Abstract
Objective
The purpose of this study was to evaluate atomoxetine treatment effects in attention-deficit/hyperactivity disorder (ADHD-only), attention-deficit/hyperactivity disorder with comorbid dyslexia (ADHD+D), or dyslexia only on ADHD core symptoms and on sluggish cognitive tempo (SCT), working memory, life performance, and self-concept.
Methods
Children and adolescents (10–16 years of age) with ADHD+D (n=124), dyslexia-only (n=58), or ADHD-only (n=27) received atomoxetine (1.0–1.4 mg/kg/day) or placebo (ADHD-only subjects received atomoxetine) in a 16 week, acute, randomized, double-blind trial with a 16 week, open-label extension phase (atomoxetine treatment only). Changes from baseline were assessed to weeks 16 and 32 in ADHD Rating Scale-IV-Parent-Version:Investigator-Administered and Scored (ADHDRS-IV-Parent:Inv); ADHD Rating Scale-IV-Teacher-Version (ADHDRS-IV-Teacher-Version); Life Participation Scale—Child- or Parent-Rated Version (LPS); Kiddie-Sluggish Cognitive Tempo (K-SCT) Interview; Multidimensional Self Concept Scale (MSCS); and Working Memory Test Battery for Children (WMTB-C).
Results
At week 16, atomoxetine treatment resulted in significant (p<0.05) improvement from baseline in subjects with ADHD+D versus placebo on ADHDRS-IV-Parent:Inv Total (primary outcome) and subscales, ADHDRS-IV-Teacher-Version Inattentive subscale, K-SCT Interview Parent and Teacher subscales, and WMTB-C Central Executive component scores; in subjects with Dyslexia-only, atomoxetine versus placebo significantly improved K-SCT Youth subscale scores from baseline. At Week 32, atomoxetine-treated ADHD+D subjects significantly improved from baseline on all measures except MSCS Family subscale and WMTB-C Central Executive and Visuo-spatial Sketchpad component scores. The atomoxetine-treated dyslexia-only subjects significantly improved from baseline to week 32 on ADHDRS-IV-Parent:Inv Inattentive subscale, K-SCT Parent and Teacher subscales, and WMTB-C Phonological Loop and Central Executive component scores. The atomoxetine-treated ADHD-only subjects significantly improved from baseline to Week 32 on ADHDRS-Parent:Inv Total and subscales, ADHDRS-IV-Teacher-Version Hyperactive/Impulsive subscale, LPS Self-Control and Total, all K-SCT subscales, and MSCS Academic and Competence subscale scores.
Conclusions
Atomoxetine treatment improved ADHD symptoms in subjects with ADHD+D and ADHD-only, but not in subjects with dyslexia-only without ADHD. This is the first study to report significant effects of any medication on SCT.
Clinical Trials Registration
This study was registered at: http://clinicaltrials.gov/ct2/home, NCT00607919.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) and dyslexia frequently co-occur (ADHD with comorbid dyslexia [ADHD+D]) (Germano et al. 2010). It has been hypothesized that common genetic influences and neuropsychological deficits are associated with an increased susceptibility for both disorders (Willcutt et al. 2007, 2010). Those shared genetic variables seem to mainly connect reading difficulties and ADHD inattention symptoms, while being largely independent of genes that contribute to general cognitive ability (Paloyelis et al. 2010). Shared cognitive deficits for both ADHD and dyslexia include weaknesses on measures of phoneme awareness, verbal reasoning, and working memory (Willcutt et al. 2010). Patients with ADHD and those with dyslexia report lower life performance and an impaired self-concept (Smith-Spark et al. 2004; Houck et al. 2011; Ridley 2011; Brod et al. 2012). It has been suggested that attention difficulties associated with ADHD may be a causal factor for reading difficulties in some patients with dyslexia (Shaywitz and Shaywitz 2008).
