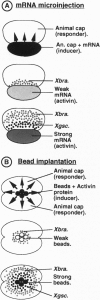
Abstract
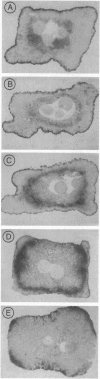
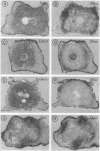
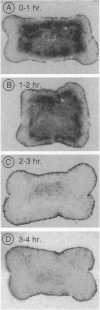
A recently described experimental system for analyzing the mode of action of a morphogen gradient involves the in situ hybridization of sectioned tissue constructs. In these constructs, a source of activin signaling induces the transcription of several mesodermal genes in blastula animal caps, according to the position of cells in a concentration gradient. New experiments show that activin-loaded beads emit a signal for only 2 hr and that the same cell can be induced to express different genes. We determine the position in the gradient and the time after the start of activin signaling at which early genes, including Mix1, Xpo, Xwnt8, Xchd, and Xlim1, are activated, relative to the previously tested genes Xbra and Xgsc.
Full text
PDF
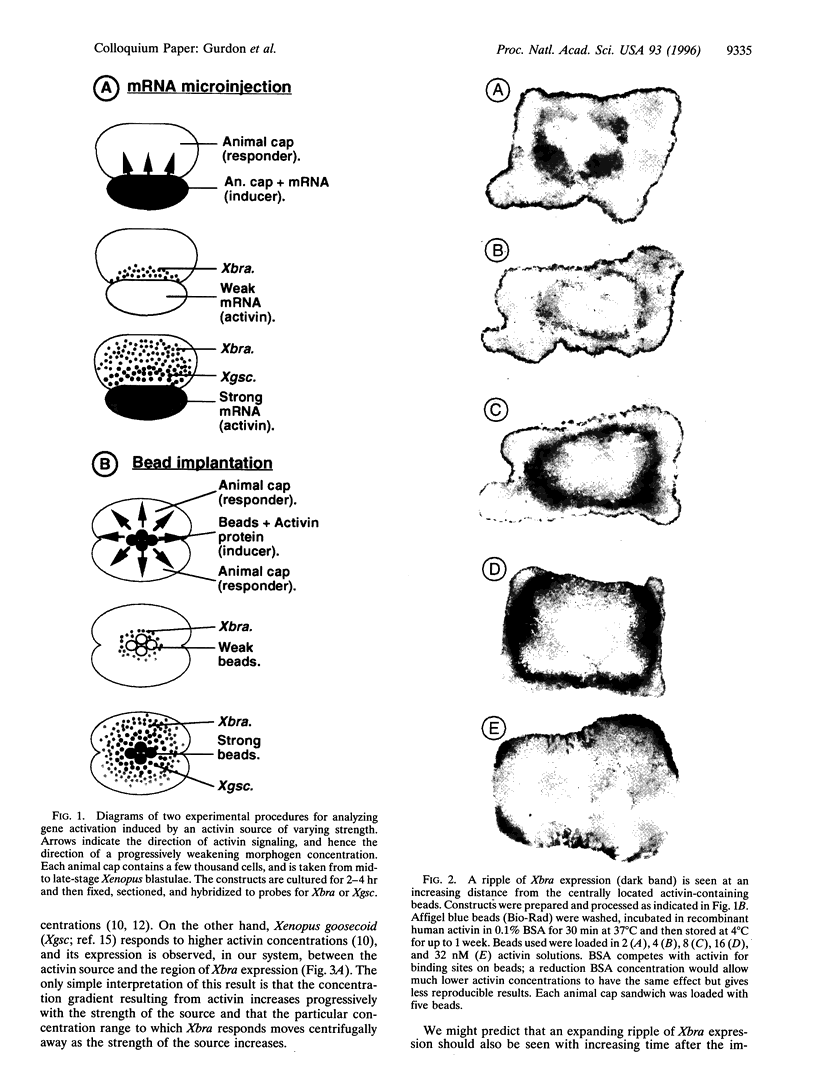
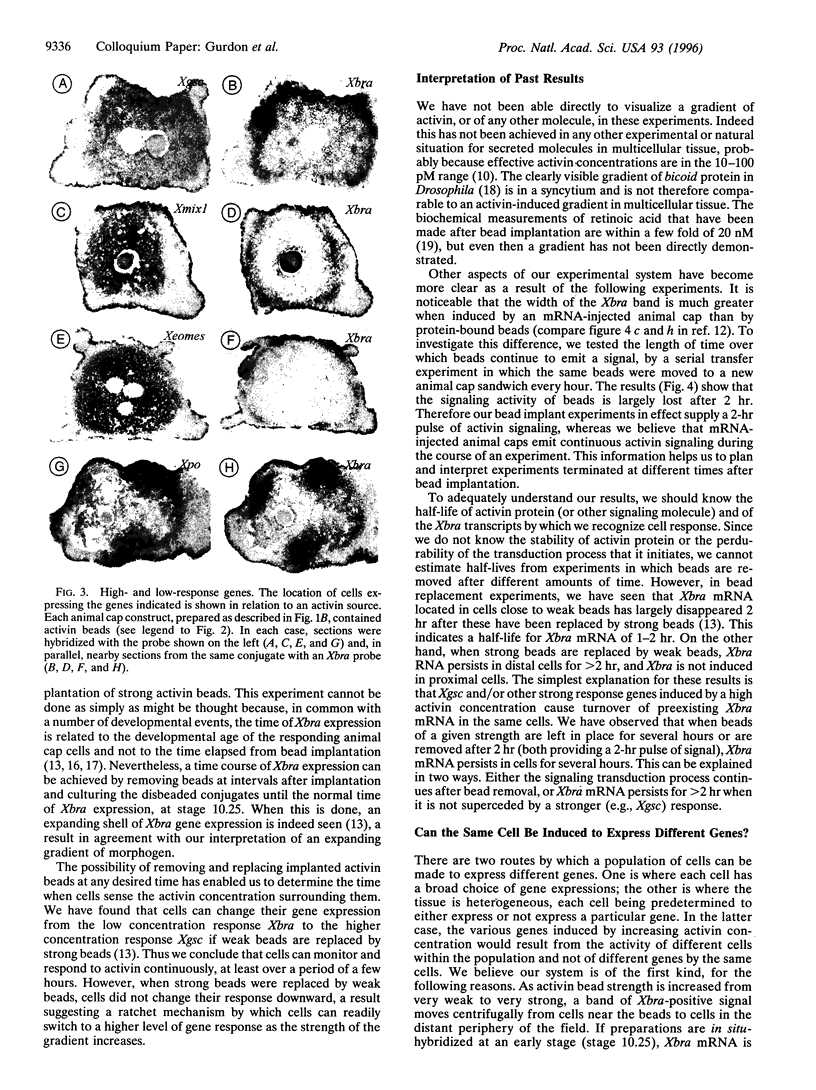


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asashima M., Nakano H., Uchiyama H., Sugino H., Nakamura T., Eto Y., Ejima D., Nishimatsu S., Ueno N., Kinoshita K. Presence of activin (erythroid differentiation factor) in unfertilized eggs and blastulae of Xenopus laevis. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6511–6514. doi: 10.1073/pnas.88.15.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. W., Blumberg B., Steinbeisser H., De Robertis E. M. Molecular nature of Spemann's organizer: the role of the Xenopus homeobox gene goosecoid. Cell. 1991 Dec 20;67(6):1111–1120. doi: 10.1016/0092-8674(91)90288-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driever W., Nüsslein-Volhard C. A gradient of bicoid protein in Drosophila embryos. Cell. 1988 Jul 1;54(1):83–93. doi: 10.1016/0092-8674(88)90182-1. [DOI] [PubMed] [Google Scholar]
- Fan C. M., Porter J. A., Chiang C., Chang D. T., Beachy P. A., Tessier-Lavigne M. Long-range sclerotome induction by sonic hedgehog: direct role of the amino-terminal cleavage product and modulation by the cyclic AMP signaling pathway. Cell. 1995 May 5;81(3):457–465. doi: 10.1016/0092-8674(95)90398-4. [DOI] [PubMed] [Google Scholar]
