Abstract
Background:
Snake bite envenomation is a major public health concern in developing countries. Acute kidney injury (AKI) is as important cause of mortality in patients with vasculotoxic snake bite.
Aims:
This study was to evaluate the clinical profile of snake bite patients and to determine the predictors of developing AKI following snake bite.
Materials and Methods:
Two hundred and eighty-one patients with snake envenomation were included. Eighty-seven patients developed AKI (Group A) and 194 (Group B) did not. History, examination findings and investigations results were recorded and compared between the two groups.
Results:
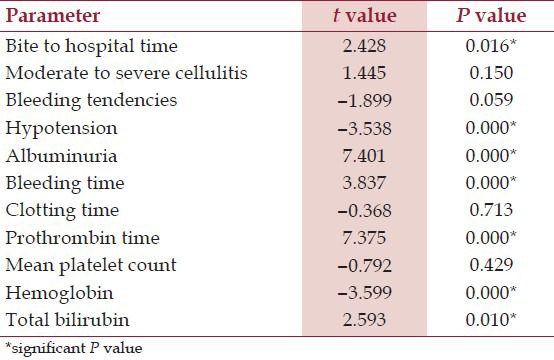
In group A, 61 (70.11%) patients were male and in group B, 117 (60.30%) patients were male. Out of 281 patients, 232 had cellulitis, 113 had bleeding tendencies, 87 had oliguria, 76 had neuroparalysis, and 23 had hypotension at presentation. After multivariate analysis, bite to hospital time (P = 0.016), hypotension (P = 0.000), albuminuria (P = 0.000), bleeding time (P = 0.000), prothrombin time (P = 0.000), hemoglobin (P = 0.000) and total bilirubin (P = 0.010) were significant independent predictors of AKI.
Conclusions:
AKI developed in 30.96% of patients with snake bite, leading to mortality in 39.08% patients. Factors associated with AKI are bite to hospital time, hypotension, albuminuria, prolonged bleeding time, prolonged prothrombin time, low hemoglobin and a high total bilirubin.
Keywords: Acute kidney injury, Bite to hospital time, Coagulopathy, Envenomation, Hemolysis, Snake bite
Introduction
More than 2700 species of snakes exist in the world, of which only 450 have front fangs making them capable of injecting venom during bite. India harbors more than 250 species and subspecies of snakes, of which about 50 are venomous. Only five venomous species of land snakes are pose a significant threat to public health in India. They are neurotoxic Elapidae, including Common Cobra (Naja Naja), King Cobra (Ophiophagus Hannah) and Krait (Bungarus Coerulus, Bungarus fasciatus); and vasculotoxic Viperidae, Russell's viper (Daboia russelii) and saw-scaled viper (Echis carinatus). In India, approximately 81,000 snake envenomings occur each year, which result in about 11,000 deaths.[1] However, these numbers may be a gross underestimation of the true burden morbidity and mortality in snake bite victims. Acute kidney injury (AKI) is one of the most significant complications developing due to snake bite. AKI is associated with bites of Russell's Viper,[2,3] saw-scaled Viper[4] Puff Adder,[5] Pit Viper,[3] Sea snake[6] and Tiger snake.[7] AKI and rhabdomyolysis have also been reported following wasp and bee stings.[8,9]
This study was undertaken to evaluate the clinical profile of snake bite patients presenting to our institute and to determine the predictors of developing AKI following snake bite.
Materials and Methods
This prospective observational study was conducted from January 2009 to August 2010 in department of Medicine of our institute. The approval from Ethics committee of our institute was obtained prior to starting the study.
Case selection
During above period, 454 patients were admitted with snake bite. Of these, 173 patients did not show signs of snake envenomation, and were discharged after 24 hours of observation. Rest 281 patients with signs and symptoms of local or systemic envenomation were included in the study (n = 281) after obtaining a complete informed consent from the patients or relatives.
