Abstract
Introduction:
Endodontic infections are polymicrobial in nature. Candida albicans is the most common fungus isolated from failed endodontic cases. The constant increase in antibiotic resistant strains and side-effects caused by synthetic drugs has prompted researchers to look for herbal alternatives such as propolis, Morinda citrifolia and Azadirachta indica (Neem) etc., since, the gold standard for irrigation, i.e., sodium hypochlorite has many disadvantages.
Materials and Methods:
Extracted human mandibular premolars were biomechanically prepared, vertically sectioned, placed in tissue culture wells exposing the root canal surface to C. albicans grown on Sabouraud Dextrose Agar to form a biofilm. At the end of 2 days, all groups were treated with test solutions and control for 10 min and evaluated for Candida growth and number of colony forming units. The readings were subjected to statistical analysis using analysis of variance and post hoc Tukey tests.
Results:
Sodium hypochlorite and propolis groups exhibited highest antimicrobial efficacy against C. albicans with no statistically significant difference. It was followed by the A. indica (Neem) group. M. citrifolia had limited antifungal action followed by the negative control group of saline.
Conclusion:
According to the results of this study, propolis can be used as an effective antifungal agent similar to that of sodium hypochlorite, although long-term in vivo studies are warranted.
Keywords: Antimicrobial efficacy, Azadirachta indica, Candida albicans, Morinda citrifolia, propolis, sodium hypochlorite
INTRODUCTION
Primary endodontic infection is caused by microorganisms colonizing the necrotic pulp tissue.[1] Endodontic infections are polymicrobial in nature dominated by obligate anaerobic bacteria.[2] Achieving predictable long-term success of root canal treatment requires effective debridement and disinfection of root canal system. This is not always achieved completely because of anatomical complexity and the limitation in accessing the canal system by instruments and irrigants.[3]
Candida albicans is the most common fungus seen in the root canals, 21% in primary infections[4] and 18% in cases of retreatments.[5] It can survive harsh conditions due to biofilm formation and the physicochemical properties of the microorganisms help them to modify according to the prevailing environmental and nutritional conditions.[6]
Biofilm helps in resisting the destruction of the fungus by making them thousand times more resistant to phagocytosis, antibodies and antimicrobial agents. This is attributed to the protective barrier provided by the extracellular matrix.[7] C. albicans is also resistant to calcium hydroxide, which is the most commonly used intracanal medicament.[6]
Sodium hypochlorite has remained a popular root canal irrigant because of its antimicrobial potential and its ability to dissolve organic matter. However, it is not only irritant to the periapical tissues,[8] but also inherently possesses certain disadvantages such as staining of instruments, burning of surrounding tissues,[2] unpleasant taste, high toxicity, corrosive to instruments,[9] inability to remove the smear layer, reduction in elastic modulus and flexural strength of dentin.[10]
Propolis is a brownish resinous substance collected by bees mainly from plants, which is used to reinforce their hives and keep the environment aseptic. It is a potent antimicrobial, antioxidant and anti-inflammatory agent due to the presence of flavonoids, phenolics and other aromatic compounds.[3]
Morinda citrifolia is commonly known as “Indian mulberry” or “noni” plant. It is indigenous to tropical countries and is considered as an important folk medicine. The fruit juice has a wide range of therapeutic effects.[11] The fruit contains polysaccharides, scopoletin, vitamins and minerals.[12]
Azadirachta indica (Neem) is the most commonly used traditional medicinal plant of India. Each part of the neem tree has some medicinal property and is thus commercially exploitable.[13] Neem elaborates a vast array of biologically active compounds that are chemically diverse and structurally complex. More than 140 compounds have been isolated from different parts of neem. Neem leaf and its constituents have been demonstrated to exhibit immunomodulatory, anti-inflammatory, antifungal, antibacterial, antiviral, antioxidant, antimutagenic and anticarcinogenic properties.[14]
The present study is aimed to explore newer herbal irrigants, which as potential antimicrobial agents in the inhibition of C. albicans in comparison to sodium hypochlorite.
MATERIALS AND METHODS
C. albicans culture preparation
A pure culture of C. albicans ATCC 10231 (Himedia, Mumbai L. No. 443-207) was inoculated on Sabouraud Dextrose Agar (Himedia, Mumbai), incubated at 37°C overnight and adjusted to an optical density of one with sterile brain-heart infusion broth.