- Ferguson E. L., Anderson K. V. Decapentaplegic acts as a morphogen to organize dorsal-ventral pattern in the Drosophila embryo. Cell. 1992 Oct 30;71(3):451–461. doi: 10.1016/0092-8674(92)90514-d. [DOI] [PubMed] [Google Scholar]
- Green J. B., New H. V., Smith J. C. Responses of embryonic Xenopus cells to activin and FGF are separated by multiple dose thresholds and correspond to distinct axes of the mesoderm. Cell. 1992 Nov 27;71(5):731–739. doi: 10.1016/0092-8674(92)90550-v. [DOI] [PubMed] [Google Scholar]
- Green J. B., Smith J. C. Graded changes in dose of a Xenopus activin A homologue elicit stepwise transitions in embryonic cell fate. Nature. 1990 Sep 27;347(6291):391–394. doi: 10.1038/347391a0. [DOI] [PubMed] [Google Scholar]
- Green J. B., Smith J. C. Growth factors as morphogens: do gradients and thresholds establish body plan? Trends Genet. 1991 Aug;7(8):245–250. doi: 10.1016/0168-9525(91)90323-I. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Harger P., Mitchell A., Lemaire P. Activin signalling and response to a morphogen gradient. Nature. 1994 Oct 6;371(6497):487–492. doi: 10.1038/371487a0. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Mitchell A., Mahony D. Direct and continuous assessment by cells of their position in a morphogen gradient. Nature. 1995 Aug 10;376(6540):520–521. doi: 10.1038/376520a0. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Mohun T. J., Fairman S., Brennan S. All components required for the eventual activation of muscle-specific actin genes are localized in the subequatorial region of an uncleaved amphibian egg. Proc Natl Acad Sci U S A. 1985 Jan;82(1):139–143. doi: 10.1073/pnas.82.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig L. S. Positional signal transmission in the developing chick limb. Nature. 1981 May 7;291(5810):72–73. doi: 10.1038/291072a0. [DOI] [PubMed] [Google Scholar]
- Johnson R. L., Tabin C. The long and short of hedgehog signaling. Cell. 1995 May 5;81(3):313–316. doi: 10.1016/0092-8674(95)90381-x. [DOI] [PubMed] [Google Scholar]
