Abstract
Administration of glucans through immersion, dietary inclusion or injection has been found to enhance many types of immune responses, resistance to bacterial and viral infections and to environmental stress in many fish species. Although the efficacy of the glucan varies with types and administration, glucan used as an immunomodulatory and mostly immunostimulatory additive has been found satisfactory in eliciting immunity in commercial aquaculture. Development of more efficient administration methods will facilitate the routine and prophylactic use of glucans as natural immunostimulants of fish. Using a PubMed search, this review has an extensive literature on glucan in fish immunity.
Keywords: Aquaculture, Fish, Glucan, Immunity, Immunostimulation, Vaccination
Introduction
β-D-glucans (hereafter referred to as “glucans”) represent part of a group of physiologically active compounds generally called “biological response modifiers.” They are highly conserved carbohydrates forming structural components of cell walls of some plants, fungi, yeast, seaweed and bacteria. Generally, glucan represents a group of chemically heterogeneous polysaccharides existing in various numbers of molecules bound together in several forms of linkage together with several forms and degrees of branching.
Glucans have a long history as natural immunomodulators. The first reports showing that some infectious diseases can have therapeutic effects on malignant processes can be found almost two hundred years ago.[1] These studies were later followed by studies of the immunomodulating properties of lipopolysaccharide (LPS). The problems with toxicity of LPS were dismissed as a result of later investigations showing that a saccharidic moiety of LPS is non-toxic but is responsible for the immunomodulating activity.[2]
The history of glucan began app. 60 years ago with two different starting points—one originated in Europe and the United States and the second in Japan. Based on historical use, the Japanese groups investigated mushroom-derived glucans, whereas the European and American groups focused on yeast-derived glucans.
The first studies showed that glucan application significantly stimulated the phagocytic system and enhanced general defense and resistance to experimental tumors. During subsequent decades of intensive research by laboratories around the world, glucans were found to significantly stimulate defense reactions against infections and cancer.[3,4] In addition, several additional effects were later shown. These included reduction of stress,[5] hypoglycemic effects, lowering cholesterol,[6] reduction of cytotoxic effects[7] and improving diseases such as ulcerative colitis.[8] Another advantage of using glucan as a stimulator of immune reactions is the fact that it has been shown to act in all species tested so far, starting with earthworms and ending with humans.[9]
Immunity of fish
As in all gnathostomeans, there are two types of immunity in fish: The innate and the adaptive. The cellular component of innate immunity represents various phagocytic cell types such as the evolutionarily ancient macrophages, and natural killer (NK) cells. In fish, there were two types of NK cell homologues: Non-specific cytotoxic cells and NK-like cells.[10] Blood leukocytes primarily form further cellular component of innate immunity. They produce a row of humoral substances, mainly cationic antimicrobial peptides, complement components, lectins, cytokines, anti-inflammatory immune mediators like IL (interleukin)-10, TGF-β and many others that are able to kill immediately altered and foreign (allogeneic or xenogeneic) cells. These substances are released into body fluids and epithelial and skin mucus.[11] These innate mechanisms are well developed in bony fish but in some cases, especially in aquacultures where it is a higher probability of spreading infectious diseases, they are not adequate without some external stimulation.
The major organs of fish adaptive immunity are the thymus, kidney, spleen and GALT (gut-associated lymphatic tissue). Conversely, to more evolutionary-advanced vertebrate taxa, they do not possess bone marrow and lymph nodes.[12] The fish thymus is the first lymphoid organ appearing in ontogeny. Structural anlage of thymus is immediately colonized by lymphoid cells. During early ontogeny, the thymus develops in an organ with the epithelial/reticular stroma, which forms a framework for thymocytes and macrophages and other accessory cells. In comparison to tetrapod thymus, fish thymic tissue is less differentiated. In many fish species, the cortex and medulla are lacking, even if in some species the middle and inner zones of thymus tightly resemble the clearly differentiated cortex and the medulla of advanced vertebrates. It also underlies histopathological degeneration during ageing.[13]
The kidney is a paired organ consisting of pronephros and mesonephros, both hemolymphopoietic, comparable to bone marrow of tetrapods. The pronefros first become erythroid. Later, the aggregates of lymphoid cells could be found among urinary tubules. In addition to these functions, fish kidney represents an immunocompetent organ with strong phagocytic capacity where antigen processing and antibody formation take place.[14] Similar to the situation in spleen, the plasmacytes, lymphocytes, monocytes, granulocytes and non-specific natural cytotoxic cells, can be found in kidney. Fish lack germinal centers but the architecture of kidney tissue ensures a suitable micromilieu for immune processes from phagocytosis, antigen presentation up to efficient humoral immune response. The kidney and particularly the well-developed GALT serve as analogs of bone marrow.[15] Antigen trapping and processing also takes place within the special structures, the ellipsoid sheets, which are described in most bony fish. It is believed that they could represent analogs or evolutionary predecessors of germinal centers of mammals.[16]
In fish species with well-developed alimentary tract, the aggregations of lymphoid cells are often present in connective tissue of the mucosa. Lymphoid cells infiltrating gut epithelia and lamina propria form clusters but are never structurally organized like Peyer's patches found in the mammalian GALT. On the other hand, the fish perienteral lymphoid tissue plays a similar role as an effective immunological barrier. In more advanced teleosts, aggregates of lymphocytes, plasmacytes, granulocytes, and macrophages occur in and under the intestinal epithelium. Together with the epithelial cells, these accumulations may form a microenvironment for food antigen collecting such as M cells.[17] So the fish GALT could functionally serve as the gut barrier of mammals. Authors seeking more information on gastrointestinal microbiota in fish should see an excellent review by.[18]
Clusters of lymphoid cells and antibody-forming cells were also identified in some other regions where the potential pathogens may invade and are phagocytized. Such predominant locations include the skin epidermis, gills and pharynx, heart, liver, and pancreas. Generally, in all above mentioned organs and tissues, the plasma cells and lymphocytes are ultrastructurally similar to those of ectotherms and endotherms.[19] The B and T cell dichotomy in fish has been documented[20] but in contrast to B cells, fish T cells are Ig- cells. The discovery of TR genes confirmed definitely the occurrence of conventional T cells in fish. Several key T cell markers as well as the typical cytokines produced by different Th subpopulations resembling the Th1, Th2 and Th17 of mammals were described. On the other hand, particular sub-populations of gut intra-epithelial lymphocytes seem to be different.[21] New studies document that the diversity of fish naive TCRβ expressed by CD8(+) and CD8(-) αβ T cells may regulated by different regulatory mechanisms than in mammals.
