, Wang H, et al. Preparation, characterization, and anti-tumor activity of paclitaxel-loaded folic acid modified and TAT peptide conjugated PEGylated polymeric liposomes. J. Drug Target. 2011;19(5):373–381. doi: 10.3109/1061186X.2010.504266. [DOI] [PubMed] [Google Scholar]
- 37.Shmeeda H, Amitay Y, Gorin J, et al. Delivery of zoledronic acid encapsulated in folate-targeted liposome results in potent in vitro cytotoxic activity on tumor cells. J. Control. Release. 2010;146(1):76–83. doi: 10.1016/j.jconrel.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 38.Ying X, Wen H, Lu WL, et al. Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals. J. Control. Release. 2010;141(2):183–192. doi: 10.1016/j.jconrel.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 39.Yang F, Lum JB, Mcgill JR, et al. Human transferrin: cDNA characterization and chromosomal localization. Proc. Natl Acad. Sci. USA. 1984;81(9):2752–2756. doi: 10.1073/pnas.81.9.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoon DJ, Chu DSH, Ng CW, et al. Genetically engineering transferrin to improve its in vitro ability to deliver cytotoxins. J. Control. Release. 2009;133(3):178–184. doi: 10.1016/j.jconrel.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakaguchi N, Kojima C, Harada A, Koiwai K, Emi N, Kono K. Effect of transferrin as a ligand of ph-sensitive fusogenic liposome – lipoplex hybrid complexes. Bioconj. Chem. 2008;19(8):1588–1595. doi: 10.1021/bc800126s. [DOI] [PubMed] [Google Scholar]
- 42.Li XM, Ding LY, Xu Y, Wang Y, Ping QN. Targeted delivery of doxorubicin using stealth liposomes modified with transferrin. Int. J. Pharm. 2009;373(1):116–123. doi: 10.1016/j.ijpharm.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 43.Zhai G, Wu J, Yu B, Guo C, Yang X, Lee RJ. A transferrin receptor-targeted liposomal formulation for docetaxel. J. Nanosci. Nanotechnol. 2010;10(8):5129–5136. doi: 10.1166/jnn.2010.2393. [DOI] [PubMed] [Google Scholar]
- 44.Cheng KT, Wang PC, Shan L. Molecular Imaging and Contrast Agent Database (MICAD) National Center for Biotechnology Information; MD, USA: 2007. Alexa Fluor 680-labeled transferrin-cationic (NBD-labeled DOPE-DOTAP) liposome-encapsulated gadopentetate dimeglumine complex. [PubMed] [Google Scholar]
- 45.Ito Y, Kimura Y, Shimahara T, et al. Disposition of TF-PEG-Liposome-BSH in tumor-bearing mice. Appl. Radiat. Isot. 2009;67(7):S109–S110. doi: 10.1016/j.apradiso.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 46.Penacho N, Rosa M, Lindman B, Miguel MG, Simões S, De Lima MCP. Physicochemical properties of transferrin-associated lipopolyplexes and their role in biological activity. Colloids Surf. B. Biointerfaces. 2010;76(1):207–214. doi: 10.1016/j.colsurfb.2009.10.034. [DOI] [PubMed] [Google Scholar]
- 47.Cardoso A, Simoes S, De Almeida L, et al. Tf-lipoplexes for neuronal siRNA delivery: a promising system to mediate gene silencing in the CNS. J. Control. Release. 2008;132(2):113–123. doi: 10.1016/j.jconrel.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 48.Düzgüneş N, De Ilarduya CT. Genetic nanomedicine: gene delivery by targeted lipoplexes. Methods Enzymol. 2012;509:355–367. doi: 10.1016/B978-0-12-391858-1.00018-6. [DOI] [PubMed] [Google Scholar]
- 49.Koshkaryev A, Piroyan A, Torchilin VP. Increased apoptosis in cancer cells in vitro and in vivo by ceramides in transferrin-modified liposomes. Cancer Biol. Ther. 2012;13(1):50–60. doi: 10.4161/cbt.13.1.18871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharma G, Modgil A, Sun C, Singh J. Grafting of cell-penetrating peptide to receptor-targeted liposomes improves their transfection efficiency and transport across blood–brain barrier model. J. Pharm. Sci. 2012;101(7):2468–2478. doi: 10.1002/jps.23152. [DOI] [PubMed] [Google Scholar]
