Abstract
Purpose
The purpose of this study was to determine locoregional control (LRC), disease-free survival (DFS), and toxicity of high-dose-rate interstitial brachytherapy (HDR-ISBT) in the treatment of locally advanced cervical cancer.
Methods and Materials
Between March 1996 and May 2009, 116 patients with cervical cancer were treated. Of these, 106 (91%) patients had advanced disease (International Federation of Gynecology and Obstetrics stage IIB-IVA). Ten patients had stage IB, 48 had stage II, 51 had stage III, and 7 had stage IVA disease. All patients were treated with a combination of external beam radiation therapy (EBRT) to the pelvis (5040 cGy) and 2 applications of HDR-ISBT to a dose of 3600 cGy to the implanted volume. Sixty-one percent of patients also received interstitial hyperthermia, and 94 (81%) patients received chemotherapy.
Results
Clinical LRC was achieved in 99 (85.3%) patients. Three-year DFS rates were 59%, 67%, 71%, and 57% for patients with stage IB, II, III, and IVA disease, respectively. The 5-year DFS and overall survival rates for the entire group were 60% and 44%, respectively. Acute and late toxicities were within acceptable limits.
Conclusions
Locally advanced cervical cancer patients for whom intracavitary BT is unsuitable can achieve excellent LRC and OS with a combination of EBRT and HDR-ISBT.
Introduction
Conventional intracavitary brachytherapy (ICBT) may not deliver adequate doses in cases of advanced cervical disease or in patients with distorted anatomy. Such cases are associated with a high incidence of local failure and complications (1, 2). These patients are often treated palliatively (3). Interstitial BT (ISBT) for patients in whom ICBT is unsuitable is well established (4-9). Previously, We reported results with low-dose-rate (LDR) ISBT in the treatment of patients with bulky cervical cancer (5).
Modern high-dose-rate (HDR) BT treatment planning systems offer radiation safety advantages as well as a fast and efficient source and dwell time optimized planning not possible with the older LDR-ICBT or ISBT treatment planning systems. In addition to practically no radiation exposure to visitors and staff, HDR ISBT results in overall shorter hospitalizations of 1 night compared to 3 days for LDR BT.
At Long Beach Memorial Medical Center (LBMMC), HDRISBT replaced LDR in 1996. Hyperthermia has been suggested as an adjuvant treatment for locally advanced cervical cancer based on experimental data showing it can potentiate the effects of radiation (10). Herein, we report outcomes with HDR-ISBT.
Methods and Materials
This was a single-institution study conducted at LBMMC institutional review board approval was obtained for this study, which analyzed the records of 116 of 121 consecutively treated patients (5 stage IVB patients were excluded) from March 1996-May 2009, with histologically proven cervical cancer, who completed at least 1 treatment of HDR-ISBT. Indications at the time of BT for HDRISBT included bulky disease; narrow vagina; obliterated vaginal fornices; extensive vaginal, parametrial, or pelvic side wall involvement; and poor tumor shrinkage after external beam radiation therapy (EBRT). Most of the patients had persistent, bulky disease involving the parametria at the time of the first BT procedure.
Pretreatment evaluation included complete history and physical examination, examination under anesthesia by a gynecologic oncologist and a radiation oncologist, cystoscopy, proctoscopy, and chemistry panel. Imaging included chest radiography and computed tomography (CT) scans of the abdomen and pelvis, bone scans if clinically indicated, and, recently, positron emission tomography-CT staging. Magnetic resonance imaging was not done routinely.
Treatment protocol
All patients received EBRT to the whole pelvis to a median dose of 5040 cGy in 28 fractions, at 5 fractions per week given with a standard 2- or 4-field box technique. There were 2 HDR-ISBT procedures, the first was performed 1-2 weeks after the completion of EBRT, and the second procedure was performed 2 weeks after the completion of the first procedure. Six patients received the first HDR-ISBT prior to the end of EBRT. Five patients refused the second interstitial implant. Seventeen patients (15%) received elective paraortic irradiation.
We previously reported the LDR-ISBT technique upon which HDR-ISBT is based (5,11,12). A brief description is provided in the supplementary text (see Supplementary Appendix E1). For the 32 patients in whom a tandem could not be used because of bulky tumor or distorted anatomy, central coverage was accomplished by 4-6 central needles.