The inattention dimension of ADHD symptoms is associated with an experimental construct termed Sluggish Cognitive Tempo (SCT), which emerges as a dimension separate from inattention and hyperactivity/impulsivity in exploratory (McBurnett et al. 2001; Hartman et al. 2004; Penny et al. 2009) and confirmatory (Hartman et al. 2004; Garner et al. 2010) factor analyses. The core features of SCT are excessive daydreaming, hypoactivity or slowness, and drowsiness. External correlates have included internalizing comorbidities (Carlson and Mann 2002; Hartman et al. 2004; Penny et al. 2009; Garner et al. 2010; Skirbekk et al. 2011) and some neuropsychological abnormalities (Hinshaw et al. 2002; Huang-Pollock et al. 2005; Yee Mikami et al. 2007; Wahlstedt and Bohlin 2010; Skirbekk et al. 2011). Neuropsychological performance in ADHD appears more affected by inattention than by other dimensions of the disease. Although SCT has often been studied as a dimensional aspect of ADHD, it has also been observed to occur in other pathologies in children. Reeves and coinvestigators observed SCT as a sequela of acute lymphoblastic leukemia in children (Reeves et al. 2007). Additionally, SCT has been described as an independent condition of ADHD, and is associated with serious impairment in adults (Barkley 2012).
To date, only a limited number of trials have evaluated possible interventions for patients with ADHD+D (Sexton et al. 2012) and no trials, to our knowledge, have evaluated the effects of medication on SCT. Recently, two small clinical trials suggested that atomoxetine is effective in the treatment of ADHD symptoms in children and adolescents with ADHD+D (de Jong et al. 2009; Sumner et al. 2009). The first study examined the effect, on reading performance and on neurocognitive function, of open-label treatment with atomoxetine in subjects with ADHD+D (n=36) or ADHD-only (n=20), 10–16 years of age (Sumner et al. 2009). Treatment with atomoxetine resulted in reduced ADHD symptoms and improved reading scores in both groups; however, the authors observed different patterns and magnitudes of improvement in the working memory component scores in the different subject groups (Sumner et al. 2009). The second study was a randomized, placebo-controlled crossover study (de Jong et al. 2009). Enrolled were subjects with ADHD+D (n=20), dyslexia-only (n=21), and ADHD-only (n=16), and healthy controls (n=26), 9–10 years of age. In this study, treatment with atomoxetine, compared with placebo, improved visuospatial working memory performance and inhibition in subjects with ADHD+D, whereas no effects were seen in the dyslexia-only and ADHD-only groups (de Jong et al. 2009).
Here, we evaluated the efficacy and safety of atomoxetine in children and adolescents with ADHD+D, dyslexia-only, and ADHD-only in a larger, randomized, placebo-controlled trial. We tested the a priori hypothesis that atomoxetine given orally once daily (QD) for ∼16 weeks would provide superior efficacy compared with placebo for the treatment of ADHD in children and adolescents with ADHD+D. Secondary objectives sought to evaluate the effects of atomoxetine in children and adolescents with dyslexia-only, and atomoxetine's effects on SCT, working memory, life performance, and self-concept in children and adolescents with ADHD+D, dyslexia-only, or ADHD-only.
Methods
Subjects
Subjects with ADHD+D and ADHD-only met Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision (American Psychiatric Association 2000) diagnostic criteria for ADHD; this was confirmed during visit 1 by the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Aged Children-Present and Lifetime Version—Behavioral Component (Kaufman et al. 1997). At visits 2 and 3, subjects with ADHD+D and ADHD-only also had an ADHD Rating Scale-IV-Parent-Version:Investigator-Administered and Scored (ADHDRS-IV-Parent:Inv) Total score ≥1.5 standard deviations above age and gender norms. Subjects with ADHD+D and dyslexia-only met criteria for dyslexia at Visit 2: ≥22-point discrepancy between the Wechsler Abbreviated Scale of Intelligence Verbal Intelligence Quotient or Performance Intelligence Quotient (whichever was higher) and the Woodcock Johnson III Basic Reading Skills score, Letter Word Identification score, or Word Attack score; or a score ≤89 on any of the aforementioned Woodcock Johnson III subscales. Excluded were subjects with a documented history of bipolar I or bipolar II disorder, psychosis, autism, Asperger's syndrome, or pervasive developmental disorder, and subjects who were currently taking anticonvulsants for seizure control.