Test solutions preparation
Propolis (Herbal Biosolutions, Delhi) was prepared by diluting a 33% commercially available alcoholic extract using warm saline in a ratio 2:1, to form an 11% alcoholic extract.[2]
About 6% concentration of pure Morinda citrifolia juice (MCJ) was taken.[3,11] (Herbal Biosolutions, Delhi).
Alcoholic extract of neem was prepared using 25 g of fresh neem leaves powder of 99% purity (The Indian Neem Tree Company, Mumbai) was added to 50 ml of absolute ethanol (Sterling Chemicals and Alcohols Pvt. Ltd., Mumbai). Mixture was macerated for 1-2 min, then extract was filtered through muslin cloth for coarse residue and then through filter paper for finer residue.[13]
Tooth samples preparation
Single rooted type 1 Vertucci's classification human mandibular premolar teeth were sectioned below the cementoenamel junction to obtain a standardized tooth length of 8 mm. The teeth were cleaned of superficial debris, calculus, tissue tags and stored in normal saline.[15]
The root canals were then instrumented using the crown down technique and rotary instruments to an apical size of ProTaper F3. A total volume of 2 ml of 5% sodium hypochlorite (Prime Dental Products Private Limited, Thane and Maharashtra) was used between each instrument during the cleaning and shaping procedure.
All teeth were vertically sectioned along the mid-sagittal plane into two halves. The concave tooth surface was minimally grounded to achieve flat surface to enable placement in tissue culture wells exposing the root canal surface to C. albicans to form a biofilm.[16]
The samples were then sterilized by ultraviolet radiation in a biosafety cabinet (Accumax India, New Delhi) and placed in the wells of tissue culture plates. The cultured yeast was inoculated in the wells containing tooth samples at 37°C for 2 days.
Grouping and assessment protocol
The samples were divided into five experimental groups with 10 samples each and irrigated with 3 ml of each irrigant for 10 min.
Group 1 — Propolis
Group 2 — MCJ
Group 3 — A. indica (Neem)
Group 4 — 5% sodium hypochlorite
Group 5 — Sterile saline.
Sterile paper point technique was used for sampling of root canals and inoculated in Sabouraud Dextrose Agar and incubated at 37°C for 24 h in a petridish, which was then analyzed by the digital colony counter (Spectronics India, Haryana) and the readings were subjected to statistical analysis using analysis of variance and post hoc Tukey tests.
RESULTS
Readings of microbial count obtained from digital colony counter after irrigation with respective irrigant were as follows: Table 1.
Table 1.
Mean and standard deviation showing inter group differences obtained from the readings of digital colony counter
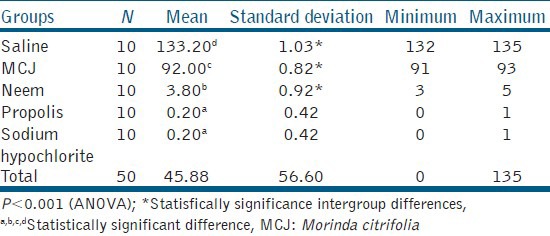
The mean and standard deviations obtained were as follows.
Microbial count was maximum in the saline group and minimum in propolis and sodium hypochlorite groups. Absolute washout of C. albicans biofilm was observed in 8 out of 10 samples of propolis and sodium hypochlorite groups. Neem group also showed a higher antimicrobial efficacy as compared with MCJ and saline groups. Saline (negative control) had least antimicrobial activity as expected.
Except for comparison between propolis and sodium hypochlorite, all the between group differences were statistically significant. The order of efficacy of different groups was as follows:
Propolis ~ Sodium hypochlorite > Neem > MCJ > Saline
DISCUSSION
This study was conducted on C. albicans because these microorganisms are commonly encountered in recalcitrant endodontic infections.[3]
C. albicans has an initial period of adherence (0-2 h) followed by subsequent microcolony formation (2-4 h). Dimorphic switching occurred thereafter with a transition from budding-yeast forms to filamentous pseudo- and true-hyphal forms (4-6 h). Micro-colonies then become interlinked by the hyphal extensions, forming a confluent monolayer (6-8 h). The complexity of the biofilm increases with time, taking on 3D architecture with spatial heterogeneity as it matured (8-48 h). The biofilm after 24 and 48 h consists of a mixture of yeast cells, pseudohyphae and true hyphae. Filamentous forms were the most important factor in the 3D architecture, with yeast cells located in the basal layer.[7] C. albicans mutants that are deficient in the production of hyphae have demonstrated an inability to form 3D biofilms. Therefore, the dimorphic switching observed in this species is a pivotal factor for biofilm formation and the pathogenic potential of C. albicans,[17,18] which is why the 48 h biofilm model was used.