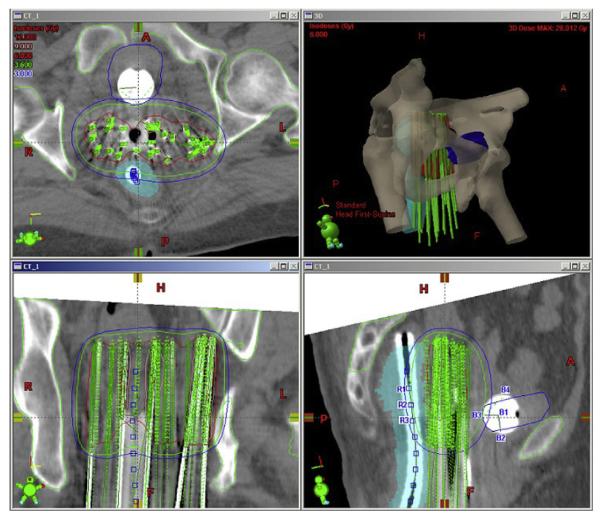
CT-based plans were optimized with BrachyVision (Varian Medical Systems, Palo Alto, CA, USA) for target volume coverage and sparing of organs at risk (Fig. 1). The treatment plan was exported to the HDR remote-afterloading machine VariSource (Varian Medical Systems, Palo Alto, CA, USA), which uses a high-activity (10 Ci max.) 192Ir source of 5-mm active length and 0.59-mm diameter. The prescription dose of 1800 cGy minimum peripheral dose per insertion was delivered in 3 equal fractions (600 cGy each) separated by a minimum of 6 h over a 24-h period. A second implant was performed in 2 weeks, delivering a similar dose. Thus, HDR-ISBT total dose was 3600 cGy in 6 fractions. Dose and fractionation parameters were obtained from LDR implant experience (5), converting the continuous LDR dose to HDR fractionated dose by using the linear quadratic model (13).
Fig. 1.
Interstitial implant optimization as described in the text.
Target volume (TV) in axial planes was taken from the implanted volume with a 3-mm margin, whereas the longitudinal extent of the target volume was obtained from the clinical examination at the time of implantation. Depending upon the extent of disease, the active lengths of the implanted needles ranged from 6-8 cm, but tandem and obturator needles were loaded only up to the external os. A CT-compatible flexible rectal marker with grooves at every centimeter was used for rectal dose determination. Several points were marked along the center of this rectal marker, and the average dose to three consecutive highest doses receiving points (a length of 2 cm) was limited to 60% of the prescription dose. A Foley balloon in the bladder (filled with 7 cc of saline/contrast solution) was used for bladder dose estimation. At the time of CT, the Foley balloon was pulled down almost to the bladder neck. Five dose points were assigned to the Foley balloon, indicating dose to its center, posterior, anterior, superior, and inferior points (Fig. 1). Average dose to the bladder was limited to 65% of the prescription dose. Dose optimization was carried out using geometric optimization (GO) with manual adjustments to ensure proper coverage of the TV, as defined above, and to limit doses to the bladder and rectum. The prescription was delivered to the implanted volume and not to point “A”; however point A by its definition was always within the TV. Rectal wall and bladder wall doses were not recorded, but from the dose distribution, it was seen that at least 2 cm of anterior rectal wall and most of the posterior bladder wall got the prescription dose.
Of 116 patients, 94 (81%) patients received concurrent chemotherapy with weekly 40 mg/m2 cisplatin. Seventy-one patients (61%) received interstitial radiofrequency hyperthermia either during the first or second implant or during both implants for 45-60 min to 41°C-42°C within 30 min of HDR treatment (10).
All patients were examined by a radiation oncologist and gynecologic oncologist after completing treatment, at 2 and then 6 weeks, then at 3-month intervals for the first year, 4-month intervals in the second year, every 6 months for up to 5 years, and yearly thereafter.
Clinical definition
Complete response (CR) was defined as total resolution of clinically visible and/or palpable tumor of the cervix and vagina. Regional control was defined as no disease recurrence in the parametria and/or pelvic lymph nodes. Locoregional control (LRC) was defined as no evidence of disease in the area of the original tumor (ie, cervix, vagina, and parametria) by clinical examination and imaging studies. CT and or magnetic resonance scans supplemented the clinical assessment if clinically indicated, with biopsy for a high suspicion of persistent or recurrent disease.