- 51.Lehtinen J, Raki M, Bergström KA, et al. Pre-targeting and direct immunotargeting of liposomal drug carriers to ovarian carcinoma. PLoS One. 2012;7(7):e41410. doi: 10.1371/journal.pone.0041410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim SK, Huang L. Nanoparticle delivery of a peptide targeting EGFR signaling. J. Control. Release. 2012;157(2): 279–286. doi: 10.1016/j.jconrel.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong A. Modified epidermal growth factor receptor (EGFR)-bearing liposomes (MRBLs) are sensitive to EGF in solution. PLoS One. 2009;4(10):e7391. doi: 10.1371/journal.pone.0007391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mamot C, Drummond DC, Noble CO, et al. Epidermal growth factor receptor-targeted immunoliposomes significantly enhance the efficacy of multiple anticancer drugs in vivo. Cancer Res. 2005;65(24):11631–11638. doi: 10.1158/0008-5472.CAN-05-1093. [DOI] [PubMed] [Google Scholar]
- 55.Gao J, Zhong W, He J, et al. Tumor-targeted PE38KDEL delivery via PEGylated anti-HER-2 immunoliposomes. Int. J. Pharm. 2009;374(1):145–152. doi: 10.1016/j.ijpharm.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 56.Paszko E, Senge M. Immunoliposomes. Curr. Med. Chem. 2012;19(31):5239–5277. doi: 10.2174/092986712803833362. [DOI] [PubMed] [Google Scholar]
- 57.Shmeeda H, Tzemach D, Mak L, Gabizon A. HER-2-targeted pegylated liposomal doxorubicin: retention of target-specific binding and cytotoxicity after in vivo passage. J. Control. Release. 2009;136(2):155–160. doi: 10.1016/j.jconrel.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 58.Chiu GNC, Edwards LA, Kapanen AI, et al. Modulation of cancer cell survival pathways using multivalent liposomal therapeutic antibody constructs. Mol. Cancer Ther. 2007;6(3):844–855. doi: 10.1158/1535-7163.MCT-06-0159. [DOI] [PubMed] [Google Scholar]
- 59.Chang DK, Chiu CY, Kuo SY, et al. Antiangiogenic targeting liposomes increase therapeutic efficacy for solid tumors. J. Biol. Chem. 2009;284(19):12905–12916. doi: 10.1074/jbc.M900280200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Katanasaka Y, Ida T, Asai T, Maeda N, Oku N. Effective delivery of an angiogenesis inhibitor by neovessel-targeted liposomes. Int. J. Pharm. 2008;360(1):219–224. doi: 10.1016/j.ijpharm.2008.04.046. [DOI] [PubMed] [Google Scholar]
- 61.Wicki A, Rochlitz C, Orleth A, et al. Targeting tumor-associated endothelial cells: anti-VEGFR2 immunoliposomes mediate tumor vessel disruption and inhibit tumor growth. Clin. Cancer Res. 2012;18(2):454–464. doi: 10.1158/1078-0432.CCR-11-1102. [DOI] [PubMed] [Google Scholar]
- 62.Li SD, Chono S, Huang L. Efficient oncogene silencing and metastasis inhibition via systemic delivery of siRNA. Mol. Ther. 2008;16(5):942–946. doi: 10.1038/mt.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang DI, Lee S, Lee JT, et al. Preparation and in vitro evaluation of anti-VCAM-1-Fab′-conjugated liposomes for the targeted delivery of the poorly water-soluble drug celecoxib. J. Microencaps. 2011;28(3):220–227. doi: 10.3109/02652048.2011.552989. [DOI] [PubMed] [Google Scholar]
- 64.Gosk S, Moos T, Gottstein C, Bendas G. VCAM-1 directed immunoliposomes selectively target tumor vasculature in vivo. Biochim. Biophys. Acta. 2008;1778(4):854–863. doi: 10.1016/j.bbamem.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 65.Djafarzadeh R, Milani V, Rieth N, et al. TIMP-1-GPI in combination with hyperthermic treatment of melanoma increases sensitivity to FAS-mediated apoptosis. Cancer Immunol. Immunother. 2009;58(3):361–371. doi: 10.1007/s00262-008-0559-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hatakeyama H, Akita H, Ishida E, et al. Tumor targeting of doxorubicin by anti-MT1-MMP antibody-modified PEG liposomes. Int. J. Pharm. 2007;342(1):194–200. doi: 10.1016/j.ijpharm.2007.04.037. [DOI] [PubMed] [Google Scholar]
- 67.Zhu L, Kate P, Torchilin VP. Matrix metalloprotease 2-responsive multifunctional liposomal nanocarrier for enhanced tumor targeting. ACS Nano. 2012;6(4):3491–3498. doi: 10.1021/nn300524f. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ Summarizes various systems that exploit internal and external stimuli to enhance drug release from nanocarriers.
