Abstract
Background:
Insulin resistance (IR) has known to be associated with coronary artery disease (CAD), but the assessment of severity of the CAD based on IR in type 2 diabetes mellitus has not been established in detail.
Aims:
The aim of our study was to establish the correlation between IR and the severity of CAD in type 2 diabetes mellitus.
Materials and Methods:
In a cross-sectional study design, 61 consecutive patients with type 2 diabetes mellitus who underwent coronary angiogram for the evaluation of CAD were recruited. Fasting blood glucose, fasting insulin levels, systolic blood pressure and total cholesterol/high density lipoprotein-cholesterol ratio were determined. Homeostasis model assessment-IR (HOMA-IR) was correlated with severity of CAD, which was measured by modified Gensini Score.
Results:
There was a significant correlation between log HOMA-IR and severity of CAD (r = 0.303, P = 0.009) in diabetic patients. Correlation of the Gensini Score with other known risk factors was not significant.
Conclusions:
The results of our study indicate that we might able to predict the severity of CAD by measure of IR.
Keywords: Coronary artery disease, Insulin resistance, Type 2 diabetes mellitus
Introduction
Diabetes mellitus is a well-established risk factor for the development of coronary artery disease (CAD).[1] CAD is the major cause of premature death in diabetic patients, both in type 1 or type 2 diabetes.[2,3,4,5] The risk of CAD in higher in type 2 diabetic patients in comparison to similarly dyslipidemia non-diabetic subjects, even after the correction of several confounders.[6] Patients with type 2 diabetes mellitus have early onset of CAD and the involved vessels show severe disease. The CAD in diabetic patients is characterized by severe, multi vessel, long segment and extensive disease. However, development and extent of CAD is not uniform among patients with CAD.
At present insulin resistance (IR), known to be a pathogenic cause that can predict the occurrence of CAD,[7] but grading of severity or assessment of severity of CAD based on IR has not been studied in detail. The evolution of IR is unique in type 2 diabetes mellitus because it precedes the onset of diabetes and remains fairly constant throughout the disease process from the time of diagnosis,[8] even after the conventional treatment for type 2 diabetes mellitus.[9,10] If significant correlation is established between IR and severity of CAD, it will help us in identifying high risk individuals and we might be able to predict the severity by measure of IR, which is a simple test. Patients with severe and extensive disease who are not candidates for angioplasty can be identified easily.
Hence, the study was designed to evaluate the correlation between IR measured by homeostasis model assessment-IR (HOMA-IR) and severity of angiographically demonstrated CAD as measured by modified Gensini Score, which is well-validated measure of severity of CAD,[11] in patients with type 2 diabetes mellitus.
Materials and Methods
This was a cross-sectional study of type 2 diabetic patients referred for coronary angiogram at a tertiary care hospital. 61 consecutive type 2 diabetic patients who underwent coronary angiogram for the evaluation of CAD were recruited in the study after obtaining informed consent. Those patients who had fluctuating glucose levels, patients on steroids, chronic kidney disease and patients on smoking were excluded from the study. The study protocol was approved by institutional ethics committee.
Fasting blood sugar, total cholesterol and high density lipoprotein-cholesterol were measured by using automated auto analyzer Hitachi P800. The coefficient of variation was < 2% and < 5% for intra- and inter-batch, respectively, in all cases. Systolic blood pressure was recorded using mercury sphygmomanometer. Fasting insulin levels were assayed using insulin enzyme-linked immunosorbent assay kit manufactured by Diagnostic Research group (DRG) legal manufacturer Germany based on sandwich principle. The coefficient of variation was < 3% for intra- and inter-batch assay respectively.
IR was measured by HOMA 2 computerized method,[12] which has been shown to correlate well with euglycemic clamp for use in cross-sectional studies.[13] Blood tests were done 2 weeks after the angiogram to achieve the steady state and to avoid the changes in IR due to the acute stress of the disease and angiographic procedure.[14] Severity of CAD was assessed and calculated by modified Gensini Scoring method.[11] Gensini Scoring was carried out by a Cardiologist, who was blind to other parameters.