Statistical analysis
Descriptive statistics were calculated for patient characteristics (age, pretreatment hemoglobin), tumor factors (histology, tumor size, grade, stage, presence of hydronephrosis, unilateral or bilateral pelvic wall involvement for stage III tumors), treatment factors (concurrent chemotherapy, paraortic irradiation, interstitial radiofrequency hyperthermia, tandem usage, treatment duration), and complications (acute and late toxicities). Categorical variables were created to represent pretreatment hemoglobin concentrations of ≤10 mg/dL vs >10 mg/dL and tumor size ≤6 cm (ie, the median value vs tumor size >6 cm). The association between response groups and these discrete variables were evaluated with the Fisher exact test. The Kaplan-Meier method (14) was applied to estimate 3- and 5-year disease-free survival (DFS) and overall survival (OS) rates for all patients and by stage. The log-rank test was applied to test for the equality of survival curves between strata. Univariate and multivariate Cox proportional hazard regression (HR) models were formed for DFS and OS, with predictors including patient characteristics, tumor factors, and treatment factors. The estimated HRs were calculated, and 95% confidence limits for HRs were obtained. The multivariate modeling strategy used backward elimination with variables removed from the model at a significance level of 0.10, while tumor size, stage, and interstitial radiofrequency hyperthermia were retained in the selection process. SAS version 9.1.3 software (SAS Cary, North Carolina) was used.
Results
Patient, tumor, and treatment factors
The median age of the patients at diagnosis was 55 years (range, 20-89 years). Tumor characteristics are presented in Table 1. Ninety-nine (85.3%) patients had squamous cell carcinoma.
Table 1.
Tumor characteristics
| Characteristic | n | % |
|---|---|---|
| Pathologic feature | ||
| Squamous cell carcinoma | 99 | 85.3 |
| Adenocarcinoma | 7 | 6.0 |
| Adenosquamous carcinoma | 7 | 6.0 |
| Mucinous adenocarcinoma | 1 | 0.9 |
| Papillary adenocarcinoma | 1 | 0.9 |
| Small cell carcinoma | 1 | 0.9 |
| Differentiation | ||
| Well | 7 | 6.0 |
| Moderate | 31 | 26.7 |
| Poor | 60 | 51.7 |
| Unknown | 18 | 15.5 |
| FIGO stage | ||
| IB1 | 6 | 5.2 |
| IB2 | 4 | 3.3 |
| IIA | 0 | 0 |
| IIB | 48 | 41.4 |
| IIIA | 7 | 6.0 |
| IIIB | 44 | 37.9 |
| IVA | 7 | 6.0 |
Abbreviation: FIGO = International Federation of Gynecology and Obstetrics.
All patients were staged according to the International Federation of Gynecology and Obstetrics (FIGO) staging system (15). The most common FIGO stage was IIB (Table 1). The median tumor size was 6 cm. Tumor size prior to treatment was ≤6 cm in 66 (58 %) patients and >6 cm in 47 (42%) patients. Tumor size prior to treatment was not associated with LRC status (Fisher’s exact test, P=.27). Twenty-six percent of the patients had hydronephrosis on CT scan.
A total of 227 HDR-ISBT procedures were performed in this study. A tandem was used during the first or second implant in 84 of 116 (72.4%) patients. Of those who refused the second implant, 3 patients had stage IIB, 1 had stage IIIB, and 1 had stage IVA cervical cancer. Four of these 5 patients achieved LRC and had no distant metastasis following treatment. One patient with stage IIB disease did not achieve LRC and had distant metastasis.
Median duration of treatment was 10.1 weeks (range, 6-41.3 weeks) for the entire group. Mean follow-up for all patients was 35.1 months (range, 3.8-138.6 months).
Response to treatment
Despite the extensive disease, distorted anatomy, and other unfavorable factors in this group of patients, CR was achieved in 105 (90.5%) patients and clinical LRC in 99 of the 116 (85.3%) patients. In the 113 subjects for whom tumor size was known, 62 of the 66 (93.9%) patients with tumor size ≤6 cm achieved CR, and 41 of the 47 (87.2%) patients with tumor size >6 cm achieved CR (Fisher’s exact test, P=.31). Among the 113 patients for whom initial hemoglobin values were known, 103 achieved a CR. Although patients were transfused to a minimum of 10 g/100 mL before treatment, there was a statistically significant difference in the percentage achieving CR for patients with an initial hemoglobin level ≤10 g/100 mL (78%) vs those with an initial hemoglobin level >10 g/100 mL (96%) (Fisher’s exact test P=.0052). Nineteen of 116 (16%) patients had a known distant relapse but no local or regional relapse at last follow-up. Of these, 4 patients had distant metastasis limited to the paraortic lymph nodes.