True bony fish (Teleostei) are the most primitive bony vertebrates containing genes for the molecules of immunoglobulin superfamily, i.e., TCRα/β TCRγ/δ, β2-microglobulin, MHC I class and MHC II class, and RAG1 (recombination activation gene 1).[22] Vβ, Dβ, Jβ, and Cβ regions are present, from which Vβ and Cβ are comparable to those of more advanced vertebrates. It was suggested that fish TCR may be close in shape to the ancestral molecule.
Teleostean B-cell subsets express either both IgM and IgD[23] or only recently discovered IgT[24] also called IgZ.[25] In contrast to endothermic vertebrates, fish are devoid of IgA.[26] IgM is regarded as the main molecule bearing antibody activity[27] but it usually is a tetramer. IgM and IgT are never co-expressed by the same B cell, which identifies two distinct lineages of B cells in fish. It appears that IgT acts like an intestinal mucosal antibody against some parasites, whereas IgM antibodies are acting mainly in the serum. In addition to immunoglobulins, the non-specific factors like (hemo) lysins, (hem) agglutinins (lectins), lytic enzymes, lysozyme, C-reactive protein, antibacterial peptides, and complement are also present. In some species, immunization results in the formation of specific antibodies not only in the serum, but also in skin and gill mucus and in the gut lamina propria.
Aquacultures and protecting against infection
For nearly 4,000 years, the farming of fish in aquacultures has been a source of human food. Aquaculture production in the past decades represents the increased importance in the food supply for a continually growing world population. At present, fish farming is the fastest growing agricultural industry. Aquacultures have expanded globally with an increase not only in absolute production (kilotons/year) but also in the number of fish species being cultured in both freshwater and marine systems. The most important farmed fish species are carp, salmon, tilapia and catfish.
On the other hand, intensification and rapid increase in aquaculture activities world-wide has provided new opportunities for the emergence and transmission of aquatic pathogenic microorganisms, both bacterial and viral.[28] The specific diseases caused by these etiological agents represent a significant limiting factor for fish aquaculture farming. Readers seeking more information on the current state of aquaculture should read the latest comprehensive.[29]
Therefore, commercial aquacultures are not only dependent on good water quality. The achievement in preventing disease outbreak and restriction of its spreading is at least of equal importance. The use of vaccines, antibiotics, and non-specific immunostimulants are three possible methods of farmed fish protection.
Efficient vaccines were developed against only several bacterial pathogens at the end of the 20th century[30,31] but no effective vaccines are available against a row of other bacterial diseases and most particularly against viral diseases.
The use of antibiotics must be considerably reduced, primarily to avoid environmental hazards and the spread of antibiotic-resistant genes. In addition, some antibiotics may suppress fish immune responses.[32]
When fish are attacked by a pathogenic microorganism, the non-specific mechanisms of natural immunity are more important than the specific response.[33] The non-specific defense in fish is similar to other vertebrates that are endowed by many elements such as phagocytic cells, granulocytes and humoral factors as complement, lysozyme, C-reactive protein, interferon and transferrin.[34] A rapid defense response through the use of antimicrobial cationic peptides and other natural immune factors is much more efficient than prolonged and relatively slow production of specific antibodies[35] that are characteristic of these cold blooded animals.
Effects of glucan on bacterial diseases
Alternative strategies to vaccination and use of antibiotics represent applications of various immunostimulatory substances as dietary supplements. Although little is known about the mechanism of their action in fish, some of them appear to enhance the non-specific killing of pathogenic microbes.[36]
At present, non-specific immunostimulants represent the primary tools in modern fish farming. They induce and enhance resistance against bacterial and viral infectious diseases by stimulating innate humoral and cellular defense mechanisms. The stimulatory action of a row of structurally non-related substances has been studied for their suitability to prevent infections in aquacultures.[37,38,39]
Several types of immunostimulants have been used in fish cultures to induce protection against a wide range of diseases. Various compounds are being added to feed, based on their possible role as immunostimulants. During the study of their prophylactic effects, of these substances the β-glucans appeared to be the most convenient for applications in aquacultures.[40,41] Therefore, in recent years, attention has focused primarily on possible immune stimulation in farmed fish by the use of β-glucans. To date, numerous studies confirming the potent immunostimulatory properties of β-glucans in many fresh and seawater fish species documenting the effects of β-glucans on the pathogen resistance, protection, survival,[42,43] and fish specific humoral immunity[44] have been published. The most successful one is glucan. It is, therefore, not surprising that feed containing glucan is routinely manufactured for commercial fisheries. The most common brands are MacroGard, Vetregard and EcoActiva.[45]
The main fish species studied were rainbow trout (Oncorhynchus mykiss).[43,46,47] African catfish[48] Channel catfish (Clarias-Gariepinus),[42] Atlantic salmon (Salmo salar L.),[49] Indian major carp (Labeo rohita),[50] turbot (Scophthalmus maximus L.),[51] pink snapper (Pagrus auratus),[52] sea bass (Dicentrarchus labrax),[53,54,55] red tail black shark (Epalzeorhynchus bicolor),[56] fathead minnows Pimephales promelas Rafinesque 1820),[57] atlantic cod (Gahus morhua L.),[58] gilthead seabream (Sparus aurata),[59] large yellow croaker (Pseudosciaena crocea),[60] carp (Cyprinus carpio),[44,61] nile tilapia (Oreochromis niloticus),[43,61] and zebrafish (Danio rerio).[62]
Administration of glucan in carp enhanced survival, most likely via stimulation of both non-specific and specific immune reactions (superoxide anion, IL-1 secretion and antibody formation), regardless of how it was administered (intraperitoneal injection, bathing and oral administration).[44] A stimulation of complement and C reactive protein responses were found in carp.[63,64] Studies of glucan-activated macrophages in trout revealed an increased ability to kill salmonid pathogen Aeromonas salmonicida, despite its virulency.[46] A more detailed study using radioactively labeled glucan showed the transfer of this material through the intestine via the epithelial cells in the lower part of the intestine. The material was later cleared from blood.[65] The exact mechanisms of glucan action on anti-infective immunity are not fully established. Recent observations suggest the role of neutrophil extracellular traps.[66]
Improvements in the composition of vaccine used in fish are still necessary. Based on the known strong effects of glucans on fish immunity, their use as part of the vaccination process is not surprising. Early studies showed that the addition of glucan to a vaccine resulted in non-specific resistance against vibriosis and yersiniosis in salmon.[67] These early studies primarily used injected glucan, and in addition to protection against infection, glucan also increased production of cytokines, complement and lysozyme production and antibody formation.[37] The direct addition of glucan into the feed resulted in reduction of mortalities caused by infections with salmon anaemia virus and Piscirickettsia salmonis. In addition, lower attachment of sea lice to fish was also observed.[68]
Similar effects were found in infection with A. salmonicida.[69] The addition of glucan to the Aeromonas vaccine significantly increased the production of antibodies in all antigens tested,[70] however, even the elevated level of antibodies did not offer sufficient protection against Aeromonas infection.
