Abstract
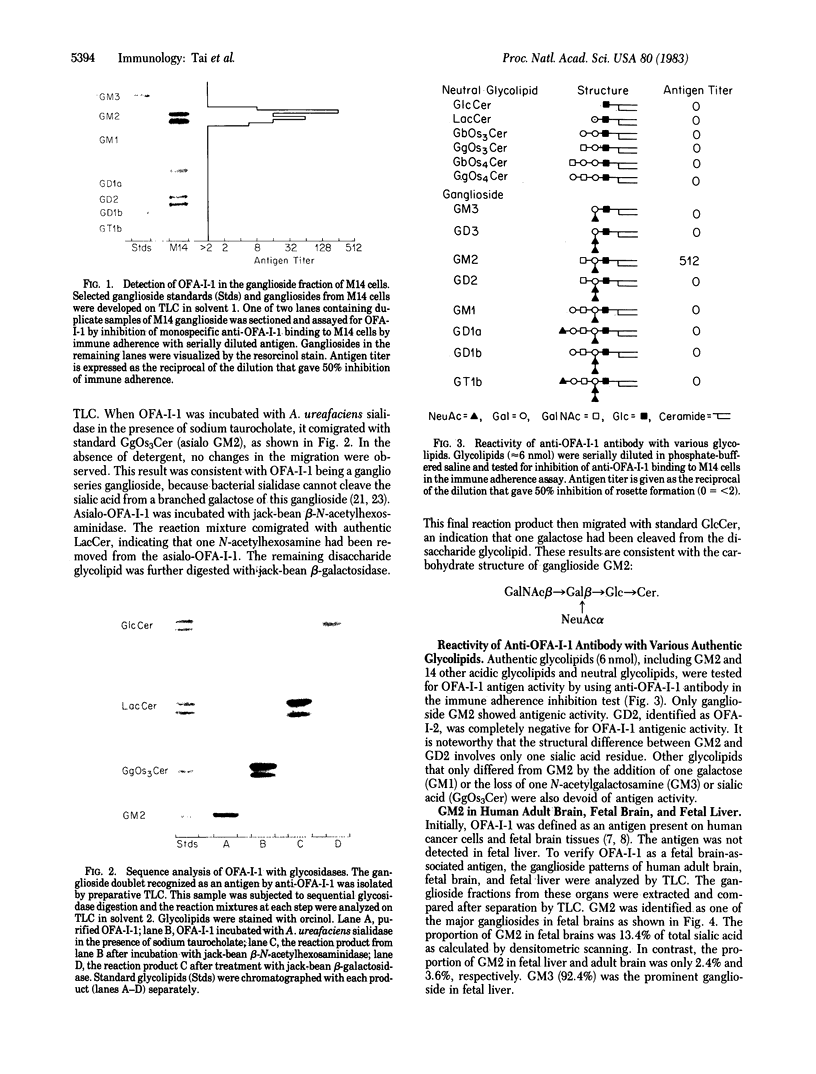
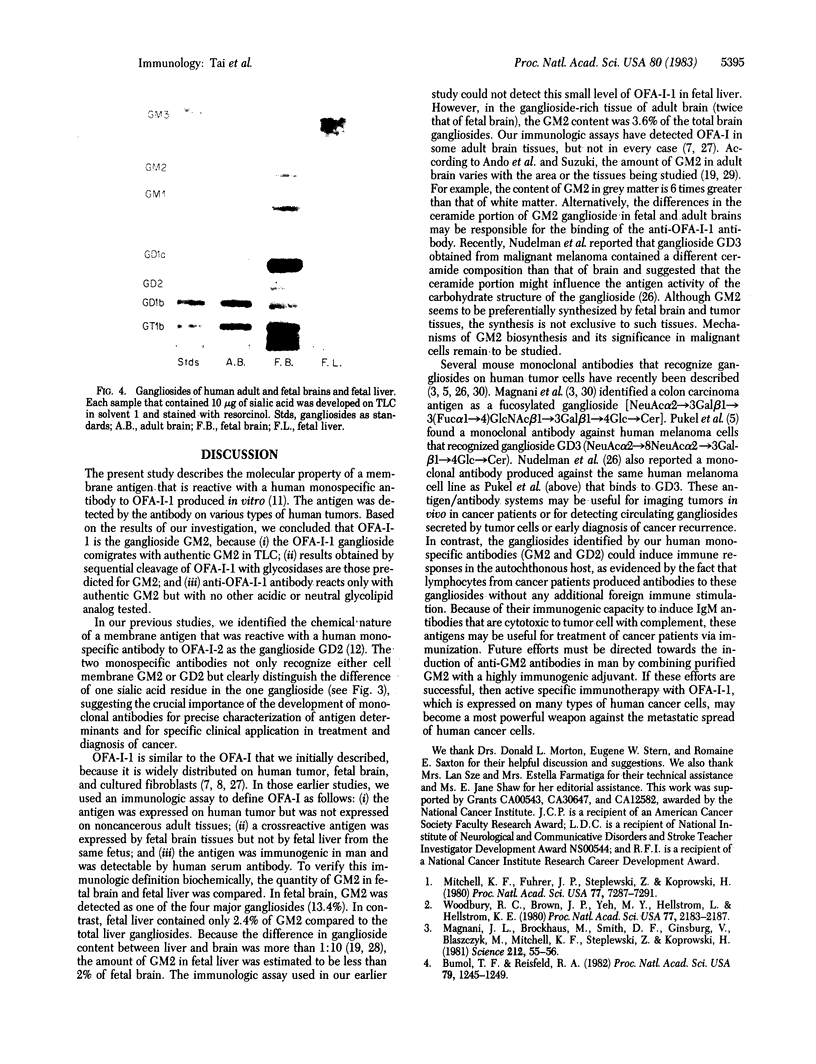
A monospecific antibody produced in vitro by a B-lymphoblastoid cell line transformed with Epstein-Barr virus has been shown to recognize a membrane antigen (OFA-I-1) on human tumors and fetal brain. This study identifies the chemical nature of OFA-I-1. The glycolipid fraction of antigen-rich spent medium of an OFA-I-1-positive melanoma cell line, M14, was extracted by chloroform/methanol/water, 4:8:3 (vol/vol), and was separated into fractions of neutral glycolipids and gangliosides by DEAE-Sephadex followed by base treatment and Bio-sil A column elution. OFA-I-1 antigens were found exclusively in the ganglioside fraction when assayed with monospecific anti-OFA-I-1 by an immune adherence inhibition test. The results obtained from thin-layer chromatography of the antigenic M14 ganglioside and sequential glycosidase digestion suggested that the antigen was a ganglioside GM2, GalNAc beta 1 leads to 4(NeuAc alpha 2 leads to 3)Gal beta 1 leads to 4Glc leads to Cer. These results were further supported by testing various authentic gangliosides and neutral glycolipids for OFA-I-1 antigenicity. Only GM2 showed positive reactivity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando S., Chang N. C., Yu R. K. High-performance thin-layer chromatography and densitometric determination of brain ganglioside compositions of several species. Anal Biochem. 1978 Sep;89(2):437–450. doi: 10.1016/0003-2697(78)90373-1. [DOI] [PubMed] [Google Scholar]
- Bumol T. F., Reisfeld R. A. Unique glycoprotein-proteoglycan complex defined by monoclonal antibody on human melanoma cells. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1245–1249. doi: 10.1073/pnas.79.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahan L. D., Irie R. F., Singh R., Cassidenti A., Paulson J. C. Identification of a human neuroectodermal tumor antigen (OFA-I-2) as ganglioside GD2. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7629–7633. doi: 10.1073/pnas.79.24.7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distler J. J., Jourdian G. W. The purification and properties of beta-galactosidase from bovine testes. J Biol Chem. 1973 Oct 10;248(19):6772–6780. [PubMed] [Google Scholar]