Defining criteria
Evidence of bite by a poisonous snake[10] included presence of fang marks consistent with a snake bite at the alleged site of bite; identification of snake if possible, either as per patient's history or if a dead snake was brought by the patient; evidence of local toxicity in form of swelling, cellulitis, gangrene, ecchymosis, blisters, blebs, or bleeding at the site of bite and area proximal to it and evidence of coagulation disturbances in form of local or systemic bleeding. Bite to hospital time was calculated as time from snake bite to the time when patient reached our hospital. Swelling at the site of bite was graded as follows: Mild – localized to the site of bite; moderate – involving more than half of involved limb and severe – presence of extensive tissue necrosis or gangrene. Neurotoxicity was defined as documented ptosis, external ophthalmoplegia, weakness of neck or bulbar muscles, use of neostigmine or ventilatory support. AKI[11] was defined as an abrupt (within 48 hours) absolute increase in the serum creatinine concentration of ≥ 0.3 mg/dL from baseline value measured after admission or elsewhere after the snake bite, or a percentage increase in the serum creatinine concentration of ≥ 50% above baseline, or oliguria of less than 0.5 mL/kg per hour for more than 6 hours, or serum creatinine more than 1.5 mg/dL or oliguria (urine output less than 400 mL/day).
Exclusion criteria
Patients were subjected to ultrasonography of abdomen and were excluded if it showed bilateral small kidneys or obstructive nephropathy or loss of corticomedullary differentiation or any other significant renal pathology. They were also excluded if they had previous records suggesting serum creatinine > 1.5 mg/dL or if they were exposed to nephrotoxic drugs or if the peripheral blood smear was positive for malaria parasite or if they were previously diagnosed to have hypertension or diabetes mellitus.
All the patients were subjected to detailed history and clinical examination. Hematological and biochemical investigations were performed in all patients, including hemoglobin, complete and differential leukocyte counts, platelet count, peripheral blood smear, bleeding and clotting times, prothrombin time (PT) and activated partial thromboplastin time (APTT), blood urea, serum creatinine, serum electrolytes, liver function tests and urine examination. Patients were administered tetanus toxoid injection, if not received previously. All patients were given anti-snake venom (ASV), administered as 100 ml infusion over 30 minutes. Patients showing signs of neuroparalysis were given injection neostigmine with prior atropine. Doses were repeated as needed based on clinical response. Supportive treatment (intravenous fluids, blood components, analgesics) was given. Patients developing AKI and having no contraindications for dialysis were subjected to peritoneal dialysis. Ventilatory support was needed for patients with respiratory failure, either due to neuroparalysis or pulmonary edema. Of 281 patients, 87 developed AKI, and were included in group A; whereas those who did not develop AKI were included in group B. Various clinical and laboratory parameters were compared between the two groups. Patients were followed until their discharge or death.
Statistical analysis
Patients were classified into group A and B based upon presence or absence of AKI. Continuous variables in the two groups were expressed as mean ± standard deviation. For comparison of categorical variables, Pearson's Chi-square test was used. Fischer exact test was used for small numbers. For continuously distributed variables, Student's t-test for the significance of difference between the means of two independent samples was used. P value of 0.05 or less was considered to be significant. To determine the factors associated with snake bite induced AKI, multivariate analysis was performed using linear regression method. Statistical analysis was performed using SPSS software version 17.0 (Chicago, IL, USA).
Results
Out of 281 patients, 87 (30.96%) developed AKI. The mean age of patients who in Group A was 36.14 ± 14.64 years and in Group B was 33.97 ± 13.72 years. This difference was not statistically significant. (P = 0.894). In group A, 61 (70.11%) patients were male and 26 (29.89%) patients were female; whereas in the group B, 117 (60.30%) patients were male and 77 (39.70%) patients were female. In group A, 55 (63.22%) patients came from rural areas, and 32 (36.78%) patients came from urban areas. Similarly, in group B, 140 (72.16%) patients came from rural areas, whereas 54 (27.84%) patients came from urban areas. Mean bite to hospital time in Group A was 20.60 ± 42.57 hours and in Group B was 7.63 ± 9.26 hours. The difference was statistically significant (P = 0.000). Thus, a prolonged bite to hospital time was associated with a significant increased risk of developing AKI. Table 1 shows the presenting features in patients with snake bite.
Table 1.
