Abstract
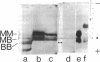
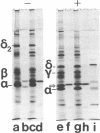
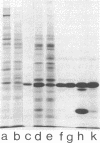
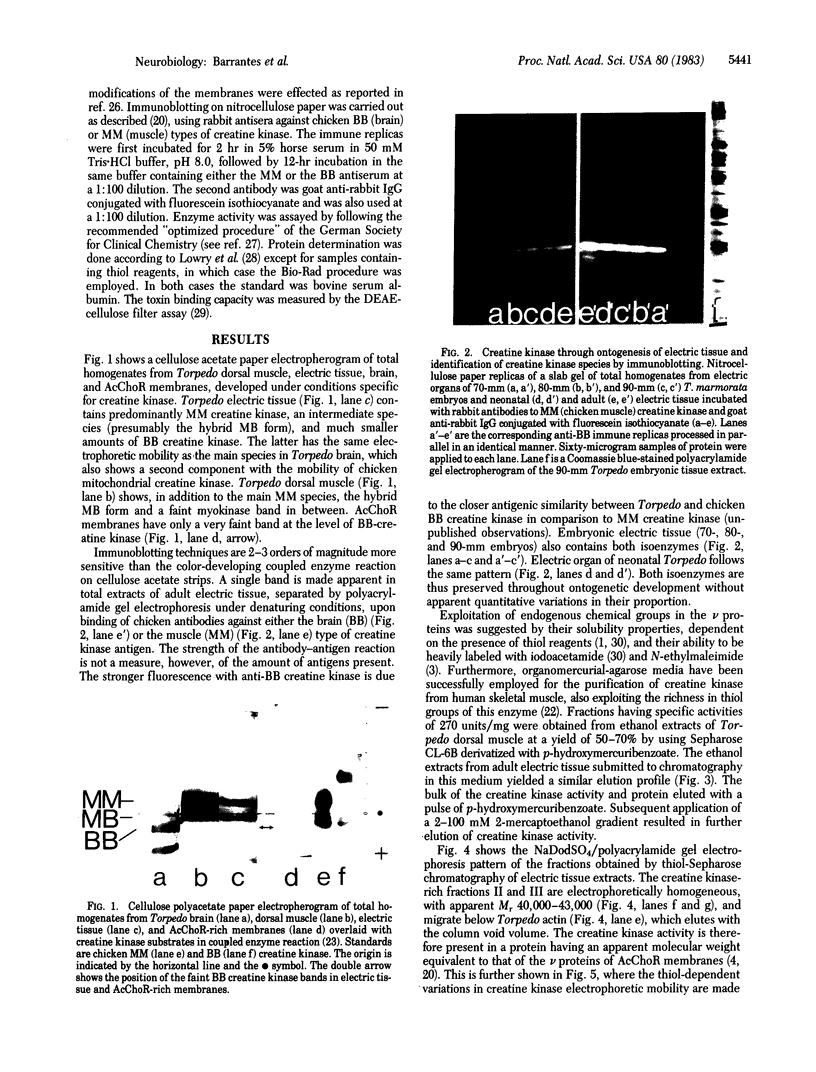
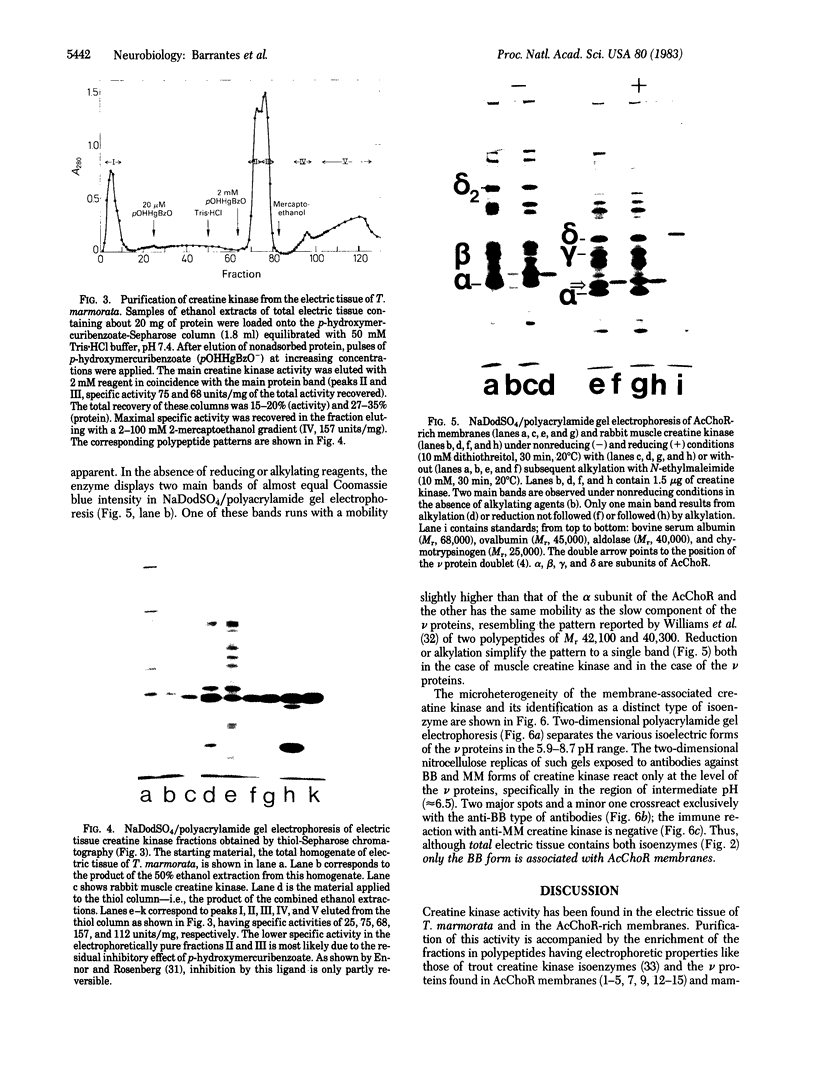
The nonreceptor, peripheral v proteins (Mr 43,000 proteins) are conspicuous components of the acetylcholine receptor-rich membranes and the Torpedo electrocyte, so far devoid of any known enzymatic function. Creatine kinase (adenosine 5'-triphosphate:creatine N-phosphotransferase, EC 2.7.3.2) is identified in distinct polypeptides belonging to the family of v proteins. Embryonic (70- to 90-mm embryos), neonatal, and adult electric organs of Torpedo marmorata contain two isoenzymes of creatine kinase: the BB (brain) and the MM (muscle) forms. The proportion of the two isoenzymes does not appear to change in the course of ontogenic and postnatal development. Only the BB isoenzyme appears to be associated with the acetylcholine-rich membranes in adult Torpedo. The creatine kinase can be purified to homogeneity by chromatographic procedures that exploit the richness in free sulfhydryl groups of the enzyme. Specific activities of 150 units/mg are obtained from electric tissue. The enzyme subunits identified by two-dimensional gel electrophoresis and immunoblotting techniques have pI values in the 6.0-6.5 region and apparent molecular weights in the 40,000-43,000 range, the latter values depending on redox conditions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrantes F. J., Mieskes G., Wallimann T. A membrane-associated creatine kinase (EC 2.7.3.2) identified as an acidic species of the non-receptor, peripheral nu-proteins in Torpedo acetylcholine receptor membranes. FEBS Lett. 1983 Feb 21;152(2):270–276. doi: 10.1016/0014-5793(83)80394-9. [DOI] [PubMed] [Google Scholar]
- Barrantes F. J. Modulation of acetylcholine receptor states by thiol modification. Biochemistry. 1980 Jun 24;19(13):2957–2965. doi: 10.1021/bi00554a022. [DOI] [PubMed] [Google Scholar]
- Barrantes F. J., Neugebauer D. C., Zingsheim H. P. Peptide extraction by alkaline treatment is accompanied by rearrangement of the membrane-bound acetylcholine receptor from Torpedo marmorata. FEBS Lett. 1980 Mar 24;112(1):73–78. doi: 10.1016/0014-5793(80)80131-1. [DOI] [PubMed] [Google Scholar]
