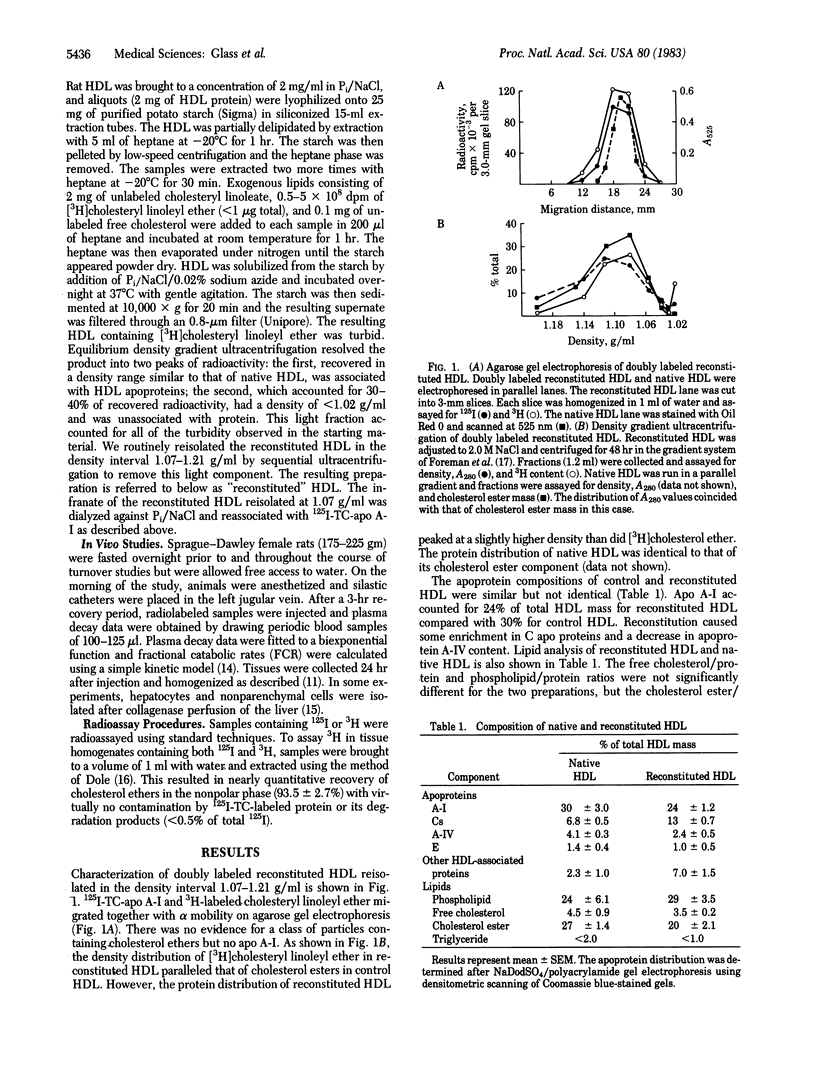
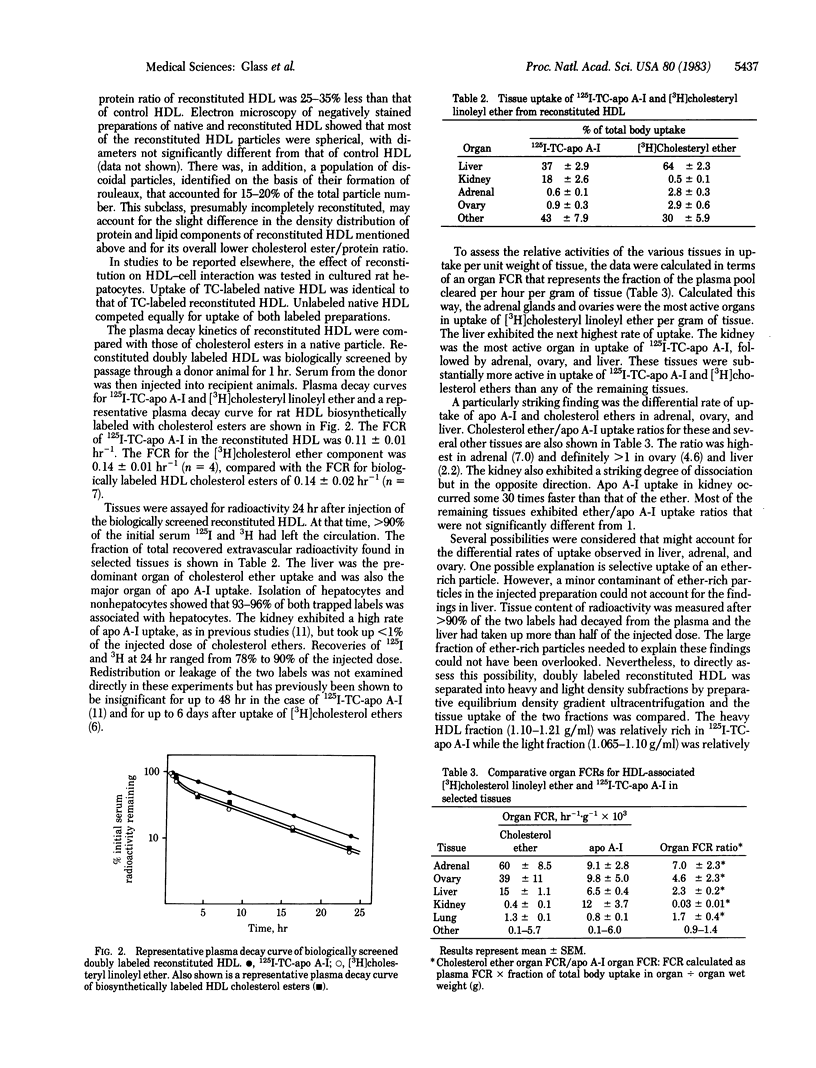
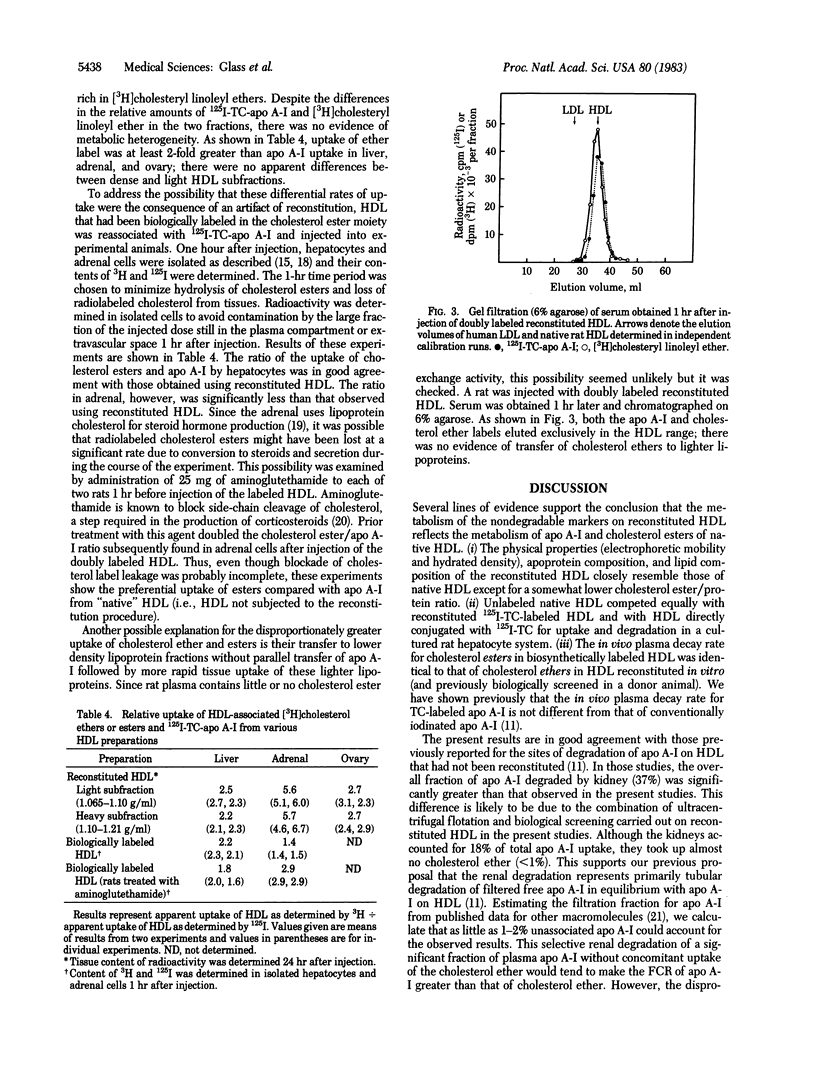
Abstract
The metabolic fate of homologous high density lipoprotein (HDL) was studied in the rat, tracing the apoprotein A-I (apo A-I) and cholesterol ester moieties simultaneously. The apo A-I was labeled with covalently linked 125I-labeled tyramine cellobiose, which accumulates in the cells degrading the apoprotein; [3H]cholesterol ethers, which cannot be hydrolyzed or mobilized after uptake, were incorporated into the lipid core of reconstituted HDL to reflect the fate of the cholesterol esters. Several lines of evidence, including direct comparison with biologically labeled HDL, are presented to support the validity of this approach. The liver was the major organ of cholesterol ether uptake, accounting for 65% of the total; the adrenal gland and ovary were the most active organs per gram (wet) of weight. Uptake of cholesterol ether was 7-fold greater than that of apo A-I in adrenal, 4-fold greater in the ovary, and greater than 2-fold greater in the liver. The remaining tissues took up apo A-I and cholesterol ethers at more nearly equal rates. Transfer of HDL-associated cholesterol ethers and 125I-labeled apo A-I to other lipoprotein fractions was not observed; thus, the results reflect direct uptake from HDL itself. Whereas uptake of low density lipoprotein appears to involve endocytosis of intact particles, uptake of HDL in at least some rat tissues involves additional, more complex, transfer mechanisms.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen J. M., Dietschy J. M. Relative importance of high and low density lipoproteins in the regulation of cholesterol synthesis in the adrenal gland, ovary, and testis of the rat. J Biol Chem. 1978 Dec 25;253(24):9024–9032. [PubMed] [Google Scholar]
