Abstract
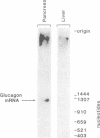
We have isolated cDNA clones encoding bovine pancreatic preproglucagon. Twenty-five putative preproglucagon clones were selected by screening 3,100 clones of a fetal bovine pancreas cDNA library with a synthetic oligodeoxynucleotide probe. The probe was a mixture of synthetic 17-base DNA oligomers constructed to correspond to the six carboxyl-terminal amino acids (residues 24-29) of mature glucagon. Restriction mapping of six of these clones suggested that they represented a single mRNA species. Primary sequence analysis of one clone containing a 1,200-base-pair DNA insert revealed that it contained an essentially full-length copy of glucagon mRNA. Analysis of the cDNA suggested a protein coding sequence of 540 nucleotides and 5'- and 3'-untranslated regions of 90 and 471 nucleotides, respectively. This cDNA sequence encoded a 20-amino acid signal sequence followed by one for glicentin, a 69-amino acid polypeptide containing an internal glucagon moiety that has been found in porcine intestines. Glicentin is followed by two additional glucagon-like peptides, each flanked by paired basic amino acids (Lys, Arg) characteristic of prohormone processing. These polypeptide sequences show striking homology with those for glucagon and other members of the glucagon family of peptides.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal K. L., Brunstedt J., Noyes B. E. A general method for detection and characterization of an mRNA using an oligonucleotide probe. J Biol Chem. 1981 Jan 25;256(2):1023–1028. [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Santerre R. F., Mullenbach G. T. Hamster preproglucagon contains the sequence of glucagon and two related peptides. Nature. 1983 Apr 21;302(5910):716–718. doi: 10.1038/302716a0. [DOI] [PubMed] [Google Scholar]
- Blobel G. Intracellular protein topogenesis. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1496–1500. doi: 10.1073/pnas.77.3.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. C. A gastric inhibitory polypeptide. I. The amino acid composition and the tryptic peptides. Can J Biochem. 1971 Feb;49(2):255–261. doi: 10.1139/o71-037. [DOI] [PubMed] [Google Scholar]
- Chan S. J., Emdin S. O., Kwok S. C., Kramer J. M., Falkmer S., Steiner D. F. Messenger RNA sequence and primary structure of preproinsulin in a primitive vertebrate, the Atlantic hagfish. J Biol Chem. 1981 Jul 25;256(14):7595–7602. [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Frazier M. L., Montagna R. A., Saunders G. F. Insulin gene expression during development of the fetal bovine pancreas. Biochemistry. 1981 Jan 20;20(2):367–371. doi: 10.1021/bi00505a022. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellerstrom C., Howell S. L., Edwards J. C., Andersson A. An investigation of glucagon biosynthesis in isolated pancreatic islets of guinea pigs. FEBS Lett. 1972 Oct 15;27(1):97–101. doi: 10.1016/0014-5793(72)80418-6. [DOI] [PubMed] [Google Scholar]
- Hellerström C., Howell S. L., Edwards J. C., Andersson A., Ostenson C. G. Biosynthesis of glucagon in isolated pancreatic islets of guinea pigs. Biochem J. 1974 Apr;140(1):13–21. doi: 10.1042/bj1400013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hers H. G. The control of glycogen metabolism in the liver. Annu Rev Biochem. 1976;45:167–189. doi: 10.1146/annurev.bi.45.070176.001123. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., Carlström A., Pettersson T., Jacobsson B., Persson M., Mutt V. Structural homologies between prealbumin, gastrointestinal prohormones and other proteins. Nature. 1981 May 21;291(5812):261–263. doi: 10.1038/291261a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Land H., Grez M., Hauser H., Lindenmaier W., Schütz G. 5'-Terminal sequences of eucaryotic mRNA can be cloned with high efficiency. Nucleic Acids Res. 1981 May 25;9(10):2251–2266. doi: 10.1093/nar/9.10.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre P. J., Luyckx A. S. Glucagon and diabetes: a reappraisal. Diabetologia. 1979 Jun;16(6):347–354. doi: 10.1007/BF01223153. [DOI] [PubMed] [Google Scholar]
- Lewin B. Units of transcription and translation: the relationship between heterogeneous nuclear RNA and messenger RNA. Cell. 1975 Jan;4(1):11–20. doi: 10.1016/0092-8674(75)90128-2. [DOI] [PubMed] [Google Scholar]
- Lomedico P. T., Saunders G. F. Preparation of pancreatic mRNA: cell-free translation of an insulin-immunoreactive polypeptide. Nucleic Acids Res. 1976 Feb;3(2):381–391. doi: 10.1093/nar/3.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund P. K., Goodman R. H., Dee P. C., Habener J. F. Pancreatic preproglucagon cDNA contains two glucagon-related coding sequences arranged in tandem. Proc Natl Acad Sci U S A. 1982 Jan;79(2):345–349. doi: 10.1073/pnas.79.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund P. K., Goodman R. H., Habener J. F. Pancreatic pre-proglucagons are encoded by two separate mRNAs. J Biol Chem. 1981 Jul 10;256(13):6515–6518. [PubMed] [Google Scholar]
- Lund P. K., Goodman R. H., Jacobs J. W., Habener J. F. Glucagon precursors identified by immunoprecipitation of products of cell-free translation of messenger RNA. Diabetes. 1980 Jul;29(7):583–586. doi: 10.2337/diab.29.7.583. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevarech M., Noyes B. E., Agarwal K. L. Detection of gastrin-specific mRNA using oligodeoxynucleotide probes of defined sequence. J Biol Chem. 1979 Aug 25;254(16):7472–7475. [PubMed] [Google Scholar]