Sample size calculations were based on the primary analysis of the difference in the ADHDRS-IV-Parent:Inv Total score between subjects with ADHD+D taking atomoxetine and those taking placebo. A last observation carried forward approach with 65 subjects per arm would allow for a two sided test at the 5% significance level, with an assumed effect size of 0.60, 90% power, and a missing data rate of 5%. At an effect size of 0.65, the power would increase to 94%; at an effect size of 0.70, the power would be 96%; and at an effect size of 0.55, the study would have 85% power. Previous studies comparing atomoxetine and placebo had effect sizes ranging from 0.63 to 0.80.
Study design
The design was a multicenter, randomized, placebo-controlled, double-blind phase 4 study of atomoxetine (0.5 mg/kg/day for 3 days, then 1.0–1.4 mg/kg/day) administered QD with food followed by a 16 week, open-label, extension phase. After nearly 2 weeks of screening, subjects with ADHD+D and dyslexia-only were randomized to atomoxetine or placebo treatment in a 1:1 ratio by a computer-generated, random sequence using an interactive voice response system. Subjects with ADHD-only received atomoxetine for 16 weeks, but they were told that at some point during the acute phase they might be placed on placebo to help mitigate the potential for an open-label bias. After finishing the acute phase, subjects could enter the extension phase and receive atomoxetine QD (0.5 mg/kg/day for a minimum of 3 days, then 1.0–1.4 mg/kg/day) with food. Before study initiation, the protocol was reviewed and approved by the appropriate institutional review boards. Parents or guardians of all patients provided written informed consent before the subjects received study medication or underwent study procedures.
Efficacy measures
Assessed were changes from baseline to weeks 16 and 32 in ADHDRS-IV-Parent:Inv (DuPaul et al. 1998) (raw scores; investigators administered the scale to parents; 18 item scale, total score ranges from 0 to 54 with each item scored on a 0–3 scale: 0=never or rarely [none]; 1=sometimes [mild]; 2=often [moderate]; 3=very often [severe]); ADHDRS-IV-Teacher-Version (raw scores; teacher completed 18 item scale, total score ranges from 0 to 54 with each item scored on a 0–3 scale:0=never or rarely [none]; 1=sometimes [mild]; 2=often [moderate]; 3=very often [severe]); Life Participation Scale—Child-, Parent-Rated Version (LPS; raw scores; 24 item scale; total score ranges from 0 to 72 with each item scored on a 0–3 scale: 0=never or seldom; 1=sometimes; 2=often; 3=very often); Kiddie-Sluggish Cognitive Tempo (K-SCT; raw scores; 17 item scale; total score ranges from 0 to 51 with each item scored on a 0–3 scale: 0=never or rarely; 1=sometimes; 2=often; 3=very often) (Lee et al. 2013); Multidimensional Self Concept Scale (MSCS; age-based standard scores; 150 item scale composed of six scales and a total score that ranges from 45 to 145; items are differentially scored based on positively worded items: 1=strongly disagree; 2=disagree; 3=agree; 4=strongly agree; or negatively worded items: 4=strongly disagree; 3=disagree; 2=agree; 1=strongly agree); and Working Memory Test Battery for Children (WMTB-C; age-based standard scores; scale consists of nine subtests that measure three components; scores for these three components range from 55 to 145).
Statistical analyses
We tested the a priori hypothesis that atomoxetine QD for ∼16 weeks would provide superior efficacy compared with placebo for the treatment of ADHD in children with ADHD+D. The prespecified primary analysis for the trial was a mixed-effects repeated measures model (MMRM) with terms for treatment, investigator, baseline score, visit, treatment by visit, and baseline score by visit, on the ADHDRS-IV-Parent:Inv Total score comparing atomoxetine and placebo in subjects with ADHD+D after 16 weeks. Only the primary analysis was conducted with MMRM.
