Abstract
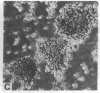
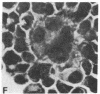
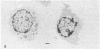
Several isolates of human T-cell leukemia/lymphoma virus (HTLV) were transmitted to normal human T cells obtained from the umbilical cord blood of newborns. T cells from seven specimens were immortalized by infection with different HTLV isolates and their properties were compared with those of activated uninfected normal T cells grown in the presence of T-cell growth factor (TCGF) and with those of HTLV-positive neoplastic T-cell lines derived from patients with T-cell malignancies. The HTLV-infected cells generally belonged to a class of mature T cells (OKT4+ and Leu 3A+) and differed from the normal uninfected cells in that they could be propagated in culture indefinitely; possessed altered morphology, including convoluted nuclei and some bi- and multinucleated giant cells; formed large clumps in culture; demonstrated a diminished requirement for TCGF; had an increased density of TCGF receptors; often became completely independent of exogenous TCGF; and expressed HLA-DR determinants. These properties of the HTLV-infected cord blood T cells contrasted to those of uncultured cord blood T cells and of cord blood cells stimulated with mitogen and grown with TCGF but resembled the characteristics of T-cell lines established previously from patients with HTLV-associated T-cell malignancies. This in vitro system offers a unique opportunity to study the basic mechanism involved in abnormal growth and neoplastic transformation of a specific class of human T cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blattner W. A., Kalyanaraman V. S., Robert-Guroff M., Lister T. A., Galton D. A., Sarin P. S., Crawford M. H., Catovsky D., Greaves M., Gallo R. C. The human type-C retrovirus, HTLV, in Blacks from the Caribbean region, and relationship to adult T-cell leukemia/lymphoma. Int J Cancer. 1982 Sep 15;30(3):257–264. doi: 10.1002/ijc.2910300302. [DOI] [PubMed] [Google Scholar]
- Catovsky D., Greaves M. F., Rose M., Galton D. A., Goolden A. W., McCluskey D. R., White J. M., Lampert I., Bourikas G., Ireland R. Adult T-cell lymphoma-leukaemia in Blacks from the West Indies. Lancet. 1982 Mar 20;1(8273):639–643. doi: 10.1016/s0140-6736(82)92200-0. [DOI] [PubMed] [Google Scholar]
- Gallo R. C., Mann D., Broder S., Ruscetti F. W., Maeda M., Kalyanaraman V. S., Robert-Guroff M., Reitz M. S., Jr Human T-cell leukemia-lymphoma virus (HTLV) is in T but not B lymphocytes from a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5680–5683. doi: 10.1073/pnas.79.18.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootenberg J. E., Ruscetti F. W., Mier J. W., Gazdar A., Gallo R. C. Human cutaneous T cell lymphoma and leukemia cell lines produce and respond to T cell growth factor. J Exp Med. 1981 Nov 1;154(5):1403–1418. doi: 10.1084/jem.154.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Miller S. E., Palker T. J., Moore J. O., Dunn P. H., Bolognesi D. P., Metzgar R. S. Identification of human T cell leukemia virus in a Japanese patient with adult T cell leukemia and cutaneous lymphomatous vasculitis. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2054–2058. doi: 10.1073/pnas.80.7.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman V. S., Sarngadharan M. G., Nakao Y., Ito Y., Aoki T., Gallo R. C. Natural antibodies to the structural core protein (p24) of the human T-cell leukemia (lymphoma) retrovirus found in sera of leukemia patients in Japan. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1653–1657. doi: 10.1073/pnas.79.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman V. S., Sarngadharan M. G., Poiesz B., Ruscetti F. W., Gallo R. C. Immunological properties of a type C retrovirus isolated from cultured human T-lymphoma cells and comparison to other mammalian retroviruses. J Virol. 1981 Jun;38(3):906–915. doi: 10.1128/jvi.38.3.906-915.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman V. S., Sarngadharan M. G., Robert-Guroff M., Miyoshi I., Golde D., Gallo R. C. A new subtype of human T-cell leukemia virus (HTLV-II) associated with a T-cell variant of hairy cell leukemia. Science. 1982 Nov 5;218(4572):571–573. doi: 10.1126/science.6981847. [DOI] [PubMed] [Google Scholar]