Estimated OS and DFS rates
For the entire group, 3- and 5-year DFS rates were 68% (95% CI, 56.3%-76.8%) and 60% (95% CI, 44.9%-72.1%), respectively. The 3- and 5-year OS rates for the entire group were 58% (95% CI, 46.7%-67.3%) and 44% (95% CI, 32.2%-55.7%), respectively. DFS and OS at 3 and 5 years according to the stage of disease are shown in Fig. 2 and in Supplementary Fig. E1.
Fig. 2.
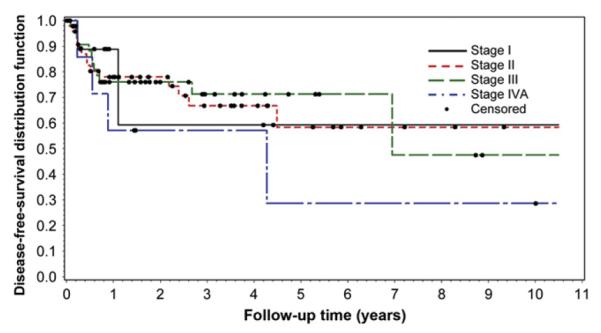
DFS by stage of disease.
The 3- and 5-year DFS rates for patients with hydronephrosis were 68% (95% CI, 46.3%-83.0%) and 46% (95% CI, 10.0%-76.5%), respectively. In contrast, these rates were 66% (95% CI, 51.0%-77.6%) and 60% (95% CI, 40.4%-74.3%) for those patients without hydronephrosis. The 3-year and 5-year OS rates for patients with hydronephrosis were 47% (95% CI, 26.1%-65.1%) and 39% (95% CI, 18.1%-59.6%), respectively, and 63% (95% CI, 48.4%-75.1%) and 50% (95% CI, 33.6%-64.6%) for those patients without hydronephrosis (Supplementary Fig. E2).
Cox proportional hazards models
Table 2 shows results of univariate analyses using Cox proportional hazards models for OS and DFS. For OS, estimated survival curves did not differ among stages (log-rank test P value = .116). Similarly, DFS curves did not differ by stage (P=.651). There were no statistically significant differences in OS or DFS curves for patients with and without hydronephrosis (log-rank test P values .116 and .560, respectively). Similarly, no differences were found in OS or DFS curves among strata representing tumor grades or other characteristics. For OS and DFS, Cox proportional HR models were formed to examine the joint effects of patient, tumor, and treatment characteristics. Adjusted for tumor size, stage, and hyperthermia, none of the additional characteristics considered added information to the multivariate models of OS or DFS (Table 3).
Table 2.
Results of the univariate analysis for overall survival and disease-free-survival (N=116)
| Overall survival |
Disease-free survival |
|||||
|---|---|---|---|---|---|---|
| Cohort |
Log-rank test |
Hazard ratioy† (95% CI) |
Cohort |
Log-rank test |
Hazard ratioy† (95% CI) |
|
| Covariate | N | P value* | N | P value* | ||
| Hemoglobin: >10 g/dL vs ≤10 g/dL‡ | 113 | .121 | 0.62 (0.333-1.143) | 109 | .268 | 0.65 (0.304-1.397) |
| Hydronephrosis: Yes vs No | 98 | .166 | 1.65 (0.878-3.114) | 95 | .560 | 1.27 (0.571-2.806) |
| Tumor size >6 cm vs ≤6 cm ‡ | 113 | .619 | 0.87 (0.492-1.525) | 109 | .253 | 1.52 (0.738-3.131) |
| Tumor grade | 98 | .766 | 94 | .318 | ||
| Grade 3 | 60 | 0.92 (0.275-3.061) | 59 | 1.49 (0.197-11.240) | ||
| Grade 2 | 31 | 1.16 (0.336-4.003) | 29 | 2.50 (0.325-19.277) | ||
| Grade 1 | 7 | 1.0 | 6 | 1.0 | ||
| Disease stage | 116 | .116 | 112 | .651 | ||
| Stage IVA | 7 | 1.52 (0.288-7.965) | 7 | 2.11 (0.383-11.592) | ||
| Stage IIIA-IIIB | 51 | 2.05 (0.486-8.655) | 48 | 1.06 (0.236-4.744) | ||
| Stage IIB | 48 | 1.02 (0.237-4.430) | 47 | 1.12 (0.252-4.938) | ||
| Stage I | 10 | 1.0 | 10 | 1.0 | ||
| Pelvic wall involvement: bilateral vs unilateral | 35 | .427 | 1.54 (0.526-4.514) | 33 | .129 | 3.46 (0.629-19.003) |
| Radiofrequency hyperthermia: yes vs no | 116 | .537 | 0.84 (0.488-1.453) | 112 | .097 | 1.88 (0.882-4.007) |
| Tandem use: yes vs no | 116 | .473 | 1.25 (0.684-2.265) | 112 | .429 | 0.75 (0.362-1.542) |
| Treatment duration: >10.1 wk vs ≤10.1 wk | 116 | .375 | 1.31 (0.720-2.388) | 112 | .961 | 0.98 (0.483-1.996) |
Abbreviation: CI = confidence interval.
