Abstract
Background:
The in situ signaling transduction within skin biopsies from patients affected by autoimmune skin blistering diseases is not well-characterized.
Aim:
In autoimmune skin blistering diseases, autoantibodies seem to trigger several intracellular signaling pathways and we investigated the presence of the phosphorylated form of ribosomal protein S6-pS240 within autoimmune skin blistering diseases biopsies.
Materials and Methods:
We utilized immunohistochemistry to evaluate the presence of S6-pS240 in lesional skin biopsies of patients affected by autoimmune skin blistering diseases including patients with an endemic and nonendemic pemphigus foliaceus (non EPF), with bullous pemphigoid (BP), pemphigus vulgaris (PV), dermatitis herpetiformis (DH), and the respective controls.
Results:
Most autoimmune bullous skin diseases biopsies stained positive for S6-pS240 around lesional blisters, including adjacent areas of the epidermis; and within upper dermal inflammatory infiltrates, and/or mesenchymal-endothelial cell junctions within the dermis.
Conclusions:
We document that S6-pS240 is expressed in lesional areas of skin biopsies from patients with autoimmune skin blistering diseases, as well as on eccrine glands and piloerector muscles. Thus, the role of this molecule in autoimmune skin blistering diseases warrants further study.
Keywords: Autoimmune skin blistering diseases, Bullous pemphigoid, Dermatitis herpetiformis, Endemic pemphigus foliaceus, Intracellular signaling pathways, Pemphigus, Ribosomal protein S6-pS240
Introduction
Autoimmune bullous skin diseases (ABDs) represent a heterogeneous group of disorders of the skin and mucosa; these disorders are commonly associated with deposits of immunoglobulins, complement, and fibrinogen, usually directed against distinct adhesion molecules. The resultant loss of adhesion between the epidermis/mucosa and dermis results in blister formation and erosions.[1]
A large number of cellular events induced in target keratinocytes by ABD autoantibodies have been described, and suggest that ABD autoantibodies bind to their target antigens and trigger a complex cascade of intracellular signaling and regulatory events.[2,3,4,5,6] Several intracellular signaling pathways, such as p38MAPK activation and RhoA inhibition, pemphigus IgG activation-translocation of protein kinase C, and imbalances in both Akt/mTOR and cyclic adenosine 5’- monophosphate signaling have been demonstrated to be altered following autoantibody binding and to be causally involved in loss of keratinocyte cohesion.[2,3,4,5,6] In our study, we evaluated potential, additional intracellular signaling by studying the presence of the phosphorylated ribosomal protein S6-pS240 in multiple ABDs.[7] Specifically, we performed a pilot study to search for the presence of this phosphorylated enzyme in the in situ immune response of ABDs by performing immunohistochemistry (IHC) staining on lesional skin biopsies.
Materials and Methods
Subjects of study
We tested 30 biopsies from patients affected by endemic pemphigus foliaceus (EPF) in El Bagre, Colombia, South America (El Bagre-EPF) and 15 skin biopsies from normal controls from the El Bagre-EPF endemic area (NCEA). Patient consents were obtained with Institutional Review Board permission.[3,4,5] We also utilized 30 control skin biopsies from healthy plastic surgery reduction patients in the USA, taken from the chest and/or abdomen normal human skin (NHS). Biopsies were fixed in 10% buffered formalin, then embedded in paraffin and cut at 4 micron thicknesses. The tissue was then submitted for hematoxylin and eosin (H and E) and immunohistochemical (IHC) staining. In addition, we tested biopsies from the archival files of two private, board certified dermatopathology laboratories in the USA; these patients underwent primary diagnostic biopsies, and were not taking immunosuppressive therapeutic medications at the time of biopsy. We evaluated 20 biopsies from bullous pemphigoid (BP) patients, 20 from patients with pemphigus vulgaris (PV), eight patient biopsies with pemphigus foliaceus (PF), and 12 from patients with dermatitis herpetiformis (DH). For all of the El Bagre area patients and controls, we obtained written consent, as well as Institutional Review Board (IRB) permission from the local hospital. The archival biopsies were IRB exempt due to the lack of patient identifiers. In both dermatopathology laboratories, each biopsy also was sent also for direct immunofluorescence (DIF) for correlation with the H and E diagnoses. The IHC stains were performed as previously described.[8,9,10,11]
IHC
We performed our IHC studies to assist in differentiation between specific pathologic autoreactivity, and nonspecific intrinsic autofluorescence (produced by the physiological presence of autofluorescent molecules). Specifically, our antibody was conjugated with horseradish peroxidase (HRP) labeled secondary antibodies. For all our IHC testing, we used a dual endogenous peroxidase blockage, with the addition of an Envision dual link to assist in chromogen attachment. We then applied the chromogen 3,3-diaminobenzidine (DAB), and counterstained with hematoxylin. The samples were run in a Dako (Carpinteria, California, USA) Autostainer Universal Staining System. Positive and negative controls were consistently performed. Our studies were specifically performed as previously described.[11,12,13] We utilized monoclonal mouse antihuman ribosomal protein antibody S6-pS240; phosphorilation site specific, clone DAK-S6-240, Dako catalog No. M7300, at a dilution of 1:50.