Sodium hypochlorite is the most commonly used irrigating solution in clinical practice because of its tissue dissolution and antimicrobial activity, making it an irrigating solution of choice irrespective of its several undesirable characteristics such as tissue toxicity, risk of emphysema, allergic potential and disagreeable smell and taste.[9,11] Ayhan et al.[19] demonstrated that sodium hypochlorite lowered colony forming units below the limit of detection after 10 s in the case of C. albicans.
Propolis exhibits antimicrobial, anti-inflammatory, healing, anesthetic and cariostatic properties. According to Takaisi-Kikuni and Schilcher,[20] it prevents fungal cell division and also breaks down fungal cell wall and cytoplasm similar to the action of some antibiotics. Kujumgiev et al.[21] reported the antimicrobial action of propolis to be due to flavonoids and esters of phenolic acids. This is in support of our study as propolis effectively inhibited Candidal biofilm. The pH chosen for propolis was six based on the results of the study conducted by Ivančajić et al.[22] since the inhibitory effect of propolis was the strongest in a slightly acidic environment (pH = 6). In an inflammatory process there is a slight decrease of pH; thus, it would be most beneficial to use the extract of propolis of slightly acidic pH.[22] Antimicrobial efficacy has been reported for other endodontic pathogens as well. Inhibitory action of propolis is solvent dependent. Among the different extracts used, acetone and ethanolic extracts were found to be more active toward most microorganisms.[22] As for neem, the ethanolic extracts performed better than the aqueous extract, therefore to standardize we prepared both our test solutions using ethanol.[23] A study conducted Grange and Davey[24] showed the antimicrobial efficacy of propolis against Enterococcus species, Staphylococcus aureus and C. albicans. It also showed antibacterial efficacy against Escherichia coli, Achromobacter, Sarcina lutea and Morganella morgani.
A. indica (Neem) (pH = 6.8) has antimicrobial properties due to the presence of alkaloids, glycosides, saponins, flavonoids, steroids, anthraquinone and tannic acid.[25] Bohora et al.[26] have concluded that neem leaf extract has a significant antimicrobial effect against C. albicans. This is in accordance of our study. Acidic ethanolic extract was found to be better than aqueous extract in cases of C. albicans.[23] Neem has a wide spectrum of antimicrobial action; against Gram-negative and Gram-positive microorganisms, including M. tuberculosis, M. pyogenes, Streptococcus mutans and Enterococcus faecalis. Ethanol extract is most effective against E. faecalis, E. coli, Proteus mirabilis. Furthermore, effective against certain human fungi including Trychophyton, Epidermophyton, Microsporum, Trichosporon, Geotricum and Candida.[23]
MCJ (pH = 3.5) has a broad range of therapeutic effects. The beneficial antimicrobial effects may be the result of acubin, L-asperuloside, alizarin, scopoletin and other anthraquinones. Banerjee et al.[27] showed that MCJ had anticandidal activity in vitro. Jayaraman et al.[28] showed that MCJ showed no significant activity against C. albicans, which might be due to the fact stated by Jainkittivong et al.[12] who showed that in case of M. citrifolia fruit longer the contact time, higher is the inhibitory effect. The contact time found to be effective was 45 min, but in our study we took 10 min to standardize the procedure and simulate a clinical situation. It has antimicrobial action against Pseudomonas aeruginosa, Proteus morgaii, S. aureus, Bacillus subtilis, E. coli, Salmonella, Shigella, E. faecalis and C. albicans.[29]
The shelf lives of ethanolic extract of propolis is 2 years, for neem leaf extract is 3 years and MCJ is 3 years when not opened and 2 years after opening, if stored properly, as per the manufacturer's instructions.
Kousedghi et al.[30] compared the antibacterial activity of propolis and calcium hydroxide against C. albicans, but the drawbacks of the study was that the biofilm model was not used thereby not simulating clinical condition and other herbal irrigants were not compared in the study. Bohora et al.[26] compared the antifungal efficacy of neem leaf extract against C. albicans, but again the biofilm model was not used.