- Barrantes F. J. Oligomeric forms of the membrane-bound acetylcholine receptor disclosed upon extraction of the Mr 43,000 nonreceptor peptide. J Cell Biol. 1982 Jan;92(1):60–68. doi: 10.1083/jcb.92.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholdi M., Barrantes F. J., Jovin T. M. Rotational molecular dynamics of the membrane-bound acetylcholine receptor revealed by phosphorescence spectroscopy. Eur J Biochem. 1981 Nov;120(2):389–397. doi: 10.1111/j.1432-1033.1981.tb05716.x. [DOI] [PubMed] [Google Scholar]
- Beis I., Newsholme E. A. The contents of adenine nucleotides, phosphagens and some glycolytic intermediates in resting muscles from vertebrates and invertebrates. Biochem J. 1975 Oct;152(1):23–32. doi: 10.1042/bj1520023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray G. M., Ferrendelli J. A. Serum creatine phosphokinase in muscle disease. An evaluation of two methods of determination and comparison with serum aldolase. Neurology. 1968 May;18(5):480–484. doi: 10.1212/wnl.18.5.480. [DOI] [PubMed] [Google Scholar]
- Cartaud J., Sobel A., Rousselet A., Devaux P. F., Changeux J. P. Consequences of alkaline treatment for the ultrastructure of the acetylcholine-receptor-rich membranes from Torpedo marmorata electric organ. J Cell Biol. 1981 Aug;90(2):418–426. doi: 10.1083/jcb.90.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado M., Eibl H., Barrantes F. J. Effects of lipids on acetylcholine receptor. Essential need of cholesterol for maintenance of agonist-induced state transitions in lipid vesicles. Biochemistry. 1982 Jul 20;21(15):3622–3629. doi: 10.1021/bi00258a015. [DOI] [PubMed] [Google Scholar]
- ENNOR A. H., ROSENBERG H. Some properties of creatine phosphokinase. Biochem J. 1954 Jun;57(2):203–212. doi: 10.1042/bj0570203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J., Blanchard S. G., Wu W., Miller J., Strader C. D., Hartig P., Moore H. P., Racs J., Raftery M. A. Purification of Torpedo californica post-synaptic membranes and fractionation of their constituent proteins. Biochem J. 1980 Mar 1;185(3):667–677. doi: 10.1042/bj1850667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppenberger H. M., Scholl A., Ursprung H. Tissue-specific isoenzyme patterns of creatine kinase (2.7.3.2.) in trout. FEBS Lett. 1971 May 20;14(5):317–319. doi: 10.1016/0014-5793(71)80289-2. [DOI] [PubMed] [Google Scholar]