P value for testing the equality of survival distributions among groups.
Univariate Cox proportional hazards regression model.
Median value for all subjects.
Table 3.
Multivariate cox proportional hazards regression model
| Variable | Likelihood ratio P value* |
Hazard ratioy† (95% CI) |
|---|---|---|
| Outcome: Overall survival cohort: n=113 | ||
| Tumor size >6 cm vs ≤6 cm | .517 | 0.83 (0.460-1.480) |
| Disease stage | .182 | |
| Stage IVA | 1.32 (0.228-7.690) | |
| Stage IIIA, IIIB | 2.02 (0.472-8.662) | |
| Stage IIB | 1.05 (0.240-4.594) | |
| Stage I | 1.0 | |
| Radiofrequency hyperthermia administered |
.685 | 0.88 (0.487-1.605) |
| Outcome: Disease-free survival cohort: n=109 | ||
| Tumor size >6 cm vs ≤6 cm | .359 | 1.42 (0.671-3.023) |
| Disease stage | .942 | |
| Stage IVA | 1.19 (0.192-7.428) | |
| Stage IIIA, IIIB | 0.84 (0.184-3.848) | |
| Stage IIB | 1.03 (0.232-4.543) | |
| Stage I | 1.0 | |
| Radiofrequency hyperthermia administered |
.114 | 1.88 (0.838-4.203) |
Abbreviation: CI = confidence interval.
P value for the likelihood ratio test of the null hypothesis that the regression coefficient is zero.
Multivariate Cox proportional hazards regression model.
Complications
Acute toxicities were defined as those occurring between the first HDR-ISBT treatment date to 90 days after the last treatment date, and late toxicities were defined as those that occurred more than 90 days after treatment. Toxicities were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 guidelines (16). The primary types of acute toxicity were grade 1 or 2 gastrointestinal and genitourinary toxicity (41.4% and 44.0%, respectively). Only 1 patient had grade 3 gastrointestinal acute toxicity. There were no grade 4 acute toxicities. Approximately 13% of patients had a grade 3 late toxicity. One patient had a grade 3 colonic stricture. Two patients experienced vaginal soft tissue necrosis; 1 had grade 2, requiring local wound care, and one had grade 3, requiring hyperbaric oxygen treatment (Table 4).
Table 4.
Acute and late toxicities in patients treated with HDR interstitial brachytherapy
| Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
|||||
|---|---|---|---|---|---|---|---|---|
| Toxicity | n | % | n | % | n | % | n | % |
| Acute toxicity | ||||||||
| Genitourinary | 48 | 41 | 3 | 2.6 | 0 | 0 | 0 | 0 |
| Gastrointestinal | 40 | 34 | 9 | 7.8 | 1 | 0.9 | 0 | 0 |
| Late toxicity | ||||||||
| Genitourinary | 7 | 6 | 4 | 3.4 | 5 | 4.3 | 0 | 0 |
| Gastrointestinal | 8 | 6.9 | 9 | 7.8 | 9 | 7.8 | 0 | 0 |
| Vaginal | 0 | 0 | 1 | 0.9 | 1 | 0.9 | 0 | 0 |
| Fistula formation | ||||||||
| Vesicovaginal | 0 | 0 | 2 | 1.7 | 2 | 1.7 | 0 | 0 |
| Rectovaginal | 0 | 0 | 0 | 0 | 1 | 0.9 | 1 | 0.9 |
Abbreviation: HDR = high-dose-rate.