Statistical analysis
For statistical analysis, the nonparametric Mann-Whitney U-test was used to calculate significant levels for all measurements. Values of P < 0.05 were considered statistically significant.
Results
Among patients with El Bagre EPF, 23/30 exhibited positive staining in spotty areas of the epidermal corneal layer, and around neurovascular supply structures of dermal eccrine glands and hair follicles. Very active clinical cases were strongly positive at within the epidermal stratum granulosum (including the middle layers of hair follicles), sebaceous glands, and especially in their base membranes. Only two controls from the endemic area displayed positive staining, specifically with focal corneal reactivity (P < 0.05); controls from the USA stained uniformly negative (P < 0.05). Among BP patients, 17/20 stained positive for S6-pS240 in dermal eccrine glands, subjacent to disease blisters, along the bases of the blisters, and within dermal endothelial-mesenchymal cell junction-like structures (P < 0.05) [Table 1]. In patients with PV, 15/20 stained positive within upper dermal inflammatory infiltrates, and both inside and around epidermal disease blisters (P < 0.05). In patient biopsies with PF, 5/8 stained positive within the epidermal stratum granulosum and in one very active clinical case was also positive at the middle layers of hair follicles), sebaceous glands and especially in their base membranes, and around dermal eccrine glands and hair follicles. Among patients with DH, 8/12 exhibited positive staining, primarily subjacent to the base membrane zone where the majority of the inflammatory infiltrate was present (P < 0.05). Figure 1 documents our most commonly observed patterns of S6-pS240 staining positivity in patients with ABDs. The majority of the normal skin controls and the controls from the endemic area of pemphigus were negative for S6-pS240 staining (P < 0.05). Our percent expressions of S6-pS240 and respective patterns of positivity are summarized in Table 1.
Table 1.
Percentages and patterns of positive IHC staining in ABDs with S6-pS240
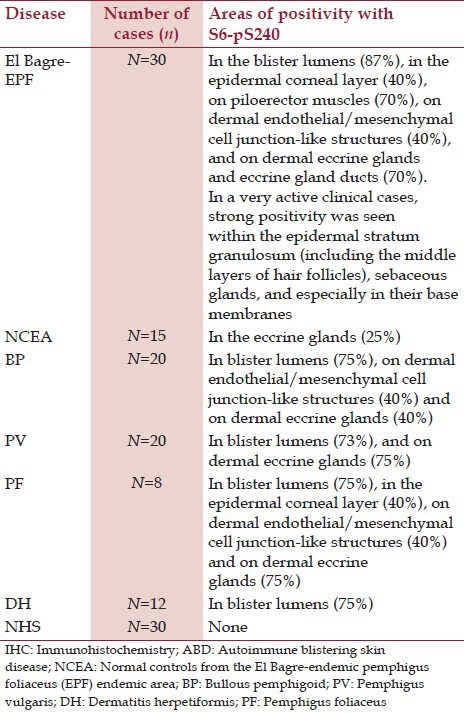
Figure 1.