Statistical analysis
Correlation between these parameters was assessed by calculating Pearson's correlation coefficient. P < 0.05 was considered to be statistically significant. HOMA-IR values were logarithmically transformed for analysis.[12] Analysis of variance (ANOVA) was performed to find out whether there is a significant relation between mean HOMA-IR and tertile values of modified Gensini Score. Data were analyzed using the statistical package for the social sciences (SPSS) version 16 (SPSS, Chicago, IL, USA). 95% confidence interval (CI) for the correlation coefficient was also determined using the online calculator.
Results
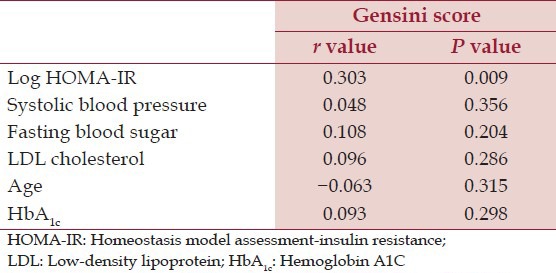
Mean age of the subjects was 58.40 ± 7.35. Median duration of diabetes was 6.00 (Interquartile range: 7.00). Median HOMA-IR among diabetic subjects was 3.37 (Interquartile range: 2.06). The overall Gensini Score ranged from 7 to 147 among diabetes. The tertile partitioning of Gensini Score in our study was defined as low (0-24), mid (25-49) and high (50-147). ANOVA for three groups of Gensini Score showed a step wise significant correlation with mean Log HOMA-IR (F = 4.158, P = 0.021) [Figure 1]. Post hoc analysis with least significant difference showed a significant correlation between lowest and the highest tertile (P = 0.006). Scatterplot depicting the relation between Gensini Score and IR in type 2 diabetic patients is shown in the Figure 2. There was a statistically significant correlation between the log of HOMA-IR and severity of CAD as assessed by Gensini Score (r = 0.303, 95% CI: 0.06-0.52, P = 0.009) in type 2 diabetic patients. Furthermore, there was no significant correlation between severity of CAD and other known risk factors of CAD in type 2 diabetic patients [Table 1].
Figure 1.
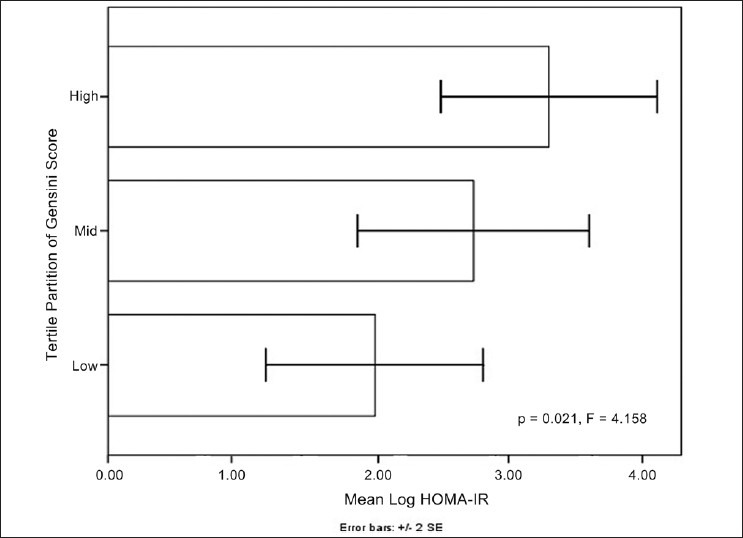
Graph showing stepwise significant correlation between mean log homeostasis model assessment-insulin resistance and tertile partitions of Gensini score
Figure 2.