Presenting features in patients with snake bite
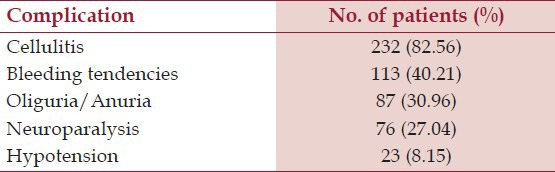
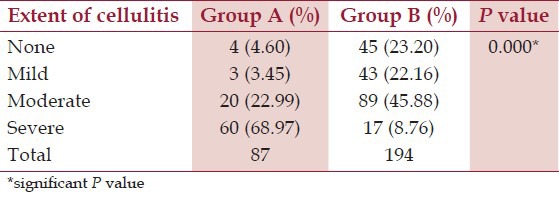
In Group A, 68 (78.16%) patients had snake bite in lower limbs and 19 (21.84%) patients had snake bite in upper limbs. Similarly, in Group B, 144 (74.23%) patients had snake bite in lower limbs and 50 (25.77%) patients had snake bite in upper limbs. The difference was not statistically significant (P = 0.577), implying that site of bite has no relation with development of AKI in patients of snake bite. Table 2 shows distribution of patients according to extent of cellulitis. Thus almost 92% patients with AKI had moderate to severe cellulitis. On the other hand, only about 55% patients without AKI had moderate to severe cellulitis.
Table 2.
Distribution of patients according to extent of cellulitis
In group A, 72 (82.76%) patients had bleeding tendencies while 15 (17.24%) patients did not have any evidence of bleeding. In group B, 41 (21.13%) patients had bleeding tendencies while 153 (78.87%) patients did not have any bleeding tendencies. The difference was statistically significant. (P = 0.000) Thus, presence of bleeding tendencies was strongly associated with AKI in patients of snake bite.
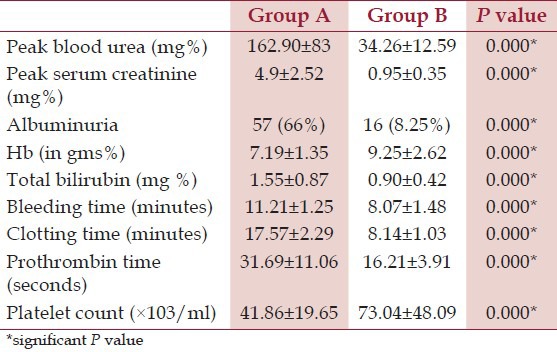
In group A, hypotension was present in 17 (19.54%) patients and absent in 70 (80.46%) patients. In group B, hypotension was present only in 6 (3.09%) patients and absent in 188 (96.91%) patients. The difference was statistically extremely significant (P < 0.0001). Thus, hypotension was more strongly associated with AKI, being present in about 20% of the patients. Laboratory investigations were also compared between the two groups, as shown in Table 3.
Table 3.
Comparison of laboratory parameters
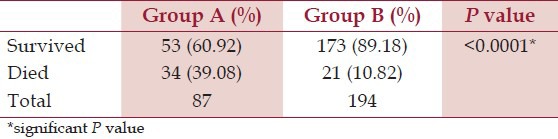
Out of 87 patients developing AKI, 48 patients having indications for dialysis were treated with peritoneal dialysis. Thirty-two patients needed ventilator support for respiratory failure due to neuroparalyis or pulmonary edema. Table 4 shows outcomes of patients in two groups. Amongst 53 patients with AKI who survived, 31 (58.49%) patients received dialysis while 22 (41.51%) did not receive the same. Similarly, among 34 patients who died, 17 (50%) had received dialysis while the rest 17 (50%) did not receive the same. The difference was not statistically significant (P = 0.437). Thus, in our study treatment with dialysis was not associated with improved outcome in patients with AKI.
Table 4.
Outcome of patients
Multivariate analysis
To determine the factors associated with development of AKI following snake bite, various clinical and laboratory parameters were compared between the two groups using linear regression method. It was observed that patients in group A had significantly longer bite to hospital time; more hypotension, albuminuria and mean hemoglobin; along with higher bleeding time, prothrombin time and serum total bilirubin [Table 5].
Table 5.
Multivariate analysis
Discussion
In India, the incidence of AKI following E. carinatus or Russell's viper bite is 13-32%.[2,5] We found that 30.96% snake bite victims developed AKI. Patil et al.,[12] noted that AKI developed in 20.48% cases of snake bite, whereas Ali et al.,[13] observed that 17% cases of viper bite were complicated by AKI. Various authors have studied AKI in snake bite, its correlation with various coagulation disturbances and the subsequent course of these patients in terms of mortality.