- 68.Du H, Cui C, Wang L, Liu H, Cui G. Novel tetrapeptide, RGDF, mediated tumor specific liposomal doxorubicin (DOX) preparations. Mol. Pharm. 2011;8(4):1224–1232. doi: 10.1021/mp200039s. [DOI] [PubMed] [Google Scholar]
- 69.Chen Z, Deng J, Zhao Y, Tao T. Cyclic RGD peptide-modified liposomal drug delivery system: enhanced cellular uptake in vitro and improved pharmacokinetics in rats. Int. J. Nanomed. 2012;7:3803. doi: 10.2147/IJN.S33541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Knudsen NØ, Schiffelers RM, Jorgensen L, Hansen J, Frokjaer S, Foged C. Design of cyclic RKKH peptide-conjugated PEG liposomes targeting the integrin α2β1 receptor. Int. J. Pharm. 2012;428(1):171–177. doi: 10.1016/j.ijpharm.2012.02.043. [DOI] [PubMed] [Google Scholar]
- 71.Garg A, Tisdale AW, Haidari E, Kokkoli E. Targeting colon cancer cells using PEGylated liposomes modified with a fibronectin-mimetic peptide. Int. J. Pharm. 2009;366(1):201–210. doi: 10.1016/j.ijpharm.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Edwards KA, Wang Y, Baeumner AJ. Aptamer sandwich assays: human α-thrombin detection using liposome enhancement. Anal. Bioanal. Chem. 2010;398(6):2645–2654. doi: 10.1007/s00216-010-3920-4. [DOI] [PubMed] [Google Scholar]
- 73.Kang H, O'Donoghue MB, Liu H, Tan W. A liposome-based nanostructure for aptamer directed delivery. Chem. Commun. 2010;46(2):249–251. doi: 10.1039/b916911c. [DOI] [PubMed] [Google Scholar]
- 74.El-Hamed F, Dave N, Liu J. Stimuli-responsive releasing of gold nanoparticles and liposomes from aptamer-functionalized hydrogels. Nanotechnology. 2011;22(49):494011. doi: 10.1088/0957-4484/22/49/494011. [DOI] [PubMed] [Google Scholar]
- 75.Cao Z, Tong R, Mishra A, et al. Reversible cell-specific drug delivery with aptamer-functionalized liposomes. Angew. Chem. Int. Ed. 2009;48(35):6494–6498. doi: 10.1002/anie.200901452. [DOI] [PubMed] [Google Scholar]
- 76.Mann AP, Bhavane RC, Somasunderam A, et al. Thioaptamer conjugated liposomes for tumor vasculature targeting. Oncotarget. 2011;2(4):298. doi: 10.18632/oncotarget.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arias JL. Drug targeting strategies in cancer treatment: an overview. Mini Rev. Med. Chem. 2011;11(1):1–17. doi: 10.2174/138955711793564024. [DOI] [PubMed] [Google Scholar]; ■ Focuses on the passive and active targeting of nanocarriers, paying particular attention to overcoming multidrug resistance.