Discussion
BT is central to the successful management of cervical cancer. The advantages of BT are the ability to deliver a maximal dose to the tumor and minimal dose to the surrounding normal tissues. However, results of treatment with EBRT and conventional ICBT in locally advanced cervical cancer remain poor. ICBT is adequate for early stage cervical cancer and in those patients who have good tumor regression with EBRT and chemotherapy. However, it does not deliver an optimal dose in those patients with bulky, locally advanced cervical cancer or in patients with distorted anatomy and poor response to EBRT and chemotherapy.
Much like LDR-ISBT, HDR-ISBT was designed to overcome the limitations of conventional ICBT by improving tumor volume coverage and improving LRC. Data for clinical outcome with LDR-ISBT and HDR-ISBT are limited to small series and are heterogeneous. The paper by Demanes et al (17) is the only published study using a similar HDR-ISBT method. That group delivered 45-50 Gy with standard four-field (midline block after 25-36 Gy depending on tumor stage), followed by two HDR fractions of either 5.5 or 6 Gy per implant. Three implantation procedures were performed, one per week, for a total of 6 HDR fractions. Patients with planned hysterectomy received only 4 HDR fractions. Compared to our results, Demanes et al (17) had a similar 5-year DFS of 48%, a 5 year OS rate of 52%, and an LRC of 81%.
Logsdon and Eifel (3) published the MD Anderson experience with the treatment of FIGO stage IIIB squamous cell carcinoma of the cervix. Of 1096 patients treated with radiation therapy, the 641 patients who received a combination of EBRT and ICBT had a 5-year DFS of 45% compared to 24% for those patients who did not receive ICBT, emphasizing the importance of BT in combination with EBRT for cervical cancer. When adequate ICBT is unfeasible, the tendency to treat these patients palliatively may prove self-defeating without reducing complications. In contrast, many patients with extensive tumors or unsuitable anatomy are treated at LBMMC where ISBT can replace ICBT with results comparable to those with patients treated with a combination of EBRT and ICBT.
In our study, LRC was achieved in 99 of 116 (85.3%) patients. The 5-year DFS rates of 58% (95% CI, 35.5%-75.6%) and 71% (95% CI, 53.0%-83.4%) were achieved for patients with stages IIB and IIIB, respectively, and 29% (95% CI, 1.4%-69.1%) for patients with stage IV disease. The combined grade 3 late complication rate was approximately 15%, with no grade 4 genitourinary complications.
Interestingly, the 5-year DFS was higher for patients with stage IIIB disease than for those with stage IIB disease. Our hypothesis is that the FIGO staging system may incompletely correlate with total tumor burden as some stage IIB lesions have bulkier central and parametrial disease without extending to the pelvic side wall(s). Total stage IIB tumor burden may be greater than some stage III lesions with only unilateral pelvic fixation. Of the 44 patients in this study with stage IIIB cervical cancer, 15 (34.1%) patients had bilateral pelvic side-wall extension.
Additionally, many patients with FIGO stage IBI were referred to our institution for ISBT after comprehensive chemoradiation to the pelvis and difficulty undergoing ICBT because of narrow vagina or extensive disease. The stage assigned to each patient by the referring physician was maintained; therefore, these were not true stage IBI patients. For example, at the time of our pretreatment evaluation prior to BT implantation, we found the central tumor to be much larger (often >4 cm; range, 2-9 cm). Also, during this time period, many patients received surgical staging. For example, 1 patient who was originally staged as IBI had a positive biopsy of parametria and surgical staging of lymph nodes. Therefore, we feel that the 3-year DFS was much lower than expected, as these patients were initially staged, and we did not change the stage to reflect the much higher burden of disease.
Compared with our LDR-ISBT results (5), both LRC and DFS have improved with the current series. We hypothesize that this may be related to increased use of concurrent chemotherapy in 81% vs 24% of the patients in the prior study (5). The LDR-ISBT study used a different toxicity scale, making it difficult to directly compare the outcomes. However, only 2% of patients developed grade 3 bladder complications with severe frequency and hematuria. Eight percent developed late grade 3 and 4 gastrointestinal complications (8 [4%] patients with bowel obstruction and 7 [4%] patients with fistula formation). Most complications occurred in patients (7 of 15 [47%]) who had undergone prior surgical intervention (5).
