Abstract
By wrapping a ligand-functionalized lipid membrane around a silica core, nanoparticles with a fluid surface are created. These combine unprecedented specificity in binding to cancer cells with the combinatorial delivery of drug cocktails.
Nanoparticles capable of specifically binding to target cells and delivering high doses of therapeutic compounds are much sought after in materials science applied to medicine. Such materials could be transforming for cancer chemotherapy and a variety of other diseases, by making therapeutic delivery into disease cells more efficient while lowering the exposure of healthy tissue to toxic side effects1,2 The complexity of this engineering challenge can be appreciated by considering some of the properties an ideal targeted nanoparticle drug carrier would exhibit, including the capability of carrying high levels of multiple, diverse molecular cargos, circulating in the blood for extended periods without elimination by the immune system and binding only to target cells while avoiding other cells. A number of nanoparticle-based therapeutics are now in use in the clinic, many of which are based on liposomes — vesicles comprising a lipid bilayer surrounding an aqueous core — which have low immunogenicity, excellent safety profiles in humans and established approaches for clinical-scale manufacturing3. However, no targeted nanoparticle system has yet been approved by the US Food and Drug Administration [AU: correct?], reflecting in part the complexity of designing particles that can meet all of the design criteria of targeted drug delivery. Attacking this problem head on, Ashley et al.4 describe in Nature Materials a new type of composite nanoparticle — a hybrid between liposomes and nanoporous silica nanoparticles. The properties engineered into this system elegantly synergize to approach the goal of an ideal targeted-delivery agent.
Despite their attractive features, the physical properties of liposomes needed to obtain efficient drug loading and stable entrapment of cargo compounds may be in conflict with the properties of carriers that would be optimal for targeted drug delivery. Targeted delivery is achieved by functionalizing the surface of nanoparticles with ligands (proteins, nucleic acids or small molecules) that will bind to specific molecules on target cells while avoiding nonspecific binding to other cells, blood or tissue components (Fig. 1; ref. 2). It has been shown how decoration of particles with multiple copies of such targeting ligands can increase the avidity of particles for target-cell binding, via the cooperative nature of multivalent binding to cell-surface receptors5-7. Unfortunately, nanoparticles with many identical copies of a molecular motif displayed on their surface structurally resemble viruses, and the immune system has evolved to strongly respond to such repeat chemical patterns. (In fact, nanoparticles displaying many copies of an antigen make excellent vaccines8.) Thus, increasing the density of targeting ligands raises the concern that an immune response will be raised against the carrier. High-avidity binding to target cells could be achieved with lower densities of targeting ligand (provided that these ligands were mobile on the particle surface) capable of clustering to bind multivalently to cell- surface receptors (Fig. 1). Liposomes are uniquely well-suited to provide this kind of mobile ligand display: phospholipid bilayers can either exist in a gel phase, where the lipids are essentially arrested with little two-dimensional mobility in the plane of the bilayer, or a fluid phase, where the lipids diffuse freely in the plane of the membrane. Targeting ligands anchored at a low surface density to the lipids of fluid-phase liposomes could thus readily achieve high-avidity binding via clustering, in theory. But here enters the design challenge: the liposomal compositions most favoured for drug delivery are generally rigid gel-phase vesicles, because fluid-phase liposomes have membranes that are poorly stable in the presence of blood components and are too permeable to be stably loaded with high levels of drug cargos3.
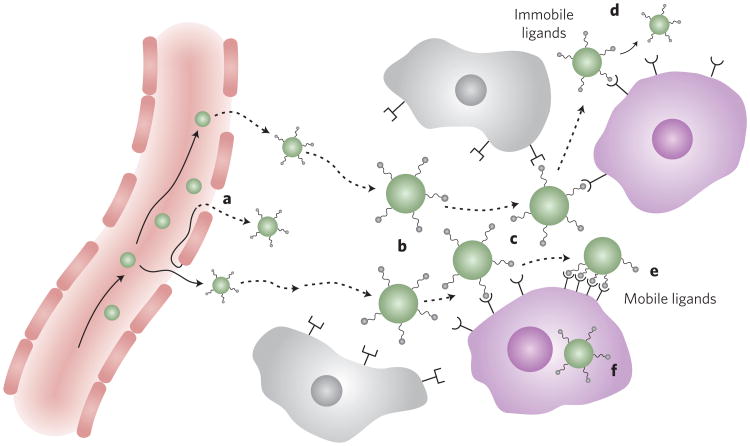
Figure 1.
A schematic outlining multivalent targeting in nanoparticle drug delivery. Drug-loaded nanoparticles carrying therapeutics to tumour sites undergo a multistep process to achieve their therapeutic goal, beginning with extravasation from leaky tumour vessels (a), diffusion past non-target cells lacking receptors for the targeting ligand (b) and initial binding to receptors on target (for example, tumour) cells (c). Particles carrying low densities of targeting ligands on their surface are less likely to elicit an immune response or bind non-specifically to non-target cells. However, if low-density ligands are immobile on the two-dimensional surface of the particle, multivalent binding to the target cell may not be possible and the lifetime of binding to the target cell may be too short to achieve internalization (d). In contrast, when ligands can diffuse over the two-dimensional particle surface, initial binding to targets can be followed by cooperative engagement of additional receptors as ligands diffuse into the particle/cell interface (e). This can lead to near-irreversible binding and rapid particle internalization for drug delivery into the cell (f).