- Moody A. J., Holst J. J., Thim L., Jensen S. L. Relationship of glicentin to proglucagon and glucagon in the porcine pancreas. Nature. 1981 Feb 5;289(5797):514–516. doi: 10.1038/289514a0. [DOI] [PubMed] [Google Scholar]
- Murphy E. C., Jr, Kopchick J. J., Watson K. F., Arlinghaus R. B. Cell-free synthesis of a precursor polyprotein containing both gag and pol gene products by Rauscher murine leukemia virus 35S RNA. Cell. 1978 Feb;13(2):359–369. doi: 10.1016/0092-8674(78)90204-0. [DOI] [PubMed] [Google Scholar]
- Mutt V., Jorpes J. E., Magnusson S. Structure of porcine secretin. The amino acid sequence. Eur J Biochem. 1970 Sep;15(3):513–519. doi: 10.1111/j.1432-1033.1970.tb01034.x. [DOI] [PubMed] [Google Scholar]
- Mutt V., Said S. I. Structure of the porcine vasoactive intestinal octacosapeptide. The amino-acid sequence. Use of kallikrein in its determination. Eur J Biochem. 1974 Mar 1;42(2):581–589. doi: 10.1111/j.1432-1033.1974.tb03373.x. [DOI] [PubMed] [Google Scholar]
- Noe B. D., Bauer G. E. Evidence for glucagon biosynthesis involving a protein intermediate in islets of the anglerfish (Lophius americanus). Endocrinology. 1971 Sep;89(3):642–651. doi: 10.1210/endo-89-3-642. [DOI] [PubMed] [Google Scholar]
- Noe B. D., Bauer G. E. Further characterization of a glucagon precursor from anglerfish islet tissue. Proc Soc Exp Biol Med. 1973 Jan;142(1):210–213. doi: 10.3181/00379727-142-36990. [DOI] [PubMed] [Google Scholar]
- Patzelt C., Tager H. S., Carroll R. J., Steiner D. F. Identification and processing of proglucagon in pancreatic islets. Nature. 1979 Nov 15;282(5736):260–266. doi: 10.1038/282260a0. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Rigopoulou D., Valverde I., Marco J., Faloona G., Unger R. H. Large glucagon immunoreactivity in extracts of pancreas. J Biol Chem. 1970 Feb 10;245(3):496–501. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L. P., Pictet R. L., Rutter W. J. Human somatostatin I: sequence of the cDNA. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4575–4579. doi: 10.1073/pnas.79.15.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields D., Warren T. G., Roth S. E., Brenner M. J. Cell-free synthesis and processing of multiple precursors to glucagon. Nature. 1981 Feb 5;289(5797):511–514. doi: 10.1038/289511a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spiess J., Rivier J., Thorner M., Vale W. Sequence analysis of a growth hormone releasing factor from a human pancreatic islet tumor. Biochemistry. 1982 Nov 23;21(24):6037–6040. doi: 10.1021/bi00267a002. [DOI] [PubMed] [Google Scholar]
- Srikant C. B., McCorkle K., Unger R. H. Characteristics of tissue IRGs in the dog. Metabolism. 1976 Nov;25(11 Suppl 1):1403–1404. doi: 10.1016/s0026-0495(76)80151-5. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Quinn P. S., Chan S. J., Marsh J., Tager H. S. Processing mechanisms in the biosynthesis of proteins. Ann N Y Acad Sci. 1980;343:1–16. doi: 10.1111/j.1749-6632.1980.tb47238.x. [DOI] [PubMed] [Google Scholar]
- Sundby F. Species variations in the primary structure of glucagon. Metabolism. 1976 Nov;25(11 Suppl 1):1319–1321. doi: 10.1016/s0026-0495(76)80132-1. [DOI] [PubMed] [Google Scholar]
- Tager H. S., Steiner D. F. Isolation of a glucagon-containing peptide: primary structure of a possible fragment of proglucagon. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2321–2325. doi: 10.1073/pnas.70.8.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatemoto K., Mutt V. Isolation and characterization of the intestinal peptide porcine PHI (PHI-27), a new member of the glucagon--secretin family. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6603–6607. doi: 10.1073/pnas.78.11.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thim L., Moody A. J. Purification and chemical characterization of a glicentin-related pancreatic peptide (proglucagon fragment) from porcine pancreas. Biochim Biophys Acta. 1982 May 3;703(2):134–141. doi: 10.1016/0167-4838(82)90041-3. [DOI] [PubMed] [Google Scholar]
- Thim L., Moody A. J. The primary structure of porcine glicentin (proglucagon). Regul Pept. 1981 May;2(2):139–150. doi: 10.1016/0167-0115(81)90007-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung A. K., Zerega F. Biosynthesis of glucagon in isolated pigeon islets. Biochem Biophys Res Commun. 1971 Oct 15;45(2):387–395. doi: 10.1016/0006-291x(71)90831-x. [DOI] [PubMed] [Google Scholar]
- Valverde I., Rigopoulou D., Marco J., Faloona G. R., Unger R. H. Characterization of glucagon-like immunoreactivity (GLI). Diabetes. 1970 Sep;19(9):614–623. doi: 10.2337/diab.19.9.614. [DOI] [PubMed] [Google Scholar]
- Valverde I., Villanueva M. L., Lozano I., Marco J. Presence of glucagon immunoreactivity in the globulin fraction of human plasma ("big plasma glucagon"). J Clin Endocrinol Metab. 1974 Dec;39(6):1090–1098. doi: 10.1210/jcem-39-6-1090. [DOI] [PubMed] [Google Scholar]
- Weinstock R., Sweet R., Weiss M., Cedar H., Axel R. Intragenic DNA spacers interrupt the ovalbumin gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1299–1303. doi: 10.1073/pnas.75.3.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]