- 78.Koren E, Apte A, Jani A, Torchilin VP. Multifunctional PEGylated 2C5-immunoliposomes containing pH-sensitive bonds and TAT peptide for enhanced tumor cell internalization and cytotoxicity. J. Control. Release. 2011;160(2):264–273. doi: 10.1016/j.jconrel.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhu L, Torchilin V. Stimulus-responsive nanopreparations for tumor targeting. Integr. Biol. (Camb.) 2012;5(1):96–107. doi: 10.1039/c2ib20135f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.May JP, Li S-D. Hyperthermia-induced drug targeting. Expert Opin. Drug Deliv. 2013;10(4):1–17. doi: 10.1517/17425247.2013.758631. [DOI] [PubMed] [Google Scholar]
- 81.Grüll H, Langereis S. Hyperthermia-triggered drug delivery from temperature-sensitive liposomes using MRI-guided high intensity focused ultrasound. J. Control. Release. 2012;161(2):317–327. doi: 10.1016/j.jconrel.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 82.Goldenbogen BR, Brodersen N, Gramatica A, et al. Reduction-sensitive liposomes from a multifunctional lipid conjugate and natural phospholipids: reduction and release kinetics and cellular uptake. Langmuir. 2011;27(17):10820–10829. doi: 10.1021/la201160y. [DOI] [PubMed] [Google Scholar]
- 83.Banerjee J, Hanson AJ, Gadam B, et al. Release of liposomal contents by cell-secreted matrix metalloproteinase-9. Bioconj. Chem. 2009;20(7):1332–1339. doi: 10.1021/bc9000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ibsen S, Benchimol M, Simberg D, Esener S. Ultrasound mediated localized drug delivery. In: Zahavy E, Ordentlich A, Yitzhaki S, Shafferman A, editors. Nano-Biotechnology for Biomedical and Diagnostic Research. Springer Netherlands; Heidelberg, The Netherlands: 2012. pp. 145–153. [Google Scholar]
- 85.Mok H, Zhang M. Superparamagnetic iron oxide nanoparticle-based delivery systems for biotherapeutics. Expert Opin. Drug Deliv. 2012;10(1):73–87. doi: 10.1517/17425247.2013.747507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Perche F, Torchilin VP. Recent trends in multifunctional liposomal nanocarriers for enhanced tumor targeting. J. Drug. Deliv. 2013;2013:705265. doi: 10.1155/2013/705265. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ Provides an extensive report on strategies employed to achieve liposome-mediated tumor targeting.
- 87.Sasaki K, Kogure K, Chaki S, et al. An artificial virus-like nano carrier system: enhanced endosomal escape of nanoparticles via synergistic action of pH-sensitive fusogenic peptide derivatives. Anal. Bioanal. Chem. 2008;391(8):2717–2727. doi: 10.1007/s00216-008-2012-1. [DOI] [PubMed] [Google Scholar]
- 88.Kullberg M, Mann K, Anchordoquy TJ. Targeting HER-2+ breast cancer cells with bleomycin immunoliposomes linked to LLO. Mol. Pharm. 2012;9(7):2000–2008. doi: 10.1021/mp300049n. [DOI] [PubMed] [Google Scholar]
- 89.Bolhassani A. Potential efficacy of cell-penetrating peptides for nucleic acid and drug delivery in cancer. Biochim. Biophys. Acta. 2011;1816(2):232–246. doi: 10.1016/j.bbcan.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 90.Mäe M, Langel Ü . Cell-penetrating peptides as vectors for peptide, protein and oligonucleotide delivery. Curr. Opin. Pharm. 2006;6(5):509–514. doi: 10.1016/j.coph.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 91.Torchilin VP. Multifunctional nanocarriers. Adv. Drug. Deliv. Rev. 2006;58(14):1532–1555. doi: 10.1016/j.addr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 92.Koshkaryev A, Piroyan A, Torchilin VP. Bleomycin in octaarginine-modified fusogenic liposomes results in improved tumor growth inhibition. Cancer Lett. 2012;334(2):293–301. doi: 10.1016/j.canlet.