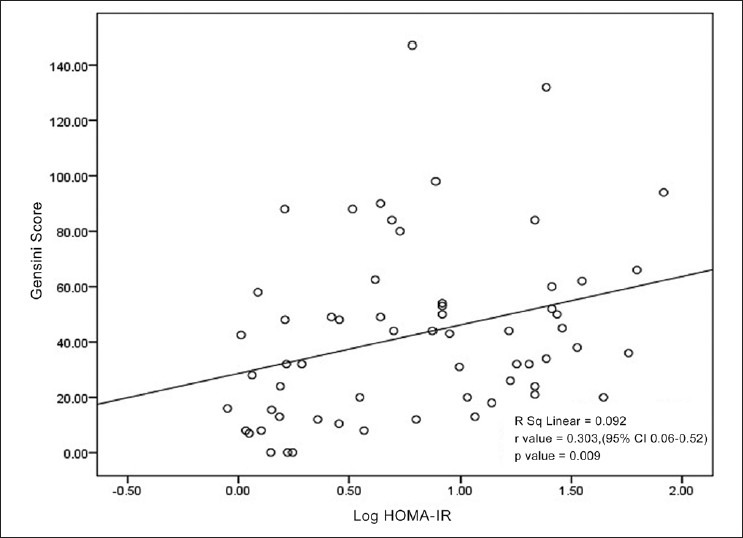
Scatter plot showing positive linear correlation between severity of coronary artery disease (Gensini score) and log of homeostasis model assessment-insulin resistance in type 2 diabetes mellitus
Table 1.
Correlation of Gensini score versus other parameters in type 2 diabetic patients
Discussion
We have evaluated the correlation between HOMA-IR and angiographic severity of CAD in 61 consecutive type 2 diabetic patients who underwent angiogram for the evaluation of coronary heart disease. IR was associated with the presence of CAD in diabetic patients,[6,7] but correlation with the severity was not studied. In this study, we have shown that there is a positive linear correlation between these parameters, which is statistically significant (r = 0.303 and P = 0.009).
Diabetic patients have early onset of CAD and the involved vessels show severe disease. Median duration of diabetes was just 6.00 (Interquartile range: 7.00) in our study.
The process is multifactorial and the known risk factors account for about 25% of the disease.[6] Other components of metabolic syndrome were not correlating well with the angiographic severity of the CAD in spite of being associated with the disease.[15] In addition to being associated with the disease, presence of positive linear correlation observed in our study, strengthens the possibility of cause-effect relationship.
Our study establishes the important role of IR in the pathogenesis of diabetic vascular disease. Numerous data also suggest that IR has a central role in the atherosclerosis.[15] IR is closely related with an increased risk of cardiovascular disease.[16] Altered insulin signaling in endothelial cells, has emerged as an important mechanism for the increased susceptibility to cardiovascular disease.[16] Endothelial dysfunction, which develops due to this alteration contributes to progressive atherosclerosis along with the proinflammatory state induced by IR.[16] Although IR is known to be a part of the metabolic syndrome, the other clinical markers like truncal obesity and body mass index has low sensitivity and specificity in identifying IR.[15]
IR is the only component of the metabolic syndrome, which remains relatively constant throughout the natural history of type 2 diabetes mellitus.[8] Even with conventional treatment of type 2 diabetes mellitus, IR as measured by HOMA-IR method has been shown to be relatively constant during the many years of treatment in UK prospective diabetes study study.[9,10] All the other anthropometric measurements and biochemical risk factors change over the period of time with or without treatment. This unique feature of the IR along with its correlation with severity of angiographic score will help us in identifying high risk individuals. A type 2 diabetic patient whose HOMA-IR, very high at the time of diagnosis may be prone to develop severe vascular disease and may benefit with very aggressive medical management.
Another implication of the study is the necessity of focusing on IR as a target for intervention in type 2 diabetes mellitus. The aggressive glucose reduction has not yielded the desired results in terms of reducing the vascular complications.[16] Instead, the association of IR with endothelial dysfunction suggests that modifying this pathological link may be an alternative therapeutic strategy.[16]
Further IR as measured by HOMA might aid in predicting the severity of CAD and its clinical relevance especially in resource limited setting. Since measurement of IR by HOMA, is easier to perform, has been shown to correlate well with euglycemic clamp method, a reference standard method for measuring IR[13] and has the potential to be used in routine clinical practice. Thus in mere future we might be able to predict the severity of CAD by means of HOMA-IR and patients with extensive and severe disease who are not candidates for angioplasty can be identified easily.
The limitation of this study is the cross-section design. Though our study did not show a significant correlation between other well-known risk factors and severity of CAD, long-term follow-up with HOMA-IR measured in the beginning of the disease and compared with angiographic findings after a few years along with other known risk factors might allow us to evaluate the strength of each factor in relation to other variables.
