Abstract
As human immunodeficiency virus (HIV)-infected women gain access to combination antiretroviral therapy throughout sub-Saharan Africa, a growing number of infants are being born HIV-exposed but uninfected. Data about neonatal mortality and the impact of premature delivery, in this population are limited. We describe the 28-day mortality outcomes in a cohort of HIV-exposed infants who had ultrasound-confirmed gestational age in rural Uganda. There were 13 deaths among 351 infants, including 9 deaths in the perinatal period. Premature delivery was a strong predictor of mortality. The prevention of HIV transmission to infants is now possible in rural low-resource settings but the frequency of neonatal death among HIV-exposed infants remains extremely high, calling for new comprehensive interventions to reduce mortality in this growing population.
Keywords: HIV, neonatal, mortality, prematurity, Africa
Background
Programs for the prevention of perinatal human immunodeficiency virus (HIV) transmission are rapidly expanding around the world. The Joint United Nations Programme on HIV/AIDS (UNAIDS) reports a 24% decline in incident perinatal HIV infections between 2009 and 2011 in the Global Plan priority countries in Sub-Saharan Africa [1]. Their bold strategy calls for the elimination of new HIV infections among children by 2015. As more women of reproductive age are living longer with HIV, and prevention strategies are effectively reducing transmission of HIV to infants, the population of infants born ‘HIV-exposed’ to infected mothers but remaining uninfected is growing.
HIV-exposed children have mortality rates that are unacceptably high, ranging from 2.5 to 7.5 deaths per 100 person-years [2, 3], yet our understanding about the causes of death in this at-risk population is limited, particularly in the countries where mortality rates are highest [4–6]. The first 4 weeks of life are a particularly vulnerable period in the developing world where malaria, poor hospital infrastructure and malnutrition are common. Prematurity is thought to be a leading contributor to neonatal death in developing countries [7], but most studies are only able to use inaccurate gestational age (GA) dating techniques such as last menstrual period (LMP) [8–10]. The provision of antiretroviral therapy (ART) to HIV-infected pregnant women might help reduce neonatal mortality, but data are limited; studies include few women receiving ART [5, 11] or are limited to retrospective analyses of clinical data [6].
The purpose of this study is to characterize risk for death during the neonatal period in a cohort of infants born to HIV-infected women treated with combination ART and followed prospectively in rural Uganda, and to assess the role of prematurity using ultrasound-confirmed GA dating.
Methods
This study included infants born in a randomized clinical trial of HIV-infected pregnant women who were enrolled between 15 December 2009 and 25 November 2012 in the rural town of Tororo, Uganda (NCT00993031). Ethical approval for the study was obtained from the Uganda National Council of Science and Technology, the Research and Ethics Committee of the School of Medicine at Makerere University and the Committee on Human Research at the University of California, San Francisco. Informed written consent for participation was obtained from all study participants at enrollment. ART-naïve women between 12 and 28 weeks gestation were randomized to receive either lopinavir-ritonavir- or efavirenz-based ART. All women received insecticide-treated bed nets and daily trimethoprim-sulfamethoxazole for prevention of opportunistic infections, and a standard prenatal care package of a safe water vessel, multivitamins and condoms as per standard of care for HIV-infected pregnant women in Uganda. GA was established using LMP and sonographic biometry (biparietal diameter, head circumference, abdominal circumference, femur length). Estimated date of delivery was based on LMP if the biometry was concordant within 7, 14 and 21 days in the first, second and third trimester, respectively, and based on ultrasound if LMP and biometry were not concordant. Women were encouraged to deliver at Tororo General Hospital (TGH), the district referral hospital. Midwives conducted deliveries and oxygen was available for resuscitation. Infants were seen every 2 weeks in the study clinic. Infants received zidovudine (AZT) prophylaxis after delivery in accordance with Ugandan Ministry of Health Guidelines, which changed during the course of the study. Seventy-six infants received AZT for 7 days, while 275 infants received AZT for 6 weeks after delivery. All infants had HIV DNA polymerase chain reaction (PCR) (Roche Amplicor HIV-1 DNA, v. 1.5) testing at birth.
Maternal medical and obstetrical history was collected at enrollment. Women were followed in the study clinic for all clinical care, including monthly routine visits. Delivery data were collected by study physicians who reviewed the medical record as recorded by the hospital staff. Infants were weighed on a calibrated scale within 12 h of delivery. Infants were followed every 2 weeks for the first month after delivery. This analysis included all neonatal deaths occurring within the first 28 days of life. Causes of neonatal deaths were determined by two pediatricians blinded to maternal study intervention arm and categorized according to criteria established by Lawn et al. [12].