Detailed studies of the adjuvant effects of glucan were done using vaccine against furunculosis. In all cases, vaccines enforced with glucan induced significantly stronger protection of salmon, as measured by serum antibodies levels against four different parts of the A. salmonicida.[49,71] A model using Flexibacter columnaris infection of trout also revealed a strong protection against mortality.[72]
Other authors tested the effects of vaccine VYS-2, a protein fraction of Aeromonas, with and without glucan and showed that glucan increased the protective effects in carp.[73] Vibrio damsela vaccine containing glucan was successfully used in turbot[51] and the feeding of glucan with bactericin Edwardsiella tarda showed strong protection in Japanese flounder (Paralichthys olivaceus).[74]
A different approach was used by.[50] These authors fed Indian major carp with glucan for 30 days followed by vaccination against E. tarda. The results showed that this combination strongly increased the specific immunity and reduced mortality in immunocompromised animals. Of the four tested, (glucan, levamisole, vitamin C, and vitamin E), the glucan was the most effective substance. A series of papers tested the effects of glucan in conjunction with vitamin C. Use of this combination following vaccination can serve as an example. The combination increased the activity of macrophages and specific antibody response.[75] Another study used a different feeding period to address the possibility that longer exposure to glucan might lead to exhausting the natural defense. All these studies led to the conclusion that glucan represents an ideal immunostimulant in the fish industry.[39]
However, not all studies were successful, Glucan with vaccine against Streptococcus bactericin was not effective in turbot[76] and glucan with vaccine against S. iniade had no effect on the Oreochromic niloticus model.[77] Trials with commercial glucan vaccine VitaStim Taito showed no effects.[78] Readers seeking more information on the role of vaccines in fish immunity should refer to[79] a comparative study of 8 different glucans found the only 1-3,1-6 β-glucans caused significant protection against A. hydrophila infection.[80] On the other hand, a comparative study of several different immunostimulators found that only glucan offered protection against white spot disease.[81]
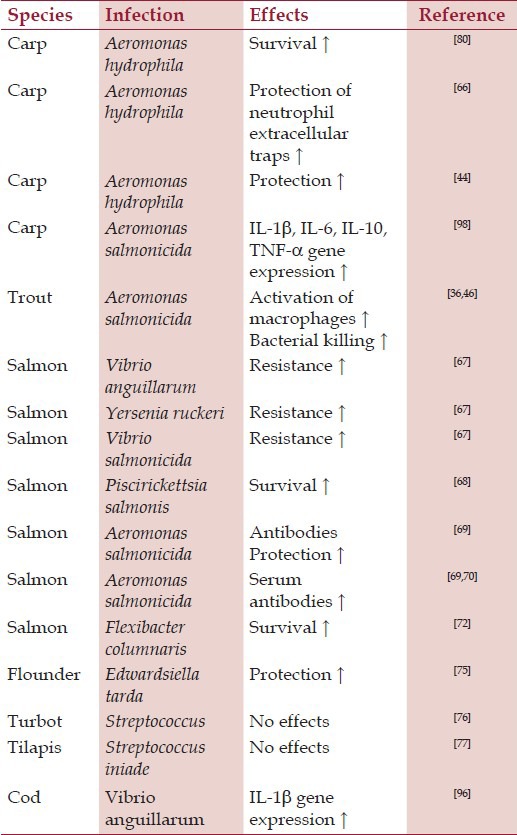
The effects of β-glucans on bacterial infections are summarized in Table 1. It is clear that the use of glucan as part of vaccines in fish is despite decades of research, still far from conclusive. This might be explained as being the result of confusion in both delivery (oral or injected administration) and dosing. The current massive use of glucan in commercial farming therefore focuses more on general stimulation of immune response than on possible adjuvant effects.
Table 1.
The most important effects of glucan on bacterial infections
Effects of glucan on viral and parasitic diseases
Main viral diseases affecting bony fish in aquacultures can occur immediately in the overwhelming majority of farmed fish.[82] Moreover, the more virulent viruses resulting in hemorrhages, ascites, and death are prone to spread globally between countries by wild fish and transmitted into new fish species [Table 1].
International fish markets and fish transported all over the world are often considered to be an even bigger threat. Antiviral vaccines have been constructed against a very limited number of pathogenic viruses. However, due to on-going emergence of new viral diseases, it is necessary to develop new vaccines.
On the contrary, the farmers must be aware that vaccines can only reduce morbidity and mortality but they do not obviate excretion of the virus or its spread. Again, this is a compelling reason to devote more research effort to study protective mechanisms of immunostimulants.
In relation to the fish production in aquacultures, viral disease control remains an important challenge. However, relatively little is known about what the producers of farmed fish can do for prevention and treatment of viral infections and determining fish defense mechanisms. Controlling the spread of viral infections in aquacultures is not sufficient due to the lack of effective vaccines commercially available and from the absence of specific therapeutics. Understanding complex interactions between environment, fish organism and pathogen also appears to be a necessary avenue to prevent disease. In the long term, alternative treatments using antiviral drugs may be developed, but the most effective way for sustainable aquaculture production relies on the production of selected animals for disease resistance and the application of immunostimulative substances from which the β-glucans seem to be the most efficient.