- Irie K., Irie R. F., Morton D. L. Evidence for in vivo reaction of antibody and complement to surface antigens of human cancer cells. Science. 1974 Nov 1;186(4162):454–456. doi: 10.1126/science.186.4162.454. [DOI] [PubMed] [Google Scholar]
- Irie R. F., Giuliano A. E., Morton D. L. Oncofetal antigen: a tumor-associated fetal antigen immunogenic in man. J Natl Cancer Inst. 1979 Aug;63(2):367–373. [PubMed] [Google Scholar]
- Irie R. F., Irie K., Morton D. L. A membrane antigen common to human cancer and fetal brain tissues. Cancer Res. 1976 Sep;36(9 Pt 2):3510–3517. [PubMed] [Google Scholar]
- Irie R. F., Sze L. L., Saxton R. E. Human antibody to OFA-I, a tumor antigen, produced in vitro by Epstein-Barr virus-transformed human B-lymphoid cell lines. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5666–5670. doi: 10.1073/pnas.79.18.5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledeen R. W., Yu R. K. Gangliosides: structure, isolation, and analysis. Methods Enzymol. 1982;83:139–191. doi: 10.1016/0076-6879(82)83012-7. [DOI] [PubMed] [Google Scholar]
- Magnani J. L., Brockhaus M., Smith D. F., Ginsburg V., Blaszczyk M., Mitchell K. F., Steplewski Z., Koprowski H. A monosialoganglioside is a monoclonal antibody-defined antigen of colon carcinoma. Science. 1981 Apr 3;212(4490):55–56. doi: 10.1126/science.7209516. [DOI] [PubMed] [Google Scholar]
- Magnani J. L., Nilsson B., Brockhaus M., Zopf D., Steplewski Z., Koprowski H., Ginsburg V. A monoclonal antibody-defined antigen associated with gastrointestinal cancer is a ganglioside containing sialylated lacto-N-fucopentaose II. J Biol Chem. 1982 Dec 10;257(23):14365–14369. [PubMed] [Google Scholar]
- Mitchell K. F., Fuhrer J. P., Steplewski Z., Koprowski H. Biochemical characterization of human melanoma cell surfaces: dissection with monoclonal antibodies. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7287–7291. doi: 10.1073/pnas.77.12.7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudelman E., Hakomori S., Kannagi R., Levery S., Yeh M. Y., Hellström K. E., Hellström I. Characterization of a human melanoma-associated ganglioside antigen defined by a monoclonal antibody, 4.2. J Biol Chem. 1982 Nov 10;257(21):12752–12756. [PubMed] [Google Scholar]