The primary outcome was neonatal death, defined as the death of a live-born infant within 28 days of birth. Perinatal death, defined as the death of a live-born infant within 7 days of life was also examined. Low birth weight (LBW) was defined as <2500 g at birth. Prematurity, or preterm birth, was defined as any infant born before 37 weeks of gestation. Infants were included in this analysis if they were born alive, and had either died or reached 28 days of life at the time of analysis. No infants were lost to follow-up. Continuous (GA, duration of ART from enrollment to delivery, maternal age, CD4 count at enrollment), dichotomous (LBW, twin gestation, infant sex, location of delivery, treatment arm) and ordinal (gravidity, 1- and 5-min Apgar scores) variables were evaluated as predictors in univariate. Adjusted odds ratios were estimated using a multivariate logistic regression model that included all predictors significant in univariate analysis. Data analysis was conducted using Stata version 9 (StataCorp LP, TX, US).
Results
Among the 342 HIV-infected pregnant women enrolled, the median age was 29 years [interquartile range (IQR): 25–33] and median gravidity was 5 (IQR: 3–6). The median baseline CD4 count was 368 cells/mm3 (IQR: 271–505) and 51.2% of the women were assigned to the protease inhibitor (PI)-based ART regimen. The median duration of ART before delivery among women was 119 days (IQR: 93–147).
There were 351 live-born infants, 77% delivered at TGH, 18% at home and 5% at another health facility. Nine (3%) of the total 306 deliveries were twin deliveries, and 3 of 351 (0.9%) infants had congenital anomalies. Overall, 192 (55%) were male and 159 (45%) were female. Of the 339 infants with a recorded birth weight, 69 (20%) were LBW. The median Apgar scores at 1 and 5 min were 9 and 10, respectively. The median GA at delivery was 38.9 weeks (number by GA was 29–30 weeks: 8, 31–32 weeks: 4, 33–34 weeks: 10, 35–36 weeks: 39, 37–38 weeks: 121, 39–40 weeks: 139, 41–43 weeks: 30); there were no live births of infants at <29 weeks GA.
Thirteen (3.7%) infants died within the first 28 days of life, with the majority (9 of 13) dying in the perinatal period. The causes of death were attributed to neonatal sepsis (n = 3), prematurity (n = 3), congenital abnormality (n = 2), birth asphyxia (n = 1) and other reasons (n = 2, convulsions, birth trauma); in two cases, the cause of death was unknown (Table 1). All infants had negative HIV DNA PCR at birth.
Table 1.
Neonatal death characteristics
| Infant numbers | GA (weeks) | Birth weight (g) | Age at death (days) | Description of events | Lawn category [12] |
|---|---|---|---|---|---|
| 1 | 31 | 1900 | 16 | Abdominal distention and tenderness, followed by fever and respiratory failure | Preterm birth |
| 2 | 37 | 2550 | 1 | Uncomplicated vaginal delivery, Apgars 9 and 10, discharged day of life 2, found dead by family | Indeterminate |
| 3 | 30 | 1440 | 1 | Prolonged rupture of membranes, severe anemia (hemoglobin 4.5 g/dl), poor suck | Preterm birth |
| 4 | 31 | 1920 | 1 | Bilateral blindness, low-set ears, cleft lip and palate and polydactly | Congenital abnormality |
| 5 | 39 | 2510 | 0 | Uncomplicated vaginal delivery, Apgars 7 and 8 found dead 3 h after delivery | Indeterminate |
| 6 | 38 | 2779 | 23 | Uncomplicated vaginal delivery and perinatal period, noticed to have convulsion and emesis | Other |