Since, the middle of the last century, when the first fish cell lines were established,[83,84] the viral origin of previously known fish disease such as Oregon sockeye disease (the first reported epidemics of infectious hematopoietic necrosis virus occurred in the United States at the Washington and the Oregon fish hatcheries during the 1950s)[85] and infectious pancreatic necrosis caused by birnavirus in Canada) were documented.[86] Recent study showed that in addition to bacterial infections, glucan-enhanced feed also strongly stimulated the defense reactions against viral hemorrhagic septicemia.[87]
The effects of glucan on parasitic infections are again less studied than on bacterial infections. Studies on hematology of carp infected with ectoparasites showed that feed containing 0.3% of glucan increase the hematocrite and red blood cells, neutrohils and monocyte counts and decreased the number of lymphocytes. At the same time, the survival rate increased from 77-91%.[88] The study using southern bluefin tuna Thunnus maccoyii focused more on physiological effects, but showed improved parasite prevalence after feeding with glucan.[89]
Additional studies focused on resistance of spotted rose snapper Lutjanus guttatus against dactylogyrids. Five weeks of feeding with feed including 0.05% glucan significantly reduced the number of dactylogyrids. Several parameters such as white blood counts, percentage of neutrophils, eosinophils and thrombocytes were also observed, but the connection is unclear.[90]
The last important study focused on rainbow trout and skin-parasitic ciliate Ichthyophthirius multifiliis. Effects of glucan isolated from Euglena gracilis were dose-dependent, but clearly lowered the number of trophonts. At the same time, lysozyme activity was elevated. The trend for upregulation of some important genes was not significant.[91]
Mechanisms of glucan action
In addition to direct stimulation of both specific and non-specific immunity, glucan can also influence expression of immune-related genes and proteins. Macrophages from Atlantic salmon and rainbow trout showed elevated levels of cytokines, but not C3.[92] Similar data were found in plasma of tilapia.[93]
The effects of glucan on gene expression are rapid and do not need long exposure. Four 45 min submersions in glucan each week caused enhanced gene expression of IL-1β, TNF-α, IL-6, IL-10 and TGF-β, sometimes even after first submersion.[94] Carp treated with glucan for 15 days, followed by injection with hemorrhage virus, showed elevated MX gene expression during early stages of infection.[95] A similar experimental model using Atlantic cod and Vibrio anguillarum challenge demonstrated that five week-long glucan immersion resulted in elevated expression of IL-1β gene in the anterior intestine and rectum, whereas the expression of IL-10 was measurable only in rectum. In the same experimental design, mannan-based oligosaccharide, additional upregulation of IL-8 and IFN-γ was observed.[96]
Glucan exposure not only helped to show the upregulation of some genes, but even to their description. Two β-defensive genes, β-defensin 1 and β-defensin 2, were described in common carp. In addition, the expression of these genes was upregulated by glucan exposure, similar to the expression of two mucin genes.[97] A study of inflammatory cytokines as a response to A. salmonicida infection showed that feeding with glucan resulted in reduction of gene expression of inflammation-related cytokines such as IL-1β, IL-6, IL-10, and TNF-α.[98]
A detailed study of the effects of dietary glucan on gene expression was done in common carp. While the 7 day incubation showed very small changes, 25 day incubation increased iNOS and Bcl-2 expression in liver and head kidney. In other organs, the effects were more pronounced with a strong increase of iNOS, Bcl-2 and Nemo in gut and iNOS, Caspase 9, Blc-2, p38 and Nemo in spleen.[99] Despite the progress, the evaluation of the effects of glucan on genomic level are limited not only with respect of species, but also of individual genes. To better understand how glucan exposure affects individual genes, the full genomic studies need to be performed.
Conclusions
Administration of glucans through various routes including immersion, feed or injection have been found to enhance many types of immune responses, resistance to bacterial and viral infections and resistance to environmental stress. Although the efficacy of the glucan to some extent varies with type and administration, glucan used as an immunomodulatory additive has been found to be active in eliciting immunity in commercial aquaculture[100] and is currently routinely used in commercial farming. Development of more efficient administration methods will facilitate the routine and prophylactic use of glucans as natural immunostimulants of fish. Lately, interest focused on mechanisms of action. However, nothing conclusive can be reached as a result of these studies and the effects of glucan on modulation of gene expression leading to stimulation of fish immunity still require elucidation. Therefore, the limited knowledge of mechanisms of glucan action on fish immunity does not currently allow better and more specific use of glucan in aquaculture.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Busch W. Verhandlungen artzlicher gesellschaften. Berl Klin Wochenschr. 1850;5:137–8. [Google Scholar]
- 2.Lam C, Schütze E, Hildebrandt J, Aschauer H, Liehl E, Macher I, et al. SDZ MRL 953, a novel immunostimulatory monosaccharidic lipid A analog with an improved therapeutic window in experimental sepsis. Antimicrob Agent Chemother. 1991;35:500–5. doi: 10.1128/aac.35.3.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Novak M, Vetvicka V. β–Glucans, history, and the present: Immunomodulatory aspects and mechanisms of action. J Immunotoxicol. 2008;5:47–57. doi: 10.1080/15476910802019045. [DOI] [PubMed] [Google Scholar]