Until date, no study has been conducted comparing all these herbal irrigants with sodium hypochlorite in a biofilm model against C. albicans and therefore, this study holds ground for future research. According to the results of the study, we see a promising herbal irrigant in propolis against C. albicans in root canal infections.
CONCLUSION
Under the limitations of this study, it was concluded that:
Propolis performed equally well as sodium hypochlorite against C. albicans biofilm formed on extracted tooth surface.
A. indica (Neem) also had a good antimicrobial action.
ACKNOWLEDGMENT
I would like to thank Dr. Vibha Pathology Laboratory, Moradabad, where the study was carried out.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Tronstad L, Sunde PT. The evolving new understanding of endodontic infections. Endod Top. 2003;6:57–77. [Google Scholar]
- 2.Shingare P, Chaugule V. Comparative evaluation of antimicrobial activity of miswak, propolis, sodium hypochlorite and saline as root canal irrigants by microbial culturing and quantification in chronically exposed primary teeth. Germs. 2011;1:12–21. doi: 10.11599/germs.2012.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kandaswamy D, Venkateshbabu N, Gogulnath D, Kindo AJ. Dentinal tubule disinfection with 2% chlorhexidine gel, propolis, Morinda citrifolia juice, 2% povidone iodine, and calcium hydroxide. Int Endod J. 2010;43:419–23. doi: 10.1111/j.1365-2591.2010.01696.x. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner JC, Watts CM, Xia T. Occurrence of Candida albicans in infections of endodontic origin. J Endod. 2000;26:695–8. doi: 10.1097/00004770-200012000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Siqueira JF, Jr, Rôças IN. Diversity of endodontic microbiota revisited. J Dent Res. 2009;88:969–81. doi: 10.1177/0022034509346549. [DOI] [PubMed] [Google Scholar]
- 6.Waltimo TM, Haapasalo M, Zehnder M, Meyer J. Clinical aspects related to endodontic yeast infections. Endod Top. 2004;9:66–78. [Google Scholar]
- 7.Baillie GS, Douglas LJ. Role of dimorphism in the development of Candida albicans biofilms. J Med Microbiol. 1999;48:671–9. doi: 10.1099/00222615-48-7-671. [DOI] [PubMed] [Google Scholar]
- 8.Spangberg L, Engström B, Langeland K. Biologic effects of dental materials. 3. Toxicity and antimicrobial effect of endodontic antiseptics in vitro. Oral Surg Oral Med Oral Pathol. 1973;36:856–71. doi: 10.1016/0030-4220(73)90338-1. [DOI] [PubMed] [Google Scholar]
- 9.Mohammadi Z. Sodium hypochlorite in endodontics: An update review. Int Dent J. 2008;58:329–41. doi: 10.1111/j.1875-595x.2008.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 10.Sim TP, Knowles JC, Ng YL, Shelton J, Gulabivala K. Effect of sodium hypochlorite on mechanical properties of dentine and tooth surface strain. Int Endod J. 2001;34:120–32. doi: 10.1046/j.1365-2591.2001.00357.x. [DOI] [PubMed] [Google Scholar]
- 11.Murray PE, Farber RM, Namerow KN, Kuttler S, Garcia-Godoy F. Evaluation of Morinda citrifolia as an endodontic irrigant. J Endod. 2008;34:66–70. doi: 10.1016/j.joen.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Jainkittivong A, Butsarakamruha T, Langlais RP. Antifungal activity of Morinda citrifolia fruit extract against Candida albicans. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:394–8. doi: 10.1016/j.tripleo.2009.05.044. [DOI] [PubMed] [Google Scholar]
- 13.Dubey S, Chaodary M, Gupta P. Comparative study of the antimicrobial efficiency of neem leaf extract, sodium hypochlorite and biopure MTAD — An in vitro study. Indian J Dent Adv. 2012;4:740–3. [Google Scholar]
- 14.Subapriya R, Nagini S. Medicinal properties of neem leaves: A review. Curr Med Chem Anticancer Agents. 2005;5:149–6. doi: 10.2174/1568011053174828. [DOI] [PubMed] [Google Scholar]