| 7 | 35 | 2800 | 0 | Concern for placental abruption, meconium-stained fluid, Apgars 2 and 0, died soon after birth | Birth asphyxia |
| 8 | 30 | 1610 | 23 | Respiratory distress, poor perfusion and fever | Sepsis/Pneumonia |
| 9 | 30 | 1260 | 22 | Respiratory distress, cyanosis, dehydration | Sepsis/Pneumonia |
| 10 | 35 | 2610 | 1 | Rupture of membranes for >4 days, maternal fever, infant fever | Sepsis/Pneumonia |
| 11 | 30 | 1450 | 2 | Breathing and feeding well, found dead 3 h later | Preterm birth |
| 12 | 40 | Unknown | 0 | No opening to buccal cavity, hypoplastic right ear, small head, fused eyelids | Congenital abnormality |
| 13 | 30 | Unknown | 0 | Hemorrhage immediately after birth related to manipulation of umbilical cord | Other |
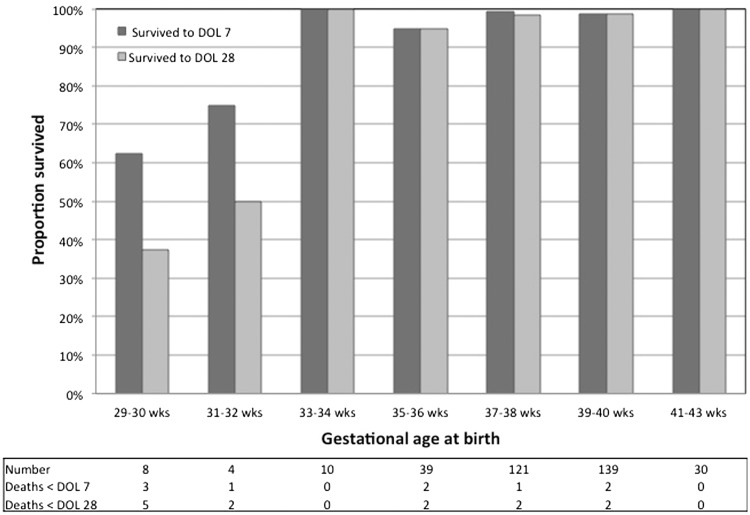
Fig. 1 shows neonatal survival by GA. While most of the deaths (7 of 13) were among children <32 weeks, all three infants born at 29 weeks survived. In univariate analysis, lower GA, LBW, lower 1- and 5-min Apgar scores, higher gravidity, twin gestation, congenital anomaly and shorter duration of ART were significant predictors of neonatal death (Table 2). In multivariate logistic regression modeling, increased GA was independently associated with reduced odds of neonatal death [odds ratio = 0.45, 95% confidence interval (CI): 0.2–0.9] after adjusting for LBW, congenital anomaly, twin gestation, gravidity, duration of ART, maternal age and 1-min Apgar score.
Fig. 1.
Perinatal and neonatal survival by completed weeks of gestation. DOL: day of life.
Table 2.
Risk factors for neonatal death
| Risk factor | Na | Unadjusted odds ratio (95% CI) | Adjusted odds ratio (95% CI)b |
|---|---|---|---|
| Infant factors | |||
| GA (weeks) | 351 | 0.6 (0.5–0.7)* | 0.5 (0.2–0.9)* |
| Preterm (<37 weeks) | 339 | 12.7 (3.8–42.7)* | |
| LBW (<2500 g) | 339 | 5.0 (1.5–17.1)* | 0.1 (0.0–2.5) |
| Congenital anomaly | 351 | 14.0 (1.2–165.3)* | 2.7 (0.1–114.7) |
| Twin | 351 | 6.5 (1.6–25.9)* | 1.6 (0.1–26.1) |
| 1-min Apgar | 289 | 0.5 (0.3–0.7)* | 0.6 (0.4–1.0) |
| 5-min Apgarc | 296 | 0.7 (0.5–0.9)* | |
| Infant sex (male) | 351 | 1.0 (0.3–2.9) | |
| Maternal factors | |||
| Increased gravidity | 351 | 1.37 (1.1–1.7)* | 1.1 (0.7–2.0) |
| Duration of ART (weeks) | 351 | 0.84 (0.7–1.0)* | 1.0 (0.8–1.2) |
| Maternal age (years) | 350 | 1.09 (1.0–1.2)* | 1.1 (0.9–1.4) |
| CD4 at enrollment (100 cells/mm3) | 348 | 0.77 (0.5–1.1) | |
| Treatment arm (PIs) | 351 | 0.66 (0.2–2.0) | |
| Delivery in health facility | 351 | 1.24 (0.3–5.7) |
aNumber with data available.
bAdjusted for all other variables listed in column.
cIncluded only 1-min Apgar in adjusted model due to covariance with 5-min Apgar.
*P<0.05
Discussion
In this cohort of infants born to HIV-infected women receiving combination ART in rural Uganda, we found a neonatal mortality rate of 37 per 1000 live births. This is higher than the national neonatal mortality rate of Uganda (26 per 1000) [13] and higher than rates reported in studies from Uganda, Tanzania and Kenya (range 17–29) [8]. While several studies have shown that children born to HIV-infected women experience elevated mortality rates during the first years of life [2, 3], few have specifically reported neonatal mortality (<28 days); one recent retrospective study of infants born to HIV-infected women in Botswana reported a neonatal mortality rate of 23 per 1000 [6].