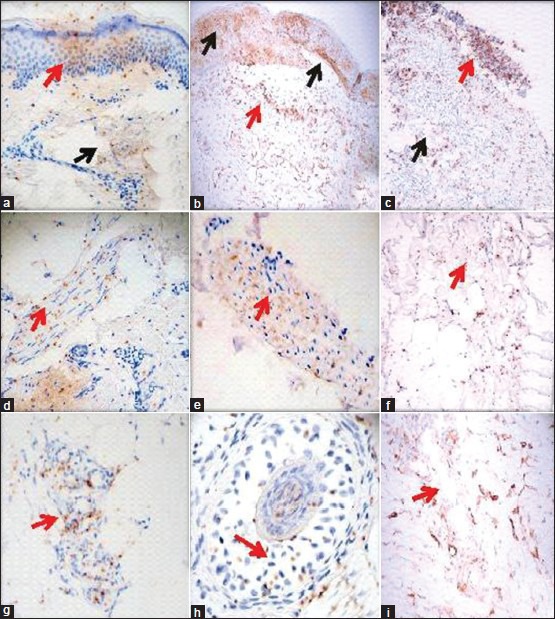
Positive staining of S6-pS240 immunohistochemistry (IHC) in multiple autoimmune blistering diseases. All IHC stains were performed utilizing the monoclonal mouse antihuman ribosomal protein S6-pS240. (a) A representative case of pemphigus foliaceus (PF), showing reactivity in a dermal eccrine sweat duct (brown staining, black arrow) and in the epidermal stratum granulosum and corneal layer (brown staining; red arrow). (b) A representative case of PV, demonstrating reactivity in the epidermis in proximity to a pathogenic blister, as well as on the blister roof (brown staining; black arrows) and in a mesenchymal/endothelial cell junction-like structure in the dermis (brown staining; red arrow). (c) A BP case, displaying positivity in a subepidermal blister area (brown staining; red arrow) and in a dermal mesenchymal/endothelial cell junction-like structure (brown staining; black arrow). (d and e) Two different cases of El Bagre-endemic pemphigus foliaceus (EPF), demonstrating reactivity in dermal piloerector muscles (brown staining; red arrows). (f) A DH case, highlighting positivity in a dermal endothelial-mesenchymal cell junction-like structure (brown staining; red arrow). (g and h) Two cases of El Bagre EPF, demonstrating reactivity within a dermal eccrine gland coil and a hair follicle, respectively (brown staining; red arrows). (i) A case of BP, displaying positivity to a dermal mesenchymal-endothelial cell junction-like structure (brown staining; red arrow)
Discussion
Skin deposition of immunoglobulins and complement is part of the pathogenic process in ABDs.[1] We found that S6-pS240 is strongly expressed in most ABS especially in what seem to be anatomical areas where active cellular processes are happening (either for immune response, cell signaling, and or for unknown reasons). This indicates the complex immune regulation and their cells signaling network. In the pathophysiology of these diseases, complex intracellular signaling networks monitor diverse environmental inputs to evoke appropriate and coordinated effectors responses. In general, defective signal transduction underlies the pathophysiology of many disorders, including autoimmune diseases.[2,3,4,5,6] Several pathways of intracellular signaling have been previously shown in ABDs, possibly triggered by the disease autoantibodies.[2,3,4,5] The major components of intracellular signaling and complex metabolic interactions between the multiple networks including protein kinases and protein phosphatases, which catalyze the reversible phosphorylation of proteins. S6-pS240 specifically represents an intracellular enzyme directly involved in protein synthesis, cell growth, and phosphorylation. S6-pS240 has been demonstrated to be present in subcorneal pustular dermatosis,[12] as well as in psoriasis where mTOR and S6-pS240 represent downstream signaling components.[14]
In our study, we document the presence of S6-pS240 in areas where the autoimmune blistering processes occur, as well as in dermal inflammatory immune response areas. Since we present a pilot study, we only can speculate that S6-pS240 may be involved in influencing protein synthesis and/or with the inflammatory/anti-inflammatory immune response in patients affected by ABDs. We have recently documented autoimmune responses, as well as protease and proteinase inhibitors in areas where the S6-pS240 was positive in the current study. These areas include disease blisters, the epidermal corneal layer, upper dermal inflammatory infiltrates (especially around neurovascular supply packages), piloerector muscles, sweat glands, and ducts and endothelial-mesenchymal cell junction-like areas.[13] Further, the aim of the current study was not to evaluate the presence of ribosomal S6-pS240 among all inflammatory skin diseases, but exclusively in ABDs. Notably, we have also reported S6-pS240 to be positive in psoriasis.[15] S6-pS240 reacts with human ribosomal protein S6 to phosphorylate at serine residue 240 (pS240). The phosphorylation of ribosomal protein S6 correlates with an increase in translation of mRNAs that encode for proteins involved in cell cycle progression and thus controlling mammalian cell growth and proliferation; thus, we speculate that this molecule could be activated for protein synthesis needed to repair lesional skin in these disorders.
Conclusion
We note that S6-pS240 is expressed in lesional skin of patients with ABDs, and also in areas where an active metabolism is happening (for unknown reasons); however, because this was a pilot study, we do not know if this activation is specific for each ABD. We further suggest that S6-pS240 intracellular signaling per se could be activated in ABDs and other inflammatory skin conditions, or could be related to a different, unspecified inflammatory signal present in these disorders.
Acknowledgments
To the staff at the Hospital Nuestra Senora del Carmen in El Bagre, Colombia, SA; to Mineros SA, Medellin, Colombia; to the mayor and the Secretary of Health in El Bagre, and to Jonathan S. Jones, HT (ASCP) at Georgia Dermatopathology Associates for excellent technical assistance.
Footnotes
Source of Support: Georgia Dermatopathology Associates, Atlanta, Georgia, USA (MSH, AMAV); Mineros SA, Medellin, Colombia, SA; Embassy of Japan in Colombia, Medellin; University of Antioquia, Medellin; Knoxville Dermatopathology Laboratory, Knoxville, Tennessee, USA (PBG)
Conflict of Interest: None declared.