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Biswas S, Dodwadkar NS, Deshpande PP, Parab S, Torchilin VP. Surface functionalization of doxorubicin-loaded liposomes with octa-arginine for enhanced anticancer activity. Eur. J. Pharm. Biopharm. 2013;84(3):517–525. doi: 10.1016/j.ejpb.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Galluzzi L, Morselli E, Kepp O, et al. Mitochondrial gateways to cancer. Mol. Aspects Med. 2010;31(1):1–20. doi: 10.1016/j.mam.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 95.Weissig V, Boddapati SV, Cheng S-M, D'Souza GGM. Liposomes and liposome-like vesicles for drug and DNA delivery to mitochondria. J. Liposome Res. 2006;16(3):249–264. doi: 10.1080/08982100600851169. [DOI] [PubMed] [Google Scholar]
- 96.Boddapati SV, D'Souza GGM, Erdogan S, Torchilin VP, Weissig V. Organelle-targeted nanocarriers: specific delivery of liposomal ceramide to mitochondria enhances its cytotoxicity in vitro and in vivo. Nano Lett. 2008;8(8):2559–2563. doi: 10.1021/nl801908y. [DOI] [PubMed] [Google Scholar]
- 97.Biswas S, Dodwadkar NS, Deshpande PP, Torchilin VP. Liposomes loaded with paclitaxel and modified with novel triphenylphosphonium–PEG–PE conjugate possess low toxicity, target mitochondria and demonstrate enhanced anti-tumor effects in vitro and in vivo. J. Control. Release. 2012;159(3):393–402. doi: 10.1016/j.jconrel.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Biswas S, Dodwadkar NS, Sawant RR, Koshkaryev A, Torchilin VP. Surface modification of liposomes with rhodamine-123-conjugated polymer results in enhanced mitochondrial targeting. J. Drug Target. 2011;19(7):552–561. doi: 10.3109/1061186X.2010.536983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yamada Y, Furukawa R, Yasuzaki Y, Harashima H. Dual function MITO-Porter, a nano carrier integrating both efficient cytoplasmic delivery and mitochondrial macromolecule delivery. Mol. Ther. 2011;19(8):1449–1456. doi: 10.1038/mt.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kirkegaard T, Jäättelä M. Lysosomal involvement in cell death and cancer. Biochim. Biophys. Acta. 2009;1793(4):746–754. doi: 10.1016/j.bbamcr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 101.Koshkaryev A, Thekkedath R, Pagano C, Meerovich I, Torchilin VP. Targeting of lysosomes by liposomes modified with octadecyl-rhodamine B. J. Drug Target. 2011;19(8):606–614. doi: 10.3109/1061186X.2010.550921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gedda L, Edwards K. Nuclisome targeting the tumor cell nucleus. Tumor Biol. 2012;33(3):661–667. doi: 10.1007/s13277-012-0341-3. [DOI] [PubMed] [Google Scholar]
- 103.Pollock S, Antrobus R, Newton L, et al. Uptake and trafficking of liposomes to the endoplasmic reticulum. FASEB J. 2010;24(6):1866–1878. doi: 10.1096/fj.09-145755. [DOI] [PubMed] [Google Scholar]
- 104.Torchilin V. Multifunctional and stimuli-sensitive pharmaceutical nanocarriers. Eur. J. Pharm. Biopharm. 2009;71(3):431–444. doi: 10.1016/j.ejpb.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jabr-Milane LS, Van Vlerken LE, Yadav S, Amiji MM. Multifunctional nanocarriers to overcome tumor drug resistance. Cancer Treat. Rev. 2008;34(7):592–602. doi: 10.1016/j.ctrv.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gindy ME, Prud'Homme RK. Multifunctional nanoparticles for imaging, delivery and targeting in cancer therapy. Expert Opin. Drug Deliv. 2009;6(8):865–878. doi: 10.1517/17425240902932908. [DOI] [PubMed] [Google Scholar]
- 107.Gubernator J. Active methods of drug loading into liposomes: recent strategies for stable drug entrapment and increased in vivo activity. Expert Opin. Drug Deliv. 2011;8(5):565–580. doi: 10.1517/17425247.2011.566552. [DOI] [PubMed] [Google Scholar]