Ashley et al. report a solution to this challenge by wrapping fluid-phase lipid membranes around a drug-loaded nanoporous silica core to create hybrid particles they dub ‘protocells’ (Fig. 2). They demonstrate that the lipid membranes of these particles retain both high in-plane two-dimensional fluidity and high stability against destabilization in the presence of blood components or leakage of drug cargos from the silica core. This ideal combination of properties is proposed to arise from gradients in adhesion and membrane tension developed in the bilayer as it is stretched over alternating nanoscale patches of silica and pure water at the surface of the nanoporous silica core. The result is both an increase in relative two-dimensional fluidity of the membranes and a suppression of nanoscale fluctuations in the bilayer topology, compared with liposomes with the same membrane composition. These factors combine to allow ligand-decorated protocells to achieve avid binding to target cells with low densities of targeting ligand, conditions where off-target binding is also lowered and immunogenicity of the particle is minimized. Ashley et al. impressively show that protocells have 100-fold greater specificity in target-cell binding than equivalent fluid-phase liposomes, suggesting that the suppression of bilayer fluctuations by the protocells' structure may be a key part of their efficient high-avidity binding to cells.
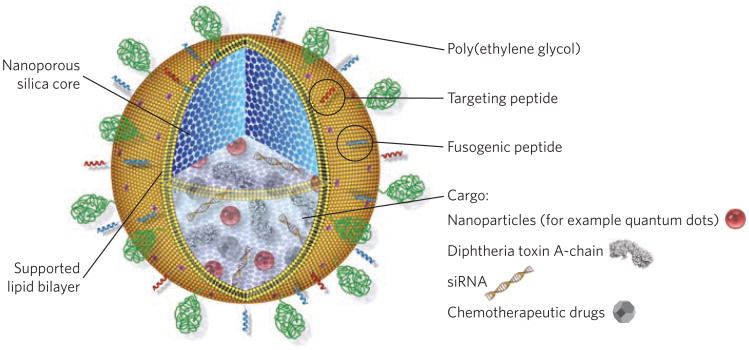
Figure 2.
The design of lipid-bilayer-wrapped nanoporous silica, termed protocells. Nanoporous silica cores are loaded with multivariate drug cargos by adsorption to the silica matrix. The drug-loaded core is then enveloped by a single lipid bilayer, which is further functionalized with poly(ethylene glycol) (to reduce non-specific interactions with its environment), peptides (to direct binding to distinct target cells) and pH-responsive peptides, which cause disruption of endosomes and the bilayer coating on particle internalization into acidic intracellular compartments, allowing drug delivery into the cytosol of the target cell. Figure adapted from ref. 4.
Another key issue in cancer drug delivery is that combinations of drugs are used clinically to maximize therapeutic efficacy while minimizing the potential for drug resistance. However, delivery of drug cocktails within liposomes remains a challenge, particularly for drug combinations with disparate physical properties such as charge, polarity and molecular weight. Here, the nanoporous silica core in the protocells plays a second key role (Fig. 2). The protocells can adsorb combinations of diverse drug cargos (quantum dots, small molecules and oligonucleotides) into their nanoporous silica cores, with reversible binding to the silica providing the means to soak up high levels of each drug cargo before enveloping the particle with its lipid shell. Strikingly, adsorption-mediated drug loading in the particle cores led to 1,000-fold greater doses of the common chemotherapy drug doxorubicin on a per-particle basis, compared with liposomes prepared by clinically employed methods. Silica binding probably also contributes to stable retention of these drug cargos following particle synthesis. It is then shown that these combinatorial cargos can be efficiently delivered into the cytosol or nucleus of target cells ‘on cue’ following internalization, by including low densities of an endosome-disrupting peptide in the surface bilayer along with their targeting ligand.
Putting these synergistic features of enhanced specificity in targeted cell binding and combinatorial high-level drug loading together, Ashley et al.4 finally demonstrate that targeted protocells carrying a cocktail of doxorubicin along with two other potent chemotherapeutics (5-fluorouracil and cisplatin) are so potent that a single particle on average per cell is sufficient to kill a model hepatic carcinoma cell; the efficient targeting allows these cells to be eliminated in cell culture under conditions that leave control healthy liver cells with >90% viability.
The experiments presented here are all in vitro and the degree to which this hybrid platform for drug delivery can advance therapeutic efficacy will need to be tested in animal models, where the complexity of events leading up to encounter of a nanoparticle with its target cell may have a significant impact on the ultimate outcome. If technically sound in vivo, it will also remain to be seen if particles with a large number of individual components as used here (PEGylation, targeting ligands, fusogenic peptides and multiple drug cargos) can be successfully translated to the clinic, from a manufacturing and intellectual-property perspective. However, this work offers the tantalizing prospect of taking highly potent yet toxic chemotherapy cocktails in use in the clinic and better focusing their action on the malignant cells for which they were intended.
References
- 1.Davis ME, Chen ZG, Shin DM. Nat Rev Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 2.Farokhzad OC, Langer R. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 3.Torchilin VP. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 4.Ashley CE, et al. Nature Mater. 2011;10:xxx–xxx. [Google Scholar]
- 5.Basha S, et al. Proc Natl Acad Sci U S A. 2006;103:13509–13513. doi: 10.1073/pnas.0509870103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho K, Lapitsky Y, Shi M, Shoichet MS. Soft Matter. 2009;5:1074–1080. [Google Scholar]
- 7.Poon Z, et al. Angew Chem Int Ed Engl. 2010;49:7266–7270. doi: 10.1002/anie.201003445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taneichi M, et al. J Immunol. 2006;177:2324–2330. doi: 10.4049/jimmunol.177.4.2324. [DOI] [PubMed] [Google Scholar]