- 4.Novak M, Vetvicka V. Glucans as biological response modifiers. Endocr Metab Immune Disord. 2009;9:67–75. doi: 10.2174/187153009787582423. [DOI] [PubMed] [Google Scholar]
- 5.Vetvicka V, Vetvickova J. Immune enhancing effects of WB365, a novel combination of Ashwagandha (Withania somnifera) and Maitake (Grifola frondosa) extracts. N Am J Med Sci. 2011;3:320–4. doi: 10.4297/najms.2011.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahar S, Swami G, Nagpal N, Nagpal MA, Singh GS. Preparation, characterization, and biological properties of β-glucans. J Adv Pharm Technol Ress. 2011;94:103. doi: 10.4103/2231-4040.82953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vetvicka V, Vetvickova J. Reversal of perfluorooctanesulfonate- caused immunotoxicity by glucan-resveratrol-vitamin C combination. Orient Pharm Exp Med. 2013;13:77–84. [Google Scholar]
- 8.Lave I, Levinson D, Peri I, Nimri L, Hadar Y, Schwartz B. Orally administered glucans from the edible muschroom Pleutorus pulmonalis reduce acute inflammation in dextran sulfate sodium-induced experimental colitis. Br J Nutr. 2010;103:393–402. doi: 10.1017/S0007114509991760. [DOI] [PubMed] [Google Scholar]
- 9.Vetvicka V, Sima P. β-Glucan in invertebrates. Inv Surv J. 2004;1:60–5. [Google Scholar]
- 10.Fischer U, Koppang EO, Nakanishi T. Teleost T and NK cell immunity. Fish Shellfish Immunol. 2013;35:197–206. doi: 10.1016/j.fsi.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Šíma P, Trebichavský I, Sigler K. Nonmammalian vertebrate antibiotic peptides. Folia Microbiol. 2003;48:709–24. doi: 10.1007/BF02931504. [DOI] [PubMed] [Google Scholar]
- 12.Větvička V, Šíma P. Basel, Switzerland: Birkhauser Verlag AG; 1998. Evolutionary mechanisms of defense reactions. [Google Scholar]
- 13.Cooper EL, Zapata A, Garcia Barrutia M, Ramirez JA. Aging changes in lymphopoieticand myelopoietic organs of the annual cyprinodont fish, Nothoranchius guentheri. Exp Geronol. 1983;18:29–38. doi: 10.1016/0531-5565(83)90048-7. [DOI] [PubMed] [Google Scholar]
- 14.Zapata A, Cooper EL. Chichester, UK: John Wiley and Sons; 1990. The immune system: Comparative histophysiology. [Google Scholar]
- 15.Zwollo P, Cole S, Bromage E, Kaattari S. B cell heterogeneity in the teleost kidney: Evidence for a maturation gradient from anterior to posterior kidney. J Immunol. 2005;174:6608–16. doi: 10.4049/jimmunol.174.11.6608. [DOI] [PubMed] [Google Scholar]
- 16.Agius C. Preliminary studies on the ontogeny of the melano-macrophages of teleost haemopoietic tissues and age-related changes. Dev Comp Immunol. 1981;5:597–606. doi: 10.1016/s0145-305x(81)80034-1. [DOI] [PubMed] [Google Scholar]
- 17.Fuglem B, Jirillo E, Bjerkas I, Kiyono H, Nochi T, Yuki Y, et al. Antigen- sampling cells in the salmoid intestinal epithelium. Dev Com Immunol. 2010;34:768–74. doi: 10.1016/j.dci.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Gatesoupe FJ. Live yeasts in the gut: Natural occurrence, dietary introduction, and their effects on fish health and development. Aquaculture. 2007;267:20–30. [Google Scholar]
- 19.Šíma P, Větvička V. Evolution of immune accessory functins. In: Fornůsek L, Vetvicka V, editors. Immune System Accessory Cells. Boca Raton, USA: CRC Press; 1992. pp. 1–55. [Google Scholar]
- 20.Koumans-van Diepen JC, Egberts E, Peixoto BR, Taverne N, Rombout JW. B cell and immunoglobulin heterogeneity in carp (Cyprinus carpio L.): An immune (cyto) chemical study. Dev Comp Immunol. 1995;19:97–108. doi: 10.1016/0145-305x(94)00061-j. [DOI] [PubMed] [Google Scholar]
- 21.Castro R, Bernard D, Lefranc MP, Six A, Benmansour A, Boudinot P. T cell diversity and TcR repertoires in teleost fish. Fish Shellfish Immunol. 2011;3:1644–54. doi: 10.1016/j.fsi.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Matsunaga T, Rahman A. What brought the adaptive immune systems to vertebrates?-The jaw hypothesis and the seahorse. Immunol Rev. 1998;166:177–86. doi: 10.1111/j.1600-065x.1998.tb01262.x. [DOI] [PubMed] [Google Scholar]
- 23.Wilson MR, Bengten E, Miller NW, Clem LW, Du Pasquier L, Warr GW. A novel chimeric Ig heavy chain from a teleost fish shares similarities to IgD. Proc Natl Acad Sci USA. 1997;94:4593–7. doi: 10.1073/pnas.94.9.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castro R, Jouneau L, Pham HP, Bouchez O, Giudicelli V, Lefranc MP, et al. Teleost fish mount complex clonal IgM and IgT responses in spleen upon systemic viral infection. PLoS Pathol. 2013;9 doi: 10.1371/journal.ppat.1003098. e1003098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danilova N, Bussmann J, Jekosch K, Steiner LA. The immunoglobulin heavy-chain locus in zebrafsh: Identifcation and expression of a previously unknown isotype, immunoglobulin. Z Nat Immunol. 2005;6:295–302. doi: 10.1038/ni1166. [DOI] [PubMed] [Google Scholar]
- 26.Flajnik MF, Kasahara M. Origin and evolution of the adaptive immune system: Genetic events and selective pressures. Nat Rev Genet. 2007;11:47–59. doi: 10.1038/nrg2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solem ST, Stenvik J. Antibody repertoire development in teleosts-A review with emphasis on salmonids and Gadus morhua. Dev Comp Immunol. 2006;30:57–76. doi: 10.1016/j.dci.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Bondad-Reantaso MG, Subasinghe RP, Arthur JR, Ogawa K, Chinabut S, Adlard R, et al. Disease and health management in Asian aquaculture. Vet Parasitol. 2005;132:249–72. doi: 10.1016/j.vetpar.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Rome: FDO UNO; 2012. The State of World Fisheries and Aquaculture 2012; p. 209. [Google Scholar]
- 30.Holm KO, Jørgensen T. A successful vaccination of Atlantic salmon, Salmo salar L, against ‘Hitra disease’ or coldwater vibriosis. J Fish Dis. 1987;10:85–90. [Google Scholar]