Age and gender distribution
Mean age of patients in Group A was 35.74 ± 14.64 years and in group B was 33.97 ± 13.72 years. The difference was not statistically significant. However, Athappan et al.,[14] found that AKI patients were older than non-AKI patients. (39.1 vs. 35.4 years, P = 0.03). A higher proportion of males in our study may be attributed to the fact that men typically go daily in the fields, are more active at night, travel wider, while women for the most part, stay in and around houses and compounds. Kulkarni et al.,[10] studied 633 cases, of which 433 (68.40%) were males while 200 (31.60%) were females. Bawaskar et al.,[15] studied 182 cases of which 114 (63%) were males and 68 (37%) were females.
Distribution of patients according to residence
We observed that rural population formed about 70% of the total patients, whereas various studies have shown a higher percentage of rural population amongst snake bite victims. Sharma et al.,[16] found that urban to rural ratio was 1:4.7. Kalantri et al.,[17] stated that 84% patients were from rural areas. This difference may be due to different geographical and demographical features of various regions. Snake bite is more common in the rural setting, primarily because farming is a major occupation in villages and farmers are most vulnerable to exposure to snakes during work. Also, residential habits, sleeping habits (in form of sleeping on the floor, in the open, in the farms) increase the risk of snake bite
Bite to hospital time
It was noted that patients who developed AKI had a significantly longer bite-to-hospital time, compared to those who did not develop AKI. Athappan et al.,[14] found that bite to needle time more than 2 hours (OR 2.10, P = 0.001) was an independent risk factor for the development of AKI. Kalantri et al.,[17] noted mean bite to hospital time of 6.5 + 10.3 hours. On the contrary, Danis et al.,[18] observed that there was no association of bite to hospital time with development of AKI. The bite to hospital time varies depending on the availability of medical facilities and the settings in which the study has been done. Snake venom, which is responsible for almost all the complications of snake bite, must be neutralized as soon as possible with ASV. This fact is well supported by various studies which show a direct relation between increased rates of complications or mortality with late arrival to hospital.
Presenting features in patients with snake bite
Important clinical features in snake bite victims were cellulitis, AKI, neuroparalysis, coagulopathy and hypotension. Snake venom, a mixture of various neurotoxins, digestive enzymes, activators or inactivators of various physiological processes is responsible for the various complications associated snake bite. In areas where vipers are predominant, cellulitis and AKI become the major complication following a snake bite. Significantly higher number of patients with AKI had moderate to severe cellulitis. The extent of cellulitis depends on various factors like the amount of venom injected into the victim's body, the type of snake, the delay in receiving ASV, and application of any harmful local measures. Moderate to severe cellulitis and swelling of the limb can accommodate many liters of extravasated blood, leading to hypovolemic shock. Underlying rhamdomyolysis, hyperkalemia, associated sepsis also contribute to AKI.
Correlation of bleeding tendencies with AKI
It was further noted that patients in group A had significantly more bleeding tendencies than those in group B, suggesting that coagulation and bleeding abnormalities are strongly associated with development of AKI in patients of snake bite. Sharma et al.,[16] studied 142 cases of snake bite, of which 52 were viper bites having hemostatic abnormalities. Of these, 27 developed AKI. Various procoagulant enzymes are found in viper venoms, which activate different steps of the clotting cascade resulting in a state of disseminated intravascular coagulopathy (DIC). Bleeding tendencies can also cause severe blood loss resulting in hypotension, further adding to the renal insult or causing one when none existed. Fibrin thrombi in renal microvasculature glomerular capillaries, microangiopathic hemolytic anemia and thrombocytopenia in patients with cortical necrosis strongly suggest that DIC plays a major pathogenetic role in snake-bite-induced cortical necrosis. Thus, bleeding tendencies secondary to DIC are a major factor in the development of AKI in patients of snake bite, especially those involving vipers.
Hypotension and AKI
We observed that significantly higher number of patients in group A had hypotension. Loss of plasma into the bitten extremity, bleeding into the tissues or externally, vasodilatation and increased capillary permeability, all results of direct and indirect effects of venom cause circulatory disturbances of shock. Rarely, hypotension can also be due to cardiotoxic effects of snake venom. Hypotension can cause pre-renal insult on the kidneys and may aggravate direct renal insult caused by the venom.