The elevated mortality of infants born to HIV-infected women is not exclusively explained by HIV transmission to the infant. Infants who become HIV-infected, especially in utero, have high mortality over the first year of life [11]. Several studies have additionally shown that infants born to HIV-infected women suffer mortality rates at least twice that of children born to HIV-uninfected women, even when they remain uninfected by HIV [3, 5]. The increased mortality among such HIV-exposed infants has been hypothesized to result from the poorer care, lower socioeconomic status and increased exposure to opportunistic infections such as tuberculosis from sick or dead parents. Conversely, one would predict that HIV-infected women receiving combination ART, as they did in our cohort, would be healthier and have, in turn, healthier infants with higher neonatal survival. One recent study from South Africa found that the mortality of children <5 years of age, born to HIV-infected women receiving combination ART could approach that of children born to HIV-uninfected women [14]. However, the neonatal mortality rate in our cohort of children born to women receiving combination ART remained tragically high.
Prematurity was a major contributor to mortality in our cohort. Using ultrasound-based GA measurements, we found that low GA was associated with increased odds of mortality (Table 2). It was encouraging that three infants born at 29 weeks GA survived; this may have been the result of the higher level of care offered by participation. However, even with those survivors, low GA was associated with increased odds of death in both unadjusted and adjusted modeling. In individual case reviews, prematurity was thought to be the primary cause in 3 of the 13 infants, but was also likely to have contributed to deaths among two (#8 and #9), which occurred in 30-week GA infants who died of sepsis (Table 1). This high proportion of deaths attributed to prematurity (5 of 13 overall) is consistent with a recent meta-analysis that reported 52% of neonatal deaths in East Africa occurring in preterm infants [8]. Our understanding of the causes of premature delivery in HIV-infected women is limited. Advanced untreated HIV disease is associated with preterm delivery [11], but one recent study suggested that combination ART may also be associated with premature delivery [6] and data are conflicting about whether different ART agents are associated with increased risk [10]. In our study, longer duration of ART seemed to decrease the risk of neonatal death. However, the effect size was small and may be related to confounding by prematurity because women who delivered prematurely would also have had a shorter duration of ART before delivery.
Infection was the primary cause of death in 3 of 13 cases (Table 1), but may also have played a role in the two cases that occurred outside of the hospital (#2 and #6), and in case #1, in which a premature infant appeared to have died from necrotizing enterocolitis (Table 2). Our findings are consistent with data identifying infection as a leading cause of neonatal mortality, responsible for 35% of neonatal deaths worldwide [12]. Some evidence suggests that HIV-exposed infants may be at elevated risk for infection, having lower levels of the protective maternal antibodies usually transferred late in gestation [15]. However a recent study of South African infants failed to find elevated risk of early or late onset sepsis among HIV-exposed infants [16]. Hospitalization and intravenous (IV) antibiotics were available to infants in this study, but distance from the hospital in the rural site often led to delays in care that may have contributed to these deaths. Of note, LBW was a significant risk factor in unadjusted, but not adjusted, analyses (Table 2), suggesting that GA, and the associated factors of fetal immaturity, is the more important predictor of mortality.
In only one infant was death attributed to complications of birth asphyxia (#7) and in that case, it is possible that other predelivery insults from infection may have also contributed. This low rate stands in contrast to data, suggesting that birth asphyxia is responsible for as much as 24% of neonatal deaths in Africa [12] and is likely related, at least in part, to the fact that women in our study were followed closely and had access to skilled birth attendance at our study hospital. However, that 8 of 13 deaths occurred within the first day of life suggests intra- and postpartum management remain important.
This study has several limitations. The small size of this study reduces the precision of our estimated mortality rate and our ability to perform multivariate analyses and parse out the interactions of prematurity, infection and other factors. There was no comparison group of infants born to HIV-uninfected women. The clinical assignment of cause of death was also limited in many cases to data from verbal reports of the family. It should also be noted that HIV DNA testing at birth would have detected children infected in utero, but not intrapartum; although with high rates of virologic suppression in women at the time of delivery (data not shown) we expect intrapartum transmission in these infants to have been unlikely.