- 31.Jones SR, MacKinnon AM, Salonius K. Vaccination of freshwater-reared Atlantic salmon reduces mortality associated with infectious salmon anaemia virus. Bull Eur Assoc Fish Pathol. 1999;19:98–101. [Google Scholar]
- 32.Rijkers GT, van Oosterom R, van Muiswinkel WB. The immune system of cyprinid fish. Oxytetracyclinc and the regulation of humoral immunity in carp (Cyprinus carpio) Vet Immunol Immunopathol. 1981;2:281–90. doi: 10.1016/0165-2427(81)90029-5. [DOI] [PubMed] [Google Scholar]
- 33.Anderson DP. Immunostimulants, adjuvants, and vaccine carriers in fish: Application to aquaculture. Ann Rev Fish Dis. 1992;1:281–307. [Google Scholar]
- 34.Fletcher TC. Non-specitic defence mechanisms of fish. Dev Comp Immunol. 1982;2:S123–32. [Google Scholar]
- 35.Zapata A, Amemiya CT. Phylogeny of lower vertebrates and their immunological structures. Curr Top Microbiol Immunol. 2000;248:67–107. doi: 10.1007/978-3-642-59674-2_5. [DOI] [PubMed] [Google Scholar]
- 36.Olivier G, Eaton CA, Campbell N. Interaction between Aeromonas salmonicida and peritoneal macrophages of brook trout (Salvelinus fontinalis) Vet Immunol Immunopathol. 1986;12:223–34. doi: 10.1016/0165-2427(86)90126-1. [DOI] [PubMed] [Google Scholar]
- 37.Raa J, Roestad G, Engstad RE, Robertsen B. The use of immunostimulants to increase resistance of aquatic organism to microbial infections. In: Shariff IM, Subasinghe RP, Arthur JR, editors. Diseases in Asian Aquaculture. Manila: Health Fish Section Asian Fisheries Society; 1992. pp. 39–50. [Google Scholar]
- 38.Siwicki AK, Morand M, Terech-Majevska E, Niemczuk W, Kazun K, Glabsky E. Influence of immunostimulant on the effectiveness of vaccines in fish: In vitro and in vivo study. J Appl Ichthyol. 1998;14:225–7. [Google Scholar]
- 39.Meena DK, Das P, Kumar S, Mandal SC, Prusty AK, Singh SK, et al. Beta-glucan: An ideal immunostimulant in aquaculture (a review) Fish Physiol Biochem. 2013;39:431–57. doi: 10.1007/s10695-012-9710-5. [DOI] [PubMed] [Google Scholar]
- 40.Raa J. Reviews in Fisheries Science. Vol. 4. Boca Raton: CRC Press; 1996. The use of immunostimulatory substances in fish and shellfish farming; pp. 229–88. [Google Scholar]
- 41.Raa J. The use of immune-stimulants in fish and shellfish feeds. In: Cruz–Suárez LE, Ricque-Marie D, Tapia-Salazar M, Olvera-Novoa MA, Civera-Cerecedo R, editors. Advances en Nutrición Acuícola V. Memorias del V Simposium Internacional de Nutrición Acuícola . Medida, Mexico: 2000. pp. 47–56. [Google Scholar]
- 42.Welker TL, Lim C, Yildrim-Aksoy M, Shelby R, Klesius PH. Immune response and resistance to stress and Edwardsiella ictaluri challenge in channel catfish, Ictalurus punctatus, fed diets containing commercial whole-cell or yeast subcomponents. J World Aquacult Soc. 2007;38:24–35. [Google Scholar]
- 43.Sealey WM, Barrows FT, Hang A, Johansen KA, Overturf K, LaPatra SE, et al. Evaluation of the ability ofbarley genotypes containing different amount of β- glucan to alter growth and disease resistance of rainbow trout (Oncorhynchus mykiss) Anim Feed Sci Tech. 2008;141:115–128. [Google Scholar]
- 44.Selvaraj V, Sampath K, Sekar V. Administration of yeast glucan enhances survival and some non-specific and specific immune parameters in carp (Cyprinus carpio) infected with Aeromonas hydrophila. Fish Shellfish Immunol. 2005;9:293–306. doi: 10.1016/j.fsi.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 45.Cook MT, Hayball PJ, Hutchison W, Nowak B, Hayball JD. The efficacy of a commercial beta-glucan preparation, EcoActiva, on stimulating respiratory burst activity of head-kidney macrophages from pink snapper (Pagrus auratus), Sparidae. Fish Shellfish Immunol. 2001;11:661–72. doi: 10.1006/fsim.2001.0343. [DOI] [PubMed] [Google Scholar]
- 46.Jorgensen JB, Sharp GJ, Secombes CJ, Robertsen B. Effects of a yeast-cell-wall glucan on the bactericidal activity of rainbow trout macropages. Fish Shellfish Immunol. 1993;3:267–77. [Google Scholar]
- 47.Djordievic B, Skugor S, Jorgensen SM, Overland M, Myland LT, Krasnov A. Modulation of splenic immune response to bacterial lipopolysaccride in rainbow trout (Oncorhynchus mykiss) fed lentinin, a β-glucan from mushroom Lentinula edodes. Fish Shellfish Immunol. 2009;26:201–9. doi: 10.1016/j.fsi.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida T, Kruger R, Inglis V. Augmentation of nonspecific protection in African catfish, Clarias-Gariepinus (Burchell), by the long-term oral-administration of immunostimulants. J Fish Dis. 1995;18:195–8. [Google Scholar]
- 49.Rorstad G, Aasjord PM, Robertsen B. Adjuvant effects of a yeast glucan in vaccines against furunculosis in Atlantic salmon (Salmo salar L.) Fish Shellfish Immunol. 1993;3:179–90. [Google Scholar]
- 50.Sahoo PK, Murkherjee SC. The effect of dietary immunomodulation upon Edwardsiella tarda vaccination in healthy and immunocompromised Indian major carp (Labeo rohita) Fish Shellfish Immunol. 2002;12:1–16. doi: 10.1006/fsim.2001.0349. [DOI] [PubMed] [Google Scholar]
- 51.Figueras A, Santarem MM, Nov B. Influence of the sequence of administration of β-glucans and a Vibrio damsela vaccine on the immune response of turbot (Scophthalmus maximus L.) Vet Immunol Immunopathol. 1998;64:59–68. doi: 10.1016/s0165-2427(98)00114-7. [DOI] [PubMed] [Google Scholar]
- 52.Cook MT, Hayball PJ, Hutchinson W, Nowak BF, Hayball JD. Administration of a commercial immunostimulant preparation, EcoActiva™ as a feed supplement enhances macrophage respiratory burst and the growth rate of snapper (Pagrus auratus, Sparidae (Bloch and Schneider)) in winter. Fish Shellfish Immunol. 2003;14:333–45. doi: 10.1006/fsim.2002.0441. [DOI] [PubMed] [Google Scholar]