Coagulation profile and parameters of intravascular hemolysis
All the parameters associated with coagulation viz. bleeding time, clotting time, platelet count and prothrombin time were deranged significantly in the group A as compared to the group B. Vijeth et al.,[19] found that renal involvement was noted in 13 (32.5%) cases, of which eight (61.5%) had primary fibrinogenolysis and five (38.5%) had DIC. Eight cases (61.5%) with renal dysfunction had incoagulable blood, whereas five (38.5%) had mild coagulation abnormality. Suchithra et al.,[20] found that abnormal coagulation profile was seen in 142 cases, 103 of these had severe coagulopathy (platelet count <50 × 109 /L, INR 1.8, aPTT 1 min or blood not coagulable). DIC is a major factor associated with snake bite resulting in AKI. Also, platelet count is reduced both due to consumptive coagulopathy and direct toxic effects of snake venom on platelets. We noted that mean hemoglobin was significantly low and mean serum bilirubin was significantly higher in group A, suggesting hemolysis. Ali et al.,[13] found that intravascular hemolysis was present in 49% of patients. Of the 1548 cases of snake bite studied by Athappan et al.,[14] 159 developed AKI and intravascular hemolysis, which was identified as an independent risk factor for the development of AKI. Intravascular hemolysis due to phospholipase A2, is a significant factor in the pathogenesis of snake bite induced AKI. It manifests by anemia, jaundice, reticulocytosis, raised plasma free hemoglobin, abnormal peripheral blood smear and hemoglobinuria.
Outcome
Overall mortality due to venomous snake bites was 19.57%, with a significantly higher mortality in victims who developed AKI. In 336 cases studied by Kularatne,[21] the mortality was 2.6% only (9 patients). In the 633 cases studied by Kulkarni et al.,[10] the mortality rate was 5.2% (33 cases). Of a total of 1548 cases studied by Athappan et al.,[14] 159 (13.5%) patients developed AKI, of which 36 (22.6%) expired. Thus, mortality rate described in various studies varies from 2.5% to 25%. The mortality rate is higher in studies involving vipers, with higher proportion of patients developing complications. It was also interesting to observe that treatment with dialysis was not associated with improved outcome in patients with snake bite induced AKI. Of the 159 patients of AKI studied in Athappan et al.,[14] series, 72 (45.3%) required dialysis and 36 (22.6%) expired (of them, 23 required dialysis). Thus even this study showed higher mortality in patients receiving dialysis.
Predictors of AKI
On multivariate analysis, bite to hospital time, Hypotension, albuminuria, bleeding time, prothrombin time, hemoglobin and total bilirubin were independent predictors of AKI. Athappan et al.,[14] had observed cellulitis, regional lymphadenopathy, intravascular hemolysis (OR 3.70, P = 0.004) and bite to needle time more than 2 hours were independent risk factors for the development of AKI. Paul and Dasgupta[22] found that black or brown urine, 20 minute whole blood clotting time > 20 minutes, and longer bite to hospital time were predictors of AKI development in snake bite victims. The association of bite to hospital time with development of AKI highlights the importance of early treatment. Earlier the patient of snake bite receives ASV, lesser is the chance of developing any of the above complication and hence ASV should be made available at peripheral centers. Furthermore, other parameters show that the extent of vasculotoxic and hemotoxic features in the victim manifesting as coagulation abnormalities and intravascular hemolysis strongly predict development of AKI.
Conclusions
AKI developed in 30.96% of patients with a history of snake bite. Snake-bite-induced AKI results in mortality in 39.08% patients. The risk factors associated with development of AKI in snake bite are bite to hospital time, hypotension, albuminuria, prolonged bleeding time, prolonged prothrombin time, low hemoglobin and a high serum bilirubin.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Kasturiratne A, Wickremasinghe AR, de Silva N, Gunawardena NK, Pathmeswaran A, Premaratna R, et al. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5:e218. doi: 10.1371/journal.pmed.0050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monteiro FN, Kanchan T, Bhagavath P, Kumar GP, Menezes RG, Yoganarasimha K. Clinico-epidemiological features of viper bite envenomation: A study from Manipal, South India. Singapore Med J. 2012;53:203–7. [PubMed] [Google Scholar]