- Barter P. J., Lally J. I. The activity of an esterified cholesterol transferring factor in human and rat serum. Biochim Biophys Acta. 1978 Nov 22;531(2):233–236. doi: 10.1016/0005-2760(78)90147-9. [DOI] [PubMed] [Google Scholar]
- Chajek-Shaul T., Friedman G., Halperin G., Stein O., Stein Y. Uptake of chylomicron [3H]cholesteryl linoleyl ether by mesenchymal rat heart cell cultures. Biochim Biophys Acta. 1981 Oct 23;666(1):147–155. doi: 10.1016/0005-2760(81)90100-4. [DOI] [PubMed] [Google Scholar]
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. A., Engelhorn S. C., Pangburn S. H., Weinstein D. B., Steinberg D. Very low density lipoprotein synthesis and secretion by cultured rat hepatocytes. J Biol Chem. 1979 Mar 25;254(6):2010–2016. [PubMed] [Google Scholar]
- Fielding C. J. Metabolism of cholesterol-rich chylomicroms. Mechanism of binding and uptake of cholesteryl esters by the vascular bed of the perfused rat heart. J Clin Invest. 1978 Jul;62(1):141–151. doi: 10.1172/JCI109099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. R., Karlin J. B., Edelstein C., Juhn D. J., Rubenstein A. H., Scanu A. M. Fractionation of human serum lipoproteins by single-spin gradient ultracentrifugation: quantification of apolipoproteins B and A-1 and lipid components. J Lipid Res. 1977 Nov;18(6):759–767. [PubMed] [Google Scholar]