- Paulson J. C., Rearick J. I., Hill R. L. Enzymatic properties of beta-D-galactoside alpha2 leads to 6 sialytransferase from bovine colostrum. J Biol Chem. 1977 Apr 10;252(7):2363–2371. [PubMed] [Google Scholar]
- Pukel C. S., Lloyd K. O., Travassos L. R., Dippold W. G., Oettgen H. F., Old L. J. GD3, a prominent ganglioside of human melanoma. Detection and characterisation by mouse monoclonal antibody. J Exp Med. 1982 Apr 1;155(4):1133–1147. doi: 10.1084/jem.155.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees W. V., Irie R. F., Morton D. L. Oncofetal antigen-I: distribution in human tumors. J Natl Cancer Inst. 1981 Sep;67(3):557–562. [PubMed] [Google Scholar]
- SVENNERHOLM L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957 Jun;24(3):604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. THE GANGLIOSIDES. J Lipid Res. 1964 Apr;5:145–155. [PubMed] [Google Scholar]
- SVENNERHOLM L. The quantitative estimation of cerebrosides in nervous tissue. J Neurochem. 1956 May;1(1):42–53. doi: 10.1111/j.1471-4159.1956.tb12053.x. [DOI] [PubMed] [Google Scholar]
- Saito M., Sugano K., Nagai Y. Action of Arthrobacter ureafaciens sialidase on sialoglycolipid substrates. Mode of action and highly specific recognition of the oligosaccharide moiety of ganglioside GM1. J Biol Chem. 1979 Aug 25;254(16):7845–7854. [PubMed] [Google Scholar]
- Saito T., Hakomori S. I. Quantitative isolation of total glycosphingolipids from animal cells. J Lipid Res. 1971 Mar;12(2):257–259. [PubMed] [Google Scholar]
- Seyfried T. N., Ando S., Yu R. K. Isolation and characterization of human liver hematoside. J Lipid Res. 1978 Jul;19(5):538–543. [PubMed] [Google Scholar]
- Suzaki K. The pattern of mammalian brain gangliosides. 3. Regional and developmental differences. J Neurochem. 1965 Dec;12(12):969–979. doi: 10.1111/j.1471-4159.1965.tb10256.x. [DOI] [PubMed] [Google Scholar]
- Svennerholm L., Fredman P. A procedure for the quantitative isolation of brain gangliosides. Biochim Biophys Acta. 1980 Jan 18;617(1):97–109. doi: 10.1016/0005-2760(80)90227-1. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Woodbury R. G., Brown J. P., Yeh M. Y., Hellström I., Hellström K. E. Identification of a cell surface protein, p97, in human melanomas and certain other neoplasms. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2183–2187. doi: 10.1073/pnas.77.4.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]