- 3.Warrell DA. Epidemiology of snake-bite in South-East Asia Region. In: Warrell DA, editor. Guidelines for the management of snakebite. New Delhi, India: WHO regional office for Southeast Asia; 2010. pp. 35–45. [Google Scholar]
- 4.Srimannarayana J, Dutta TK, Sahai A, Badrinath S. Rational use of anti-snake venom (ASV): Trial of various regimens in hemotoxic snake envenomation. J Assoc Physicians India. 2004;52:788–93. [PubMed] [Google Scholar]
- 5.Lavonas EJ, Tomaszewski CA, Ford MD, Rouse AM, Kerns WP., 2nd Severe puff adder (Bitis arietans) envenomation with coagulopathy. J Toxicol Clin Toxicol. 2002;40:911–8. doi: 10.1081/clt-120016963. [DOI] [PubMed] [Google Scholar]
- 6.Tamiya N, Yagi T. Studies on sea snake venom. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87:41–52. doi: 10.2183/pjab.87.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isbister GK, O’Leary MA, Elliott M, Brown SG. Tiger snake (Notechis spp) envenoming: Australian Snakebite Project (ASP-13) Med J Aust. 2012;197:173–7. doi: 10.5694/mja11.11300. [DOI] [PubMed] [Google Scholar]
- 8.Rachaiah NM, Jayappagowda LA, Siddabyrappa HB, Bharath VK. Unusual case of acute renal failure following multiple wasp stings. N Am J Med Sci. 2012;4:104–6. doi: 10.4103/1947-2714.93380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deshpande PR, Ahsan Farooq KK, Bairy M, Prabhu RA. Acute renal failure and/or rhabdomyolysis due to multiple bee stings: A retrospective study. N Am J Med Sci. 2013;5:235–9. doi: 10.4103/1947-2714.109202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulkarni ML, Anees S. Snake venom poisoning: Experience with 633 cases. Indian Pediatr. 1994;31:1239–43. [PubMed] [Google Scholar]
- 11.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patil TB, Bansod YV. Snake bite-induced acute renal failure: A study of clinical profile and predictors of poor outcome. Ann Trop Med Public Health. 2012;5:335–9. [Google Scholar]
- 13.Ali G, Kak M, Kumar M, Bali SK, Tak SI, Hassan G, et al. Acute renal failure following echis carinatus (saw-scaled viper) envenomation. Indian J Nephrol. 2004;14:177–81. [Google Scholar]
- 14.Athappan G, Balaji MV, Navaneethan U, Thirumalikolundusubramanian P. Acute renal ailure in snake envenomation: A large prospective study. Saudi J Kidney Dis Transpl. 2008;19:404–10. [PubMed] [Google Scholar]
- 15.Bawaskar HS, Bawaskar PH, Punde DP, Inamdar MK, Dongare RB, Bhoite RR. Profile of snakebite envenoming in rural Maharashtra, India. J Assoc Physicians India. 2008;56:88–95. [PubMed] [Google Scholar]
- 16.Sharma N, Chauhan S, Faruqi S, Bhat P, Varma S. Snake envenomation in a north Indian hospital. Emerg Med J. 2005;22:118–20. doi: 10.1136/emj.2003.008458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalantri S, Singh A, Joshi R, Malamba S, Ho C, Ezoua J, et al. Clinical predictors of in-hospital mortality in patients with snake bite: A retrospective study from a rural hospital in central India. Trop Med Int Health. 2006;11:22–30. doi: 10.1111/j.1365-3156.2005.01535.x. [DOI] [PubMed] [Google Scholar]
- 18.Danis R, Ozmen S, Celen MK, Akin D, Ayaz C, Yazanel O. Snakebite-induced acute kidney injury: Data from Southeast Anatolia. Ren Fail. 2008;30:51–5. doi: 10.1080/08860220701742021. [DOI] [PubMed] [Google Scholar]
- 19.Vijeth SR, Dutta TK, Shahapurkar J. Correlation of renal status with hematologic profile in viperine bite. Am J Trop Med Hyg. 1997;56:168–70. doi: 10.4269/ajtmh.1997.56.168. [DOI] [PubMed] [Google Scholar]
- 20.Suchithra N, Pappachan JM, Sujathan P. Snakebite envenoming in Kerala, South India: Clinical profile and factors involved in adverse outcomes. Emerg Med J. 2008;25:200–4. doi: 10.1136/emj.2007.051136. [DOI] [PubMed] [Google Scholar]
- 21.Kularatne SA. Common krait (Bungarus caeruleus) bite in Anuradhapura, Sri Lanka: A prospective clinical study, 1996-98. Postgrad Med J. 2002;78:276–80. doi: 10.1136/pmj.78.919.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paul J, Dasgupta S. Early Prediction of acute kidney injury by clinical features of snakebite patients at the time of hospital admission. N Am J Med Sci. 2012;4:216–20. doi: 10.4103/1947-2714.95903. [DOI] [PMC free article] [PubMed] [Google Scholar]