The present study is limited by the fact that it is a retrospective review of a single-institution experience with relatively short follow-up (mean, 35.1 months (95% CI, 3.8-138.6 months)). While the follow-up period may be shorter in the current series, there is also better CT-based dosimetry and the use of a stepping source for better control over dose distribution.
In most series of irradiation in cervical cancer studies, the success of the ICBT outcome depends on the size of the tumor. In our study, there was no difference in CR achieved among patients with tumor size smaller than the median of 6 cm vs >6 cm. This is further proof of the efficacy of HDR-ISBT and its unique ability to adequately treat bulky disease.
Another limitation of ICBT particularly with larger lesions is its reliance upon sufficient tumor regression to allow the uterus to accommodate an intrauterine tandem. In our study, a tandem was usable in 65.5% of patients during the first implantation and in 66.7% of patients during the second implantation. Contrary to the findings by Viswanathan et al (18) there was no statistically significant difference in OS between the patients in which a tandem was used versus those in which a tandem could not be used due to unsuitable geometry. By placing BT sources directly into the central portion of the tumor despite the absence of the uterine canal to carry the tandem, the technique of ISBT makes the treatment of larger tumors with unsuitable geometry both feasible and successful without a tandem.
The GO technique results in uniform dose distribution in a volume implantation with equal separation of implanted needles and sources. With concentric partial circular implant geometry, GO resulted in slightly (~125%) higher dose in the center, which was preferable. In implants where there was bulky central disease, the dose in the central region of the implant was increased to 150% of the prescription dose. With several implanted needles in place of vaginal ovoids, the source distributions can be tailored to the desired dose distribution, resulting in better target coverage.
Moore et al (19) recently reported a 47.8% incidence of vesicovaginal fistula formation among 23 women treated for stage IVA cervical carcinoma. Eleven of 23 women developed a fistula within an average of 2 months postdiagnosis. Nine of the 11 patients had received HDR-ICBT treatment. That rate of fistulae formation is higher than those of other reports of 4%-22% (20, 21). This may be the necessary tradeoff for the improved CR rates possible with modern chemoradiation techniques (19). However, it may also be related to the much higher central radiation dose inherent in ICBT. Although the numbers are small, only 1 of the 7 stage IVA patients (14%) in our study developed a vesicovaginal fistula. This could possibly be attributed to better control over dose distribution with HDR-ISBT treatment planning (12-14,19-21).
Conclusions
Improved OS compared to the previous experience at LBMMC is probably due to advances in chemotherapy and dosimetric advantages of HDR-ISBT. Patients with locally advanced cervical cancer unsuitable for conventional ICBT can achieve excellent long-term LRC and acceptable morbidity with HDR-ISBT. Traditionally, these patients might not have been treated with curative intent. However, our experience with HDR-ISBT has shown that these patients can also achieve comparable results.
Supplementary Material
Acknowledgment
The authors thank the Tumor Registry staff at Long Beach Memorial Medical Center for their assistance with data collection and the Biostatistical Shared Resource of the Chao Family Comprehensive Cancer Center for assistance with statistical analyses and interpretation of results.
Footnotes
Supplementary material for this article can be found at www.redjournal.org.
Conflict of interest: none.