- Roelink H., Porter J. A., Chiang C., Tanabe Y., Chang D. T., Beachy P. A., Jessell T. M. Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of sonic hedgehog autoproteolysis. Cell. 1995 May 5;81(3):445–455. doi: 10.1016/0092-8674(95)90397-6. [DOI] [PubMed] [Google Scholar]
- Rosa F. M. Mix.1, a homeobox mRNA inducible by mesoderm inducers, is expressed mostly in the presumptive endodermal cells of Xenopus embryos. Cell. 1989 Jun 16;57(6):965–974. doi: 10.1016/0092-8674(89)90335-8. [DOI] [PubMed] [Google Scholar]
- Sasai Y., Lu B., Steinbeisser H., Geissert D., Gont L. K., De Robertis E. M. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994 Dec 2;79(5):779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S. M., Sargent T. D. Localized and inducible expression of Xenopus-posterior (Xpo), a novel gene active in early frog embryos, encoding a protein with a 'CCHC' finger domain. Development. 1991 Jul;112(3):747–753. doi: 10.1242/dev.112.3.747. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Price B. M., Green J. B., Weigel D., Herrmann B. G. Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell. 1991 Oct 4;67(1):79–87. doi: 10.1016/0092-8674(91)90573-h. [DOI] [PubMed] [Google Scholar]
- Smith W. C., Harland R. M. Injected Xwnt-8 RNA acts early in Xenopus embryos to promote formation of a vegetal dorsalizing center. Cell. 1991 Nov 15;67(4):753–765. doi: 10.1016/0092-8674(91)90070-f. [DOI] [PubMed] [Google Scholar]
- Taira M., Jamrich M., Good P. J., Dawid I. B. The LIM domain-containing homeo box gene Xlim-1 is expressed specifically in the organizer region of Xenopus gastrula embryos. Genes Dev. 1992 Mar;6(3):356–366. doi: 10.1101/gad.6.3.356. [DOI] [PubMed] [Google Scholar]
- Thaller C., Eichele G. Identification and spatial distribution of retinoids in the developing chick limb bud. Nature. 1987 Jun 18;327(6123):625–628. doi: 10.1038/327625a0. [DOI] [PubMed] [Google Scholar]
- Tickle C., Alberts B., Wolpert L., Lee J. Local application of retinoic acid to the limb bond mimics the action of the polarizing region. Nature. 1982 Apr 8;296(5857):564–566. doi: 10.1038/296564a0. [DOI] [PubMed] [Google Scholar]