- 108.Mufamadi MS, Pillay V, Choonara YE, et al. A review on composite liposomal technologies for specialized drug delivery. J. Drug. Deliv. 2011;2011:939851. doi: 10.1155/2011/939851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Blanco E, Hsiao A, Mann AP, Landry MG, Meric-Bernstam F, Ferrari M. Nanomedicine in cancer therapy: innovative trends and prospects. Cancer Sci. 2011;102(7):1247–1252. doi: 10.1111/j.1349-7006.2011.01941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Roggers RA, Lin VSY, Trewyn BG. Chemically reducible lipid bilayer coated mesoporous silica nanoparticles demonstrating controlled release and HeLa and normal mouse liver cell biocompatibility and cellular internalization. Mol. Pharm. 2012;9(9):2770–2777. doi: 10.1021/mp200613y. [DOI] [PubMed] [Google Scholar]
- 111.Cauda V, Engelke H, Sauer A, et al. Colchicine-loaded lipid bilayer-coated 50 nm mesoporous nanoparticles efficiently induce microtubule depolymerization upon cell uptake. Nano Lett. 2010;10(7):2484–2492. doi: 10.1021/nl100991w. [DOI] [PubMed] [Google Scholar]
- 112.Al-Jamal WT, Kostarelos K. Liposomes: from a clinically established drug delivery system to a nanoparticle platform for theranostic nanomedicine. Acc. Chem. Res. 2011;44(10):1094–1104. doi: 10.1021/ar200105p. [DOI] [PubMed] [Google Scholar]
- 113.Tarn D, Ashley CE, Xue M, Carnes EC, Zink JI, Brinker CJ. Mesoporous silica nanoparticle nanocarriers: biofunctionality and biocompatibility. Acc. Chem. Res. 2013;46(3):792–801. doi: 10.1021/ar3000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Barenholz Y. Doxil® – the first FDA-approved nano-drug: lessons learned. J. Control. Release. 2012;160(2):117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 115.Zhang Q, Huang X-E, Gao L-L. A clinical study on the premedication of paclitaxel liposome in the treatment of solid tumors. Biomed. Pharmacother. 2009;63(8):603–607. doi: 10.1016/j.biopha.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 116.Petre CE, Dittmer DP. Liposomal daunorubicin as treatment for Kaposi's sarcoma. Int. J. Nanomedicine. 2007;2(3):277. [PMC free article] [PubMed] [Google Scholar]
- 117.Park J. Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res. 2002;4(3):95–99. doi: 10.1186/bcr432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Batist G, Ramakrishnan G, Rao CS, et al. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized, multicenter trial of metastatic breast cancer. J. Clin. Oncol. 2001;19(5):1444–1454. doi: 10.1200/JCO.2001.19.5.1444. [DOI] [PubMed] [Google Scholar]
- 119.Ayen W, Kumar N. In vivo evaluation of doxorubicin-loaded (peg)3-pla nanopolymersomes (PolyDoxSome) using dmba-induced mammary carcinoma rat model and comparison with marketed LipoDox™. Pharm. Res. 2012;29(9):2522–2533. doi: 10.1007/s11095-012-0783-8. [DOI] [PubMed] [Google Scholar]
- 120.Chou H-H, Wang K-L, Chen C-A, et al. Pegylated liposomal doxorubicin (Lipo-Dox®) for platinum-resistant or refractory epithelial ovarian carcinoma: a Taiwanese gynecologic oncology group study with long-term follow-up. Gynecol. Oncol. 2006;101(3):423–428. doi: 10.1016/j.ygyno.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 121.Silverman J, Deitcher S. Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother. Pharmacol. 2013;71(3):555–564. doi: 10.1007/s00280-012-2042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rodriguez MA, Pytlik R, Kozak T, et al. Vincristine sulfate liposomes injection (Marqibo) in heavily pretreated patients with refractory aggressive non-Hodgkin lymphoma. Cancer. 2009;115(15):3475–3482. doi: 10.1002/cncr.24359. [DOI] [PubMed] [Google Scholar]