- 15.Pujar M, Patil C, Kadam A. Comparison of antimicrobial efficacy of triphala, green tea polyphenols and 3% of sodium hypochlorite on Enterococcus faecalis biofilms formed on tooth substrate: In vitro. JIOH. 2011;3:23–9. [Google Scholar]
- 16.Prabhakar J, Senthilkumar M, Priya MS, Mahalakshmi K, Sehgal PK, Sukumaran VG. Evaluation of antimicrobial efficacy of herbal alternatives (Triphala and green tea polyphenols), MTAD, and 5% sodium hypochlorite against Enterococcus faecalis biofilm formed on tooth substrate: An in vitro study. J Endod. 2010;36:83–6. doi: 10.1016/j.joen.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 17.Nikawa H, Nishimura H, Hamada T, Makihira S, Samaranayake LP. Relationship between thigmotropism and candida biofilm formation in vitro. Mycopathologia. 1998;144:125–9. doi: 10.1023/a:1007073930933. [DOI] [PubMed] [Google Scholar]
- 18.Davies JM, Stacey AJ, Gilligan CA. Candida albicans hyphal invasion: Thigmotropism or chemotropism? FEMS Microbiol Lett. 1999;171:245–9. doi: 10.1111/j.1574-6968.1999.tb13439.x. [DOI] [PubMed] [Google Scholar]
- 19.Ayhan H, Sultan N, Cirak M, Ruhi MZ, Bodur H. Antimicrobial effects of various endodontic irrigants on selected microorganisms. Int Endod J. 1999;32:99–102. doi: 10.1046/j.1365-2591.1999.00196.x. [DOI] [PubMed] [Google Scholar]
- 20.Takaisi-Kikuni NB, Schilcher H. Electron microscopic and microcalorimetric investigations of the possible mechanism of the antibacterial action of a defined propolis provenance. Planta Med. 1994;60:222–7. doi: 10.1055/s-2006-959463. [DOI] [PubMed] [Google Scholar]
- 21.Kujumgiev A, Tsvetkova I, Serkedjieva Y, Bankova V, Christov R, Popov S. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J Ethnopharmacol. 1999;64:235–40. doi: 10.1016/s0378-8741(98)00131-7. [DOI] [PubMed] [Google Scholar]
- 22.Ivančajić S, Mileusnić I, Milošević DC. In vitro antibacterial activity of propolis extracts on 12 different bacteria in conditions of 3 various pH values. Arch Biol Sci Belgrade. 2010;62:915–34. [Google Scholar]
- 23.Nayak A, Nayak RN, Soumya GB, Bhat K, Kudalkar M. Evaluation of antibacterial and anticandidal efficacy of aqueous and alcoholic extract of neem (Azadirachta indica): An in vitro study. IJRAP. 2011;2:230–5. [Google Scholar]
- 24.Grange JM, Davey RW. Antibacterial properties of propolis (bee glue) J R Soc Med. 1990;83:159–60. doi: 10.1177/014107689008300310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polaquini SR, Svidzinski TI, Kemmelmeier C, Gasparetto A. Effect of aqueous extract from neem (Azadirachta indica A. Juss) on hydrophobicity, biofilm formation and adhesion in composite resin by Candida albicans. Arch Oral Biol. 2006;51:482–90. doi: 10.1016/j.archoralbio.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Bohora A, Hedge V, Kokate S. Comparison of the antibacterial efficiency of neem leaf extract and 2% sodium hypochlorite against E. faecalis, C. albicans and mixed culture — An in vitro study. Endodontology. 2010;22(1):8–12. [Google Scholar]
- 27.Banerjee S, Johnson AD, Csiszar K, Wansley DL, McGeady P. An extract of Morinda citrifolia interferes with the serum-induced formation of filamentous structures in Candida albicans and inhibits germination of Aspergillus nidulans. Am J Chin Med. 2006;34:503–9. doi: 10.1142/S0192415X0600403X. [DOI] [PubMed] [Google Scholar]
- 28.Jayaraman SK, Manoharan MS, Illanchezian S. Antibacterial, antifungal and tumour cell suppression potential of Morinda citrifolia fruit extracts. Int J Integr Biol. 2008;3:44–9. [Google Scholar]
- 29.Neelkantan P, Jagannathan N, Nazar N. Ethnopharmacological approach in endodontic treatment: A focused review. Int J Drug Dev Res. 2011;3:68–77. [Google Scholar]
- 30.Kousedghi H, Ahangari Z, Eslami G, Ayatolahi A. Antibacterial activity of propolis and Ca(OH)2 against Lactobacillus, Enterococus faecalis, Peptostreptococus and Candida albicans. Afr J Microbiol Res. 2012;6:3510–5. [Google Scholar]