In summary, we report a high neonatal mortality rate in a cohort of children born to HIV-infected women receiving combination ART in rural Uganda, with prematurity and infection the leading causes of death. As treatment of HIV-infected pregnant women expands throughout Africa, it will be important that strategies to minimize both the incidence of and mortality from premature delivery and postnatal infection be developed to optimize the outcomes of HIV-exposed infants.
Funding
Eunice Kennedy Shriver National Institute of Child Health and Human Development [grant number PO1HD059454-03, K236045901A2 to T.D.R] and National Institute of Allergy and Infectious Disease [grant number T32AI065388 to V.A.] at the National Institutes of Health.
Acknowledgements
We are grateful to women who agreed to participate in this study and the PROMOTE study team. We also thank AbbVie for the donation of lopinavir/ritonavir.
References
- 1.UNAIDS. UNAIDS Report on the global AIDS epidemic. 2012. http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/gr2012/20121120_UNAIDS_Global_Report_2012_en.pdf.
- 2.Taha TE, Dadabhai SS, Sun J, et al. Child mortality levels and trends by HIV status in Blantyre, Malawi: 1989–2009. J Acquir Immune Defic Syndr. 2012;61:226–34. doi: 10.1097/QAI.0b013e3182618eea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marinda E, Humphrey JH, Iliff PJ, et al. Child mortality according to maternal and infant HIV status in Zimbabwe. Pediatr Infect Dis J. 2007;26:519–26. doi: 10.1097/01.inf.0000264527.69954.4c. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Neonatal and perinatal mortality 2004: country, regional and global estimates. 2007. http://www.who.int/reproductivehealth/publications/monitoring/9789241596145/en/index.html.
- 5.Landes M, van Lettow M, Chan AK, et al. Mortality and health outcomes of HIV-exposed and unexposed children in a PMTCT cohort in Malawi. PLoS One. 2012;7:e47337. doi: 10.1371/journal.pone.0047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen JY, Ribaudo HJ, Souda S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J Infect Dis. 2012;206:1695–705. doi: 10.1093/infdis/jis553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawn JE, Kerber K, Enweronu-Laryea C, et al. 3.6 million neonatal deaths–what is progressing and what is not? Semin Perinatol. 2010;34:371–86. doi: 10.1053/j.semperi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Marchant T, Willey B, Katz J, et al. Neonatal mortality risk associated with preterm birth in East Africa, adjusted by weight for gestational age: individual participant level meta-analysis. PLoS Med. 2012;9:e1001292. doi: 10.1371/journal.pmed.1001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geirsson RT. Ultrasound instead of last menstrual period as the basis of gestational age assignment. Ultrasound Obstet Gynecol. 1991;1:212–9. doi: 10.1046/j.1469-0705.1991.01030212.x. [DOI] [PubMed] [Google Scholar]
- 10.van der Merwe K, Hoffman R, Black V, et al. Birth outcomes in South African women receiving highly active antiretroviral therapy: a retrospective observational study. J Int AIDS Soc. 2011;14:42. doi: 10.1186/1758-2652-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HY, Kasonde P, Mwiya M, et al. Pregnancy loss and role of infant HIV status on perinatal mortality among HIV-infected women. BMC Pediatr. 2012;12:138. doi: 10.1186/1471-2431-12-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawn JE, Wilczynska-Ketende K, Cousens SN. Estimating the causes of 4 million neonatal deaths in the year 2000. Int J Epidemiol. 2006;35:706–18. doi: 10.1093/ije/dyl043. [DOI] [PubMed] [Google Scholar]
- 13. United Nations Children’s Fund (UNICEF). The State of the World's children. In: Aslam A, Szczuka J (eds). New York, New York, 2012. http://www.unicef.org/sowc2012/pdfs/SOWC%202012-Main%20Report_EN_13Mar2012.pdf.
- 14.Ndirangu J, Newell ML, Thorne C, et al. Treating HIV-infected mothers reduces under 5 years of age mortality rates to levels seen in children of HIV-uninfected mothers in rural South Africa. Antivir Ther. 2012;17:81–90. doi: 10.3851/IMP1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones CE, Naidoo S, De Beer C, et al. Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. JAMA. 2011;305:576–84. doi: 10.1001/jama.2011.100. [DOI] [PubMed] [Google Scholar]
- 16.Cutland CL, Schrag SJ, Zell ER, et al. Maternal HIV infection and vertical transmission of pathogenic bacteria. Pediatrics. 2012;130:e581–90. doi: 10.1542/peds.2011-1548. [DOI] [PubMed] [Google Scholar]