- 123.Sarris AH, Hagemeister F, Romaguera J, et al. Liposomal vincristine in relapsed non-Hodgkin's lymphomas: early results of an ongoing Phase II trial. Ann. Oncol. 2000;11(1):69–72. doi: 10.1023/a:1008348010437. [DOI] [PubMed] [Google Scholar]
- 124.Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomedicine. 2006;1(3):297. [PMC free article] [PubMed] [Google Scholar]
- 125.Fasol U, Frost A, Büchert M, et al. Vascular and pharmacokinetic effects of EndoTAG-1 in patients with advanced cancer and liver metastasis. Ann. Oncol. 2012;23(4):1030–1036. doi: 10.1093/annonc/mdr300. [DOI] [PubMed] [Google Scholar]
- 126.Stathopoulos GP, Boulikas T. Lipoplatin formulation review article. J. Drug Deliv. 2012;2012:581363. doi: 10.1155/2012/581363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fantini M, Gianni L, Santelmo C, et al. Lipoplatin treatment in lung and breast cancer. Chemother. Res. Pract. 2011;2011:125192. doi: 10.1155/2011/125192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stathopoulos GP, Antoniou D, Dimitroulis J, Stathopoulos J, Marosis K, Michalopoulou P. Comparison of liposomal cisplatin versus cisplatin in non-squamous cell non-small-cell lung cancer. Cancer Chemother. Pharmacol. 2011;68(4):945–950. doi: 10.1007/s00280-011-1572-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Newman MS, Colbern GT, Working PK, Engbers C, Amantea MA. Comparative pharmacokinetics, tissue distribution, and therapeutic effectiveness of cisplatin encapsulated in long-circulating, pegylated liposomes (SPI-077) in tumor-bearing mice. Cancer Chemother. Pharmacol. 1999;43(1):1–7. doi: 10.1007/s002800050855. [DOI] [PubMed] [Google Scholar]
- 130.Seetharamu N, Kim E, Hochster H, Martin F, Muggia F. Phase II study of liposomal cisplatin (SPI-77) in platinum-sensitive recurrences of ovarian cancer. Anticancer Res. 2010;30(2):541–545. [PubMed] [Google Scholar]
- 131.Yarmolenko PS, Zhao Y, Landon C, et al. Comparative effects of thermosensitive doxorubicin-containing liposomes and hyperthermia in human and murine tumours. Int. J. Hyperthermia. 2010;26(5):485–498. doi: 10.3109/02656731003789284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Poon RTP, Borys N. Lyso-thermosensitive liposomal doxorubicin: an adjuvant to increase the cure rate of radiofrequency ablation in liver cancer. Future Oncol. 2011;7(8):937–945. doi: 10.2217/fon.11.73. [DOI] [PubMed] [Google Scholar]
- 133.Staruch R, Chopra R, Hynynen K. Localised drug release using MRI-controlled focused ultrasound hyperthermia. Int. J. Hyperthermia. 2011;27(2):156–171. doi: 10.3109/02656736.2010.518198. [DOI] [PubMed] [Google Scholar]
- 134.Pal A, Khan S, Wang Y-F, et al. Preclinical safety, pharmacokinetics and antitumor efficacy profile of liposome-entrapped SN-38 formulation. Anticancer Res. 2005;25(1A):331–341. [PubMed] [Google Scholar]
- 135.Booser D, Esteva F, Rivera E, et al. Phase II study of liposomal annamycin in the treatment of doxorubicin-resistant breast cancer. Cancer Chemother. Pharmacol. 2002;50(1):6–8. doi: 10.1007/s00280-002-0464-0. [DOI] [PubMed] [Google Scholar]
- 136.Apostolidou E, Swords R, Alvarado Y, Giles FJ. Treatment of acute lymphoblastic leukaemia: a new era. Drugs. 2007;67(15):2153–2171. doi: 10.2165/00003495-200767150-00004. [DOI] [PubMed] [Google Scholar]
- 137.Semple SC, Leone R, Wang J, et al. Optimization and characterization of a sphingomyelin/cholesterol liposome formulation of vinorelbine with promising antitumor activity. J. Pharm. Sci. 2005;94(5):1024–1038. doi: 10.1002/jps.20332. [DOI] [PubMed] [Google Scholar]
- 138.Use of nanoscale delivery systems to maintain synergistic drug ratios in vivo. Expert Opin. Drug Deliv. 2010;7(12):1329–1341. doi: 10.1517/17425247.2010.538678. [DOI] [PubMed] [Google Scholar]