- 53.Bagni M, Romano N, Finoia MG, Abelli L, Scapigliati G, Tiscar PG, et al. Short- and long-term effects of a dietary yeast beta-glucan (Macrogard) and alginic acid (Ergosan) preparation on immune response in sea bass (Dicentrarchus labrax) Fish Shellfish Immunol. 2005;18:311–25. doi: 10.1016/j.fsi.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 54.Bagni M, Archetti L, Amadori M, Marin G. Effect of long-term oral administration of an immunostimulant diet on innate immunity in sea bass (Dicentrarchus labrax) J Vet Med B. 2008;47:745–51. doi: 10.1046/j.1439-0450.2000.00412.x. [DOI] [PubMed] [Google Scholar]
- 55.Bonaldo A, Thompson KD, Manfrin A, Adams A, Murano AL, Gatta PP. The influence of dietary beta-glucans on the adaptive and innate immune responses of European sea bass (Dicentrarchus labrax) vacinated against vibriosis. Ital J Anim Sci. 2007;6:151–64. [Google Scholar]
- 56.Russo R, Yanong RP, Mitchell H. Dietary beta-glucans and nucleotides enhance resistance of red-tail black shark (Epalzeorhynchus bicolor, fam. Cyprinidae) to Streptococcus iniae infection. J World Aquacult Soc. 2006;37:298–306. [Google Scholar]
- 57.Palic D, Andreasen CB, Herolt DM, Menzel BW, Roth JA. Immunomodulatory effects of beta-glucan on neutrophil action in fathead minnows (Pimephales promelas Rafinesque 1820) Dev Comp Immunol. 2006;30:817–30. doi: 10.1016/j.dci.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 58.Skjermo J, Storseth TR, Hansen K, Handa A, Oie G. Evaluation of beta-(1-3, 1-6)-glucans and high-M alginate used as immunostimulatory dietary supplement during first feeding and weaning of Atlantic code (Gahus morhua L.) Aquaculture. 2006;261:1088–101. [Google Scholar]
- 59.Cuesta A, Rodriguez A, Salinas I, Mesequer J, Esteban MA. Early local and systemic innate responses in the teleost gilthead seabream after intraperitoneal injection of whole yeast cells. Fish Shelfish Immunol. 2007;22:242–51. doi: 10.1016/j.fsi.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 60.Ai Q, Mai K, Zhang L, Tan B, Zhang W, Xu W, et al. Effects of dietary β-1, 3 glucan on innate immune response of large yellow croaker, Pseudosciaena crocea. Fish Shellfish Immunol. 2007;22:394–402. doi: 10.1016/j.fsi.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 61.Shelby RA, Lim C, Yildirim-Aksoy M, Welker TL, Klesius PH. Effects of yeast oligosaccharide diet supplements on growth, and disease resistance in Nile tilapia, Oreochromis niloticus. J Appl Aquacult. 2009;21:61–71. [Google Scholar]
- 62.Rodriguez I, Chamorro R, Novoa B, Figueras A. Beta-glucan administration enhances disease resistance and some innate immune responses in zebrafish (Danio rerio) Fish Shelfish Immunol. 2009;27:369–73. doi: 10.1016/j.fsi.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 63.Pionnier N, Falco A, Miest J, Frost P, Imazarow I, Shrive A, et al. Dietary β-glucan stimulates complement and C-reactive protein acute phase responses in common carp (Cyprinus carpio) during an Aeromonas salmonidica infection. Fish Shellfish Immunol. 2013;34:819–31. doi: 10.1016/j.fsi.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 64.Przybylska-Diaz DA, Schmidt JG, Vera-Jimenez NI, Steinhagen D, Nielsen ME. β-Glucan enriched bath directly stimulates the wound healing process in common carp (Cyprinus carpio L.) Fish Shellfish Immunol. 2013;35:998–1006. doi: 10.1016/j.fsi.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 65.Sveinbjornsson B, Smerdsrod B, Berg T, Seljelid R. Intestinal uptake and organ distribution of immunomodulatory aminated β-1,3-D-polyglucose in Atlantic salmon (Salmo salar L.) Fish Shellfish Immunol. 1995;5:39–50. [Google Scholar]
- 66.Brogden G, von Kockritz-Blickwede M, Adamek M, Reuner F, Jung-Schroers V, Naim HY, et al. β-Glucan protects neutrophil extracellular traps against degradation by Aeromonas hydrophila in carp (Cyprinus carpio) Fish Shellfish Immunol. 2012;33:1060–4. doi: 10.1016/j.fsi.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 67.Robertsen B, Rorstad G, Engstad R, Raa J. Enhancement of non-specific resistance in Atlantic salmon, Salmo salar L., by a glucan from Saccharomyces cerevisiae cell walls. J Fish Dis. 1990;13:391–400. [Google Scholar]
- 68.Burrells C, Williams PD, Forno PF. Dietary nucleotides: A novel supplement in fish feeds. I. Effect on resistance to disease in salmonids. Aquaculture. 2001;199:159–69. [Google Scholar]
- 69.Nikl L, Albright LJ, Evelyn TP. Influence of seven immunostimulants on the immune response of coho salmon to Aeromonas salmonicida. Dis Aquat Organ. 1991;12:7–12. [Google Scholar]
- 70.Aakre R, Wergeland HI, Aasjord PM, Endresen C. Enhanced antibody response in Atlantic salmon (Salmo salar L.) to Aeromonas salmonicida cell wall antigens using a bacterin containing β-1,3-M-glucan as adjuvant. Fish Shellfish Immunol. 1994;4:47–61. [Google Scholar]
- 71.Midtlyng PJ, Reitan LJ, Speilberg L. Experimental studies on the efficacy and side-effects of intraperitoneal vaccination of Atlantic salmon (Salmo salar L.) against furunculosis. Fish Shellfish Immunol. 1996;6:335–50. [Google Scholar]
- 72.Jeney G, Galeotti M, Volpatti D, Jeney Z, Anderson DP. Prevention of stress in rainbow trout (Oncorhynchus mykiss) fed diets containing different doses of glucan. Aquaculture. 1997;154:1–15. [Google Scholar]
- 73.Guseva NV, Galash VT, Ford LA. Effects of VYS-2 and glucan on the immune functions of carp. In: Gudding R, Lillehaug A, Midtlyng PJ, Brown F, editors. Fish Vaccinology. Basel: Karger; 1997. p. 447. [Google Scholar]
- 74.Ashida T, Okimasu E, Ui M, Heguri M, Oyama Y, Amemura A. Protection of Japanese flounder Paralichthys olivaceus against experimental Edwardsiellosis by formalin-killed Edwardsiella tarda in combination with oral administration of immunostimulants. Fish Sci. 199(65):527–30. [Google Scholar]
- 75.Verlhac V, Gabaudan J, Obach A, Schuep W, Hole R. Influence of dietary glucan and vitamin C on non-specific and specific immune responses of rainbow trout (Oncorhynchus mykiss) Aquaculture. 1996;43:123–33. [Google Scholar]
- 76.Romalde JL, Magarinos B, Toranzo AE. Prevention of streptococcosis in turbot by intraperitoneal vaccination: A review. J Appl Ichthyol. 1999;15:153–8. [Google Scholar]