- Froehner S. C., Gulbrandsen V., Hyman C., Jeng A. Y., Neubig R. R., Cohen J. B. Immunofluorescence localization at the mammalian neuromuscular junction of the Mr 43,000 protein of Torpedo postsynaptic membranes. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5230–5234. doi: 10.1073/pnas.78.8.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gysin R., Wirth M., Flanagan S. D. Structural heterogeneity and subcellular distribution of nicotinic synapse-associated proteins. J Biol Chem. 1981 Nov 25;256(22):11373–11376. [PubMed] [Google Scholar]
- Hamilton S. L., McLaughlin M., Karlin A. Formation of disulfide-linked oligomers of acetylcholine receptor in membrane from torpedo electric tissue. Biochemistry. 1979 Jan 9;18(1):155–163. doi: 10.1021/bi00568a024. [DOI] [PubMed] [Google Scholar]
- Jerusalem F., Engel A. G., Gomez M. R. Duchenne dystrophy. II. Morphometric study of motor end-plate fine structure. Brain. 1974 Mar;97(1):123–130. doi: 10.1093/brain/97.1.123. [DOI] [PubMed] [Google Scholar]
- Klymkowsky M. W., Heuser J. E., Stroud R. M. Protease effects on the structure of acetylcholine receptor membranes from Torpedo californica. J Cell Biol. 1980 Jun;85(3):823–838. doi: 10.1083/jcb.85.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lo M. M., Garland P. B., Lamprecht J., Barnard E. A. Rotational mobility of the membrane-bound acetylcholine receptor of Torpedo electric organ measured by phosphorescence depolarisation. FEBS Lett. 1980 Mar 10;111(2):407–412. doi: 10.1016/0014-5793(80)80838-6. [DOI] [PubMed] [Google Scholar]
- Madelian V., Warren W. A. Affinity chromatography of creatine kinase. Anal Biochem. 1975 Apr;64(2):517–520. doi: 10.1016/0003-2697(75)90462-5. [DOI] [PubMed] [Google Scholar]
- Neubig R. R., Krodel E. K., Boyd N. D., Cohen J. B. Acetylcholine and local anesthetic binding to Torpedo nicotinic postsynaptic membranes after removal of nonreceptor peptides. Proc Natl Acad Sci U S A. 1979 Feb;76(2):690–694. doi: 10.1073/pnas.76.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- PEARSON C. M. Histopathological features of muscle in the preclinical stages of muscular dystrophy. Brain. 1962 Mar;85:109–120. doi: 10.1093/brain/85.1.109. [DOI] [PubMed] [Google Scholar]
- Richardson G. P., Krenz W. D., Kirk C., Fox G. Q. Organotypic culture of embryonic electromotor system tissues from Torpedo marmorata. Neuroscience. 1981;6(6):1181–1200. doi: 10.1016/0306-4522(81)90082-8. [DOI] [PubMed] [Google Scholar]