- Kramarsky B., Sarkar N. H., Moore D. H. Ultrastructural comparison of a virus from a Rhesus-monkey mammary carcinoma with four oncogenic RNA viruses. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1603–1607. doi: 10.1073/pnas.68.7.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Uchiyama T., Smith K. A., Waldmann T. A., Greene W. C. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor; partial characterization of the receptor. Nature. 1982 Nov 18;300(5889):267–269. doi: 10.1038/300267a0. [DOI] [PubMed] [Google Scholar]
- Manzari V., Gallo R. C., Franchini G., Westin E., Ceccherini-Nelli L., Popovic M., Wong-Staal F. Abundant transcription of a cellular gene in T cells infected with human T-cell leukemia-lymphoma virus. Proc Natl Acad Sci U S A. 1983 Jan;80(1):11–15. doi: 10.1073/pnas.80.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzgar R. S., Bertoglio J., Anderson J. K., Bonnard G. D., Ruscetti F. W. Detection of HLA-DRw (Ia-like) antigens on human T lymphocytes grown in tissue culture. J Immunol. 1979 Mar;122(3):949–953. [PubMed] [Google Scholar]
- Mier J. W., Gallo R. C. Purification and some characteristics of human T-cell growth factor from phytohemagglutinin-stimulated lymphocyte-conditioned media. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6134–6138. doi: 10.1073/pnas.77.10.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi I., Kubonishi I., Yoshimoto S., Akagi T., Ohtsuki Y., Shiraishi Y., Nagata K., Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981 Dec 24;294(5843):770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Mier J. W., Woods A. M., Gallo R. C. T-cell lines established from human T-lymphocytic neoplasias by direct response to T-cell growth factor. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6815–6819. doi: 10.1073/pnas.77.11.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontén J. The relationship between in vitro transformation and tumor formation in vivo. Biochim Biophys Acta. 1976 Dec 23;458(4):397–422. doi: 10.1016/0304-419x(76)90009-3. [DOI] [PubMed] [Google Scholar]
- Popovic M., Reitz M. S., Jr, Sarngadharan M. G., Robert-Guroff M., Kalyanaraman V. S., Nakao Y., Miyoshi I., Minowada J., Yoshida M., Ito Y. The virus of Japanese adult T-cell leukaemia is a member of the human T-cell leukaemia virus group. Nature. 1982 Nov 4;300(5887):63–66. doi: 10.1038/300063a0. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarin P. S., Robert-Gurroff M., Kalyanaraman V. S., Mann D., Minowada J., Gallo R. C. Isolation and transmission of human retrovirus (human t-cell leukemia virus). Science. 1983 Feb 18;219(4586):856–859. doi: 10.1126/science.6600519. [DOI] [PubMed] [Google Scholar]
- Reitz M. S., Jr, Poiesz B. J., Ruscetti F. W., Gallo R. C. Characterization and distribution of nucleic acid sequences of a novel type C retrovirus isolated from neoplastic human T lymphocytes. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1887–1891. doi: 10.1073/pnas.78.3.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Guroff M., Nakao Y., Notake K., Ito Y., Sliski A., Gallo R. C. Natural antibodies to human retrovirus HTLV in a cluster of Japanese patients with adult T cell leukemia. Science. 1982 Feb 19;215(4535):975–978. doi: 10.1126/science.6760397. [DOI] [PubMed] [Google Scholar]
- Robert-Guroff M., Ruscetti F. W., Posner L. E., Poiesz B. J., Gallo R. C. Detection of the human T cell lymphoma virus p19 in cells of some patients with cutaneous T cell lymphoma and leukemia using a monoclonal antibody. J Exp Med. 1981 Dec 1;154(6):1957–1964. doi: 10.1084/jem.154.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti F. W., Mier J. W., Gallo R. C. Human T-cell growth factor: parameters for production. J Supramol Struct. 1980;13(2):229–241. doi: 10.1002/jss.400130211. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Okada M., Koyanagi Y., Kannagi M., Hinuma Y. Transformation of human leukocytes by cocultivation with an adult T cell leukemia virus producer cell line. Science. 1982 Aug 20;217(4561):737–739. doi: 10.1126/science.6980467. [DOI] [PubMed] [Google Scholar]
- Yokoi T., Miyawaki T., Yachie A., Ohzeki S., Taniguchi N. Discrepancy in expression ability of Tac antigen and Ia determinants defined by monoclonal antibodies on activated or cultured cord blood T lymphocytes. J Immunol. 1982 Oct;129(4):1441–1445. [PubMed] [Google Scholar]
- Yoshida M., Miyoshi I., Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]