Conclusion
Based on the limitations, the study highlights the importance of IR being a major risk factor for CAD in type 2 diabetes mellitus. Thus, we might be able to predict the severity by measure of IR which is a simple test. Patients with severe and extensive disease who are not the candidate for angioplasty can be identified easily.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Fein F, Scheuer J. Heart disease in diabetes. In: Rifkin H Jr, editor. Diabetes Mellitus: Theory and Practice. New York, NY: Elsevier Science Publishing Co, Inc; 1990. pp. 812–23. [Google Scholar]
- 2.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241:2035–8. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 3.Welborn TA, Wearne K. Coronary heart disease incidence and cardiovascular mortality in Busselton with reference to glucose and insulin concentrations. Diabetes Care. 1979;2:154–60. doi: 10.2337/diacare.2.2.154. [DOI] [PubMed] [Google Scholar]
- 4.Eschwege E, Richard JL, Thibult N, Ducimetière P, Warnet JM, Claude JR, et al. Coronary heart disease mortality in relation with diabetes, blood glucose and plasma insulin levels. The Paris prospective study, ten years later. Horm Metab Res Suppl. 1985;15:41–6. [PubMed] [Google Scholar]
- 5.Pyörälä K, Savolainen E, Kaukola S, Haapakoski J. Plasma insulin as coronary heart disease risk factor: Relationship to other risk factors and predictive value during 9 1/2-year follow-up of the Helsinki policemen study population. Acta Med Scand Suppl. 1985;701:38–52. doi: 10.1111/j.0954-6820.1985.tb08888.x. [DOI] [PubMed] [Google Scholar]
- 6.Bonora E, Formentini G, Calcaterra F, Lombardi S, Marini F, Zenari L, et al. HOMA-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: Prospective data from the verona diabetes complications study. Diabetes Care. 2002;25:1135–41. doi: 10.2337/diacare.25.7.1135. [DOI] [PubMed] [Google Scholar]
- 7.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 8.Kendall DM, Cuddihy RM, Bergenstal RM. Clinical application of incretin-based therapy: Therapeutic potential, patient selection and clinical use. Am J Med. 2009;122:S37–50. doi: 10.1016/j.amjmed.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Matthews DR, Cull CA, Stratton IM, Holman RR, Turner RC. UKPDS 26: Sulphonylurea failure in non-insulin-dependent diabetic patients over six years. UK prospective diabetes study (UKPDS) group. Diabet Med. 1998;15:297–303. doi: 10.1002/(SICI)1096-9136(199804)15:4<297::AID-DIA572>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 10.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia. 2003;46:3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 11.Kim JY, Mun HS, Lee BK, Yoon SB, Choi EY, Min PK, et al. Impact of metabolic syndrome and its individual components on the presence and severity of angiographic coronary artery disease. Yonsei Med J. 2010;51:676–82. doi: 10.3349/ymj.2010.51.5.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–95. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 13.Nishio K, Fukui T, Tsunoda F, Kawamura K, Itoh S, Konno N, et al. Insulin resistance as a predictor for restenosis after coronary stenting. Int J Cardiol. 2005;103:128–34. doi: 10.1016/j.ijcard.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 14.Kwon K, Choi D, Koo BK, Ryu SK. Decreased insulin sensitivity is associated with the extent of coronary artery disease in patients with angina. Diabetes Obes Metab. 2005;7:579–85. doi: 10.1111/j.1463-1326.2004.00438.x. [DOI] [PubMed] [Google Scholar]
- 15.Zornitzki T, Ayzenberg O, Gandelman G, Vered S, Yaskil E, Faraggi D, et al. Diabetes, but not the metabolic syndrome, predicts the severity and extent of coronary artery disease in women. QJM. 2007;100:575–81. doi: 10.1093/qjmed/hcm066. [DOI] [PubMed] [Google Scholar]
- 16.Aziz A, Wheatcroft S. Insulin resistance in Type 2 diabetes and obesity: Implications for endothelial function. Expert Rev Cardiovasc Ther. 2011;9:403–7. doi: 10.1586/erc.11.20. [DOI] [PubMed] [Google Scholar]