- Friedman G., Chajek-Shaul T., Stein O., Olivecrona T., Stein Y. The role of lipoprotein lipase in the assimilation of cholesteryl linoleyl ether by cultured cells incubated with labeled chylomicrons. Biochim Biophys Acta. 1981 Oct 23;666(1):156–164. doi: 10.1016/0005-2760(81)90101-6. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Pittman R. C., Keller G. A., Steinberg D. Tissue sites of degradation of apoprotein A-I in the rat. J Biol Chem. 1983 Jun 10;258(11):7161–7167. [PubMed] [Google Scholar]
- Glomset J. A. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res. 1968 Mar;9(2):155–167. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Gordon T., Castelli W. P., Hjortland M. C., Kannel W. B., Dawber T. R. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977 May;62(5):707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- Gwynne J. T., Hess B. The role of high density lipoproteins in rat adrenal cholesterol metabolism and steroidogenesis. J Biol Chem. 1980 Nov 25;255(22):10875–10883. [PubMed] [Google Scholar]
- Gwynne J. T., Strauss J. F., 3rd The role of lipoproteins in steroidogenesis and cholesterol metabolism in steroidogenic glands. Endocr Rev. 1982 Summer;3(3):299–329. doi: 10.1210/edrv-3-3-299. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger M., Brown M. S., Faust J. R., Goldstein J. L. Replacement of endogenous cholesteryl esters of low density lipoprotein with exogenous cholesteryl linoleate. Reconstitution of a biologically active lipoprotein particle. J Biol Chem. 1978 Jun 25;253(12):4093–4101. [PubMed] [Google Scholar]
- MATTHEWS C. M. The theory of tracer experiments with 131I-labelled plasma proteins. Phys Med Biol. 1957 Jul;2(1):36–53. doi: 10.1088/0031-9155/2/1/305. [DOI] [PubMed] [Google Scholar]