- 77.Whittington R, Lim C, Klesius PH. Effect of dietary β-glucan levels on the growth response and efficacy of Streptococcus iniae vaccine in Nile tilapia, Oreochromis niloticus. Aquaculture. 2005;248:217–25. [Google Scholar]
- 78.Nikl L, Evelyn TP, Albright LJ. Trials with an orally and immersion- administered β-1,3 glucan as an immunoprophylactic against Aeromonas salmonicida in juvenile chinook salmon Oncorhynchus tshawytscha. Dis Aquat Organ. 1993;17:191–6. [Google Scholar]
- 79.Anderson DP. Immunostimulants, adjuvants, and vaccine carrier in fish: Applications to aquaculture. Annu Rev Fish Dis. 1992;2:281–307. [Google Scholar]
- 80.Yano T, Matsuyama H, Mangindaan RE. Polysaccharide- induced protection of carp, Cyprinus carpio L., against bacterial infection. J Fish Dis. 1991;14:577–82. [Google Scholar]
- 81.Xueqin J, Kania PW, Buchmann K. Comparative effects of four feed types on white spot disease susceptibility and skin immune parameters in rainbow trout, Oncorhynchus mykiss (Walbaum) J Fish Dis. 2012;35:127–35. doi: 10.1111/j.1365-2761.2011.01329.x. [DOI] [PubMed] [Google Scholar]
- 82.Finstad B, Bjørn PA, Grimnes A, Hvidsten NA. Laboratory and field investigations of salmon lice (Lepeophtheirus salmonis [Krøyer]) infestations on Atlantic salmon (Salmosalar L.) post-smolts. Aquacult Res. 2000;31:795–803. [Google Scholar]
- 83.Wolf K, Quimby MC. Established eurythermic line of fish cells in vitro. Science. 1962;135:1065–6. doi: 10.1126/science.135.3508.1065. [DOI] [PubMed] [Google Scholar]
- 84.Gravell M, Malsberger RG. A permanent cell line from fathead minnow (Pimephales promelas) Ann NY Acad Sci. 1965;126:555–65. doi: 10.1111/j.1749-6632.1965.tb14302.x. [DOI] [PubMed] [Google Scholar]
- 85.Rucker RR, Whipple WJ, Parvin JR, Evans CA. A contagious disease of salmon possibly of virus origin. Fishery Bull. 1953;76:35–46. [Google Scholar]
- 86.M’Gonigle RH. Acute catarrhal enteritis of salmonid fingerlings. Trans Am Fish Soc. 1941;70:297–303. [Google Scholar]
- 87.Beaulaurier J, Bickford N, Gregg JL, Grady CA, Gannam AL, Winton JR, et al. Susceptibility of pacific herring to viral hemorrhagic septicemia is induced by diet. J Aquat Anim Health. 2012;24:43–8. doi: 10.1080/08997659.2012.668511. [DOI] [PubMed] [Google Scholar]
- 88.Sahan A, Duman S. Effects of β glucan on haematology of common carp (Cyprinus carpio) infected by ectoparasites. Mediterr Aquacult J. 2010;1:1–7. [Google Scholar]
- 89.Kirchhoff NT, D’Antigana T, Leef MJ, Hayward CJ, Wilkinson RJ, Nowak BF. Effects of immunostimulans on ranched southern bluefin tuna Thunnus maccoyii: Immune response, health and performance. J Fish Biol. 2011;79:331–55. doi: 10.1111/j.1095-8649.2011.03019.x. [DOI] [PubMed] [Google Scholar]
- 90.Del Rio-Zaragoza OB, Fajer-Avila EJ, Almazan-Rueda P. Influence of β-glucan on innate immunity and resistance of Lutjanus guttatus to an experimental infection of dactylogyrid monogeneans. Parasite Immunol. 1011;33:483–94. doi: 10.1111/j.1365-3024.2011.01309.x. [DOI] [PubMed] [Google Scholar]
- 91.Jaafar RM, Skov J, Kania PW, Buchmann K. Dose dependent effects of dietary immunostimulants on rainbow trout immune parameters and susceptibility to the parasite Ichthyophthirius multifillis. J Aquacult Res Dev. 2011 In press. [Google Scholar]
- 92.Lovoll M, Fischer U, Mathisen GS, Bogwald J, Ototake M, Dalmo RA. The C3 subtype are differentially regulated after immunostimulation in rainbow trout, but head kidney macrophages do not contribute to C3 transcription. Vet Immunol Immunopathol. 2007;117:84–295. doi: 10.1016/j.vetimm.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 93.Chansue N, Endo M, Kono T, Sakai M. The stimulation of cytokine-like protein in tilapia (Oreochromis niloticus) orally treated with β-1,3 glucan. Asian Fish Sci. 2000;13:271–8. [Google Scholar]
- 94.Hardie LJ, Chappell LH, Secombes CJ. Human tumor necrosis factor α influences rainbow trout Oncorhynchus mykiss leukocyte resposes. Vet Immunol Immunopathol. 1994;40:73–84. doi: 10.1016/0165-2427(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 95.Kim YS, Ke F, Zhang QY. Effect of β-glucan on activity of antioxidant enzymes and Mx gene expression in vitus infected grass carp. Fish Shellfish Immunol. 2009;27:336–40. doi: 10.1016/j.fsi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 96.Lokesh J, Fernandes JM, Korsner K, Bergh O, Brinchmann MF, Kiron V. Transcriptional regulation of cytokines in the intestine of Atlantic cod fed yeast derived mannan oligosaccharide or β-glucan and challenged with Vibrio anguillarum. Fish Shellfish Immunol. 2012;33:626–31. doi: 10.1016/j.fsi.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 97.Van der Marel M, Adamek M, Gonzalez SF, Frost P, Rombout JH, Wiegertjes GF, et al. Molecular cloning and expression of two β-defensin and two mucin genes in common carp (Cypinus carpio L.) and their up-regulation after β-glucan feeding. Fish Shellfish Immunol. 2012;32:494–501. doi: 10.1016/j.fsi.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 98.Falco A, Frost P, Miest J, Pionnier N, Irnazarow I, Hoole D. Reduced inflammatory response to Aeromonas salmonicida infection in common carp (Cypinus carpio L.) fed with β-glucan supplements. Fish Shellfish Immunol. 2012;32:1051–7. doi: 10.1016/j.fsi.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 99.Miest JJ, Falco A, Pionnier NP, Frost P, Irnazarow I, Williams GT, et al. The influence of dietary β-glucan, PAMP exposure and Aeromonas salmonicida on apoptosis in common carp. Fish Shellfish Immunol. 2012;33:846–56. doi: 10.1016/j.fsi.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 100.Dalmo RA, Bogwald J. β-glucans as conductors on immune symphonies. Fish Shellfish Immunol. 2008;23:384–96. doi: 10.1016/j.fsi.2008.04.008. [DOI] [PubMed] [Google Scholar]