References
- 1.Kneisel A, Hertl M. Autoimmune bullous skin diseases. Part 2: Diagnosis and therapy. J Dtsch Dermatol Ges. 2011;9:927–47. doi: 10.1111/j.1610-0387.2011.07809.x. [DOI] [PubMed] [Google Scholar]
- 2.Osada K, Seishima M, Kitajima Y. Pemphigus IgG activates and translocates protein kinase C from the cytosol to the particulate/cytoskeleton fractions in human keratinocytes. J Invest Dermatol. 1997;108:482–7. doi: 10.1111/1523-1747.ep12289726. [DOI] [PubMed] [Google Scholar]
- 3.Chernyavsky AI, Arredondo J, Kitajima Y, Sato-Nagai M, Grando SA. Desmoglein versus non-desmoglein signaling in pemphigus acantholysis: Characterization of novel signaling pathways downstream of pemphigus vulgaris antigens. J Biol Chem. 2007;282:13804–12. doi: 10.1074/jbc.M611365200. [DOI] [PubMed] [Google Scholar]
- 4.Spindler V, Waschke J. Role of Rho GTPases in desmosomal adhesion and pemphigus pathogenesis. Ann Anat. 2011;193:177–80. doi: 10.1016/j.aanat.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Spindler V, Vielmuth F, Schmidt E, Rubenstein DS, Waschke J. Protective endogenous cyclic adenosine 5ș-monophosphate signaling triggered by pemphigus autoantibodies. J Immunol. 2010;185:6831–8. doi: 10.4049/jimmunol.1002675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pretel M, España A, Marquina M, Pelacho B, López-Picazo JM, López-Zabalza MJ. An imbalance in Akt/mTOR is involved in the apoptotic and acantholytic processes in a mouse model of pemphigus vulgaris. Exp Dermatol. 2009;18:771–80. doi: 10.1111/j.1600-0625.2009.00893.x. [DOI] [PubMed] [Google Scholar]
- 7.Ruvinsky I, Meyuhas O. Ribosomal protein S6 phosphorylation: From protein synthesis to cell size. Trends Biochem Sci. 2006;31:342–8. doi: 10.1016/j.tibs.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Abrèu-Velez AM, Beutner EH, Montoya F, Bollag WB, Hashimoto T. Analyses of autoantigens in a new form of endemic pemphigus foliaceus in Colombia. J Am Acad Dermatol. 2003;49:609–14. doi: 10.1067/s0190-9622(03)00852-1. [DOI] [PubMed] [Google Scholar]
- 9.Abreu-Velez AM, Robles EV, Howard MS. A new variant of endemic pemphigus foliaceus in El-Bagre, Colombia: The Hardy-Weinberg-Castle law and linked short tandem repeats. N Am J Med Sci. 2009;1:169–78. doi: 10.4297/najms.2009.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abrèu-Velez AM, Hashimoto T, Bollag WB, Tobón Arroyave S, Abrèu-Velez CE, Londoño ML, et al. A unique form of endemic pemphigus in northern Colombia. J Am Acad Dermatol. 2003;49:599–608. doi: 10.1067/s0190-9622(03)00851-x. [DOI] [PubMed] [Google Scholar]
- 11.Abreu-Velez AM, Howard MS, Yi H, Gao W, Hashimoto T, Grossniklaus HE. Neural system antigens are recognized by autoantibodies from patients affected by a new variant of endemic pemphigus foliaceus in Colombia. J Clin Immunol. 2011;31:356–68. doi: 10.1007/s10875-010-9495-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abreu Velez AM, Smith JG, Jr, Howard MS. Subcorneal pustular dermatosis an immunohistopathological perspective. Int J Clin Exp Pathol. 2011;4:526–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Abreu Velez AM, Googe PB, Jr, Jiao Z, Duque-Ramírez M, Arias LF, Howard MS. Tissue inhibitor of metalloproteinase 1, matrix metalloproteinase 9, α-1 antitrypsin, metallothionein and urokinase type plasminogen activator receptor in skin biopsies from patients affected by autoimmune blistering diseases. Our Dermatol Online. 2013;4:275–80. [Google Scholar]
- 14.Buerger C, Malisiewicz B, Eiser A, Hardt K, Boehncke WH. mTOR and its downstream signalling components are activated in psoriatic skin. Br J Dermatol. 2013;169:156–9. doi: 10.1111/bjd.12271. [DOI] [PubMed] [Google Scholar]
- 15.Abreu Velez AM, Howard WR, Howard MS. Upregulation of anti-human ribosomal protein S6-P240, topoisomerase II ά, cyclin D1, BCL-2 and anti-corneal antibodies in acute psoriasis. N Dermatol Online. 2011;3:113–7. [Google Scholar]