- O'Hare M. J., Neville A. M. The steroidogenic response of adult rat adrenocortical cells in monolayer culture. J Endocrinol. 1973 Mar;56(3):537–549. doi: 10.1677/joe.0.0560537. [DOI] [PubMed] [Google Scholar]
- Pittman R. C., Carew T. E., Glass C. K., Green S. R., Taylor C. A., Jr, Attie A. D. A radioiodinated, intracellularly trapped ligand for determining the sites of plasma protein degradation in vivo. Biochem J. 1983 Jun 15;212(3):791–800. doi: 10.1042/bj2120791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool G. L., French M. E., Edwards R. A., Huang L., Lumb R. H. Use of radiolabeled hexadecyl cholesteryl ether as a liposome marker. Lipids. 1982 Jun;17(6):448–452. doi: 10.1007/BF02535225. [DOI] [PubMed] [Google Scholar]
- Stein O., Halperin G., Stein Y. Biological labeling of very low density lipoproteins with cholesteryl linoleyl ether and its fate in the intact rat. Biochim Biophys Acta. 1980 Nov 7;620(2):247–260. doi: 10.1016/0005-2760(80)90206-4. [DOI] [PubMed] [Google Scholar]
- Stein Y., Stein O., Halperin G. Use of 3H-cholesteryl linoleyl ether for the quantitation of plasma cholesteryl ester influx into the aortic wall in hypercholesterolemic rabbits. Arteriosclerosis. 1982 Jul-Aug;2(4):281–289. doi: 10.1161/01.atv.2.4.281. [DOI] [PubMed] [Google Scholar]
- Uzgiris V. I., Graves P. E., Salhanick H. A. Ligand modification of corpus luteum mitochondrial cytochrome P-450 spectra and cholesterol monooxygenation: an assay of enzyme-specific inhibitors. Biochemistry. 1977 Feb 22;16(4):593–600. doi: 10.1021/bi00623a006. [DOI] [PubMed] [Google Scholar]