References
- 1.Fletcher GH, Brown TC, Rutledge FN. Clinical significance of rectal and bladder dose measurements in radium therapy of cancer of the uterine cervix. AJR Am J Roentgenol. 1978;79:421–450. [PubMed] [Google Scholar]
- 2.Villasanta U. Complications of radiotherapy for carcinoma of the uterine cervix. Am J Obstet Gynecol. 1972;114:717–726. doi: 10.1016/0002-9378(72)90892-7. [DOI] [PubMed] [Google Scholar]
- 3.Logsdon MD, Eifel PJ. FIGO IIIB squamous cell carcinoma of the cervix: an analysis of the prognostic factors emphasizing the balance between EBRT and ICBT. Int J Radiat Oncol Biol Phys. 1999;43:763–775. doi: 10.1016/s0360-3016(98)00482-9. [DOI] [PubMed] [Google Scholar]
- 4.Nag S, Martinez-Monge R, Selman AE, et al. ISBT in the management of primary carcinoma of the cervix and vagina. Gynecol Oncol. 1998;70:27–32. doi: 10.1006/gyno.1998.5032. [DOI] [PubMed] [Google Scholar]
- 5.Syed AM, Puthawala AA, Abdelaziz NN, et al. Long-term results of low-dose-rate interstitial-intracavitary brachytherapy in the treatment of carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2002;54:67–78. doi: 10.1016/s0360-3016(02)02900-0. [DOI] [PubMed] [Google Scholar]
- 6.Syed AM, Feder BH. Technique of after-loading interstitial implants. Radiol Clin (Basel) 1977;46:458–475. [PubMed] [Google Scholar]
- 7.Syed AMN, Puthawala AA, Neblett D. Transperineal interstitialintracavitary “Syed-Neblett” application in the treatment of carcinoma of the uterine cervix. Endocurie Hyperthem Oncol. 1998;2:1–13. [Google Scholar]
- 8.Feder B, Syed ANA, Neblett D. Treatment of extensive carcinoma of the cervix with “Transperineal parametrial butterfly”: Preliminary report on the revival of Waterman’s approach. Int J Radiat Oncol Biol Phys. 1978;4:735–742. doi: 10.1016/0360-3016(78)90204-3. [DOI] [PubMed] [Google Scholar]
- 9.Martinez A, Cox RS, Edmunson GK. A multiple-site perineal applicator (MUPIT) for treatment of prostatic, anorectal and gynecological malignancies. Int J Radiat Oncol Biol Phys. 1984;10:297–305. doi: 10.1016/0360-3016(84)90016-6. [DOI] [PubMed] [Google Scholar]
- 10.Bloss JD, Berman ML, Syed AM. Treatment of Advanced Carcinoma of the Uterine Cervix with Interstitial Radiotherapy and Hyperthermia. Endocurie Hyperthem Oncol. 1992;8:145–150. [Google Scholar]
- 11.Syed AM, Neblett DL. Interstitial-intracavitary applicator in the management of gynecologic malignancy. Proceedings of the Pacific Endocurietherapy Society Winter Meeting; Mazatlan, Mexico. December 5-7, 1979. [Google Scholar]
- 12.Disaia PJ, Creasman WT. Clinical gynecology oncology. 5th ed Mosby Year Book; St. Louis: 1997. pp. 51–106. [Google Scholar]
- 13.Dale RG. The application of the linear-quadratic dose effect equation to fractional and protracted radiotherapy. Br J Radiol. 1985;58:515–528. doi: 10.1259/0007-1285-58-690-515. [DOI] [PubMed] [Google Scholar]
- 14.Kalbfleish JD, Prentice RL. The Statistical Analysis of Failure Time Data. John Wiley & Sons; New York: 1980. [Google Scholar]
- 15.International Federation of Gynecology and Obstetrics Staging announcement: FIGO staging of gynecologic cancers; cervical and vulva. Int J Gynecol Cancer. 1995;5:319. [Google Scholar]
- 16.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effect of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 17.Demanes DJ, Rodriguez RR, Bendre DD, et al. High dose rate transperineal interstitial brachytherapy for cervical cancer: high pelvic control and low complication rates. Int J Radiat Oncol Biol Phys. 1999;45:105–112. doi: 10.1016/s0360-3016(99)00124-8. [DOI] [PubMed] [Google Scholar]
- 18.Viswanathan A, Cormack R, Rawal B, et al. Increasing brachy-therapy dose predicts survival for interstitial and tandem-based radiation for stage IIIB cervical cancer. Int J Gynecol Cancer. 2009;19:1402–1406. doi: 10.1111/IGC.0b013e3181b62e73. [DOI] [PubMed] [Google Scholar]
- 19.Moore K, Gold M, McMeekin S, et al. Vesicovaginal fistula formation in patients with stage IVA cervical carcinoma. Gynecol Oncol. 2007;106:498–501. doi: 10.1016/j.ygyno.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 20.Millon RR, Rutledge F, Fletcher GH. Stage IV carcinoma of the cervix with bladder invasion. Am J Obstet Gynecol. 1972;113(2):239–246. doi: 10.1016/0002-9378(72)90773-9. [DOI] [PubMed] [Google Scholar]
- 21.Kramer C, Peschel RE, Goldberg N, et al. Radiation treatment of FIGO stage IVA carcinoma of the cervix. Gynecol Oncol. 1989;32(3):323–326. doi: 10.1016/0090-8258(89)90633-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.