- Rousselet A., Cartaud J., Devaux P. F. Importance des interactions protéine-protéine dans les maintien de la structure des fragments excitables de l'organe électrique de Torpedo marmorata. C R Seances Acad Sci D. 1979 Sep 24;289(5):461–463. [PubMed] [Google Scholar]
- Saitoh T., Wennogle L. P., Changeux J. P. Factors regulating the susceptibility of the acetylcholine receptor protein to heat inactivation. FEBS Lett. 1979 Dec 15;108(2):489–494. doi: 10.1016/0014-5793(79)80595-5. [DOI] [PubMed] [Google Scholar]
- Schmidt J., Raftery M. A. A simple assay for the study of solubilized acetylcholine receptors. Anal Biochem. 1973 Apr;52(2):349–354. doi: 10.1016/0003-2697(73)90036-5. [DOI] [PubMed] [Google Scholar]
- Sealock R. Cytoplasmic surface structure in postsynaptic membranes from electric tissue visualized by tannic-acid-mediated negative contrasting. J Cell Biol. 1982 Feb;92(2):514–522. doi: 10.1083/jcb.92.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel A., Heidmann T., Hofler J., Changeux J. P. Distinct protein components from Torpedo marmorata membranes carry the acetylcholine receptor site and the binding site for local anesthetics and histrionicotoxin. Proc Natl Acad Sci U S A. 1978 Jan;75(1):510–514. doi: 10.1073/pnas.75.1.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel A., Weber M., Changeux J. P. Large-scale purification of the acetylcholine-receptor protein in its membrane-bound and detergent-extracted forms from Torpedo marmorata electric organ. Eur J Biochem. 1977 Oct 17;80(1):215–224. doi: 10.1111/j.1432-1033.1977.tb11874.x. [DOI] [PubMed] [Google Scholar]
- Szasz G., Gruber W., Bernt E. Creatine kinase in serum: 1. Determination of optimum reaction conditions. Clin Chem. 1976 May;22(5):650–656. [PubMed] [Google Scholar]
- Wennogle L. P., Changeux J. P. Transmembrane orientation of proteins present in acetylcholine receptor-rich membranes from Torpedo marmorata studied by selective proteolysis. Eur J Biochem. 1980 May;106(2):381–393. doi: 10.1111/j.1432-1033.1980.tb04584.x. [DOI] [PubMed] [Google Scholar]
- Williamson J., Greene J., Chérif S., Milner-White E. J. Heterogeneity of rabbit muscle creatine kinase and limited proteolysis by proteinase K. Biochem J. 1977 Dec 1;167(3):731–737. doi: 10.1042/bj1670731. [DOI] [PMC free article] [PubMed] [Google Scholar]