Secondary objectives sought to evaluate the effects of atomoxetine in children and adolescents with dyslexia-only, and atomoxetine's effects on SCT, working memory, life performance, and self-concept in children and adolescents with ADHD-only, dyslexia-only, or ADHD-only. These efficacy data were analyzed with last observation carried forward analyses that used fixed-effects analysis of covariance (ANCOVA) models with terms for treatment group, investigator, sex, baseline score, age, and baseline score-by-treatment interaction. Similar ANCOVA models were used to assess diagnostic group differences with terms for diagnosis, investigator, sex, baseline score, age, and baseline score-by-diagnosis interaction in both acute and open-label phases. Type III sums of squares were used for between-treatment tests. Changes within treatment were assessed using Student's t test applied to the least-squares mean for the group from the ANCOVA model. In retrospect, the adjustment for baseline scores may not have been an appropriate analysis for scales that specifically measure ADHD symptoms, as all patients did not have ADHD; therefore, this adjustment could have obscured a difference when an overall mean was used across diagnoses in calculation of least-squares mean, thereby inflating the scores in the dyslexia-only group, up to levels consistent with ADHD+D. Also, effects of baseline and baseline score-by-treatment interaction could have been overinfluenced by ADHD+D patients, given the larger variability of baseline values for this group of patients. To evaluate this possibility, means and p values that ignore baseline were also examined for ADHDRS-IV-Parent:Inv, ADHDRS-IV-Teacher-Version, and LPS with the ANCOVA approach described, excluding the terms for baseline score and baseline score-by-treatment interaction. Secondary end-points were not adjusted for testing of multiple hypotheses, as we wanted to show the actual results that could identify areas in which more research could be warranted.
To determine whether improvements in ancillary measures were a byproduct of the ADHD improvement, Pearson's correlation coefficients were determined between changes in K-SCT scores (Parent, Teacher, and Youth subscales) and ADHDRS-IV-Parent:Inv/ADHDRS-IV-Teacher-Version (Total and Inattentive and Hyperactive/Impulsive subscales), as well as among demographic baseline parameters (age, gender, income status, education, and ADHD subtype) and all outcome measures at 16 and 32 weeks.
Results
Subjects' disposition and baseline demographics
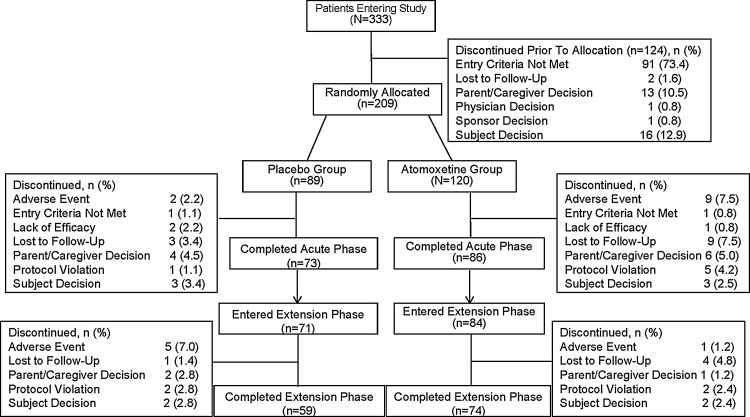
A total of 333 subjects were screened for study eligibility, of which 209 subjects were randomized for the acute treatment phase (Fig. 1). The acute treatment phase was completed by 86 subjects in the atomoxetine group and 73 subjects in the placebo group. Of these subjects, 84 subjects who had received atomoxetine and 71 subjects who had received placebo entered the extension phase. The extension phase was completed by a total of 133 subjects (Fig. 1).
FIG. 1.
Flow diagram of subject disposition during the acute and extension phases.
During the acute treatment phase, ∼62% of subjects in both the atomoxetine and placebo groups were male, with a mean age of 12 years. Most subjects were diagnosed with inattentive ADHD (atomoxetine: 50%; placebo: 54%), followed by combined ADHD (atomoxetine: 49%; placebo: 43%). These demographic parameters were similar during the extension phase (Supplementary Table 1) (see online Supplementary Material at http://www.liebertonline.com).
Efficacy results – acute phase
The result of the primary MMRM analysis was significant (p<0.001) and showed greater improvement on the ADHDRS-IV-Parent:Inv Total score for atomoxetine-treated subjects with ADHD+D than for placebo-treated subjects with ADHD+D (−20.0 vs. −12.3, respectively). When data were analyzed using ANCOVA with an adjustment for baseline scores, significant (p<0.05) improvements on the ADHDRS-IV-Parent:Inv Total score, and Inattentive and Hyperactive/Impulsive subscale scores, were seen in response to treatment with atomoxetine in subjects with ADHD+D, dyslexia-only, and ADHD-only. When compared with subjects on placebo, however, improvements after treatment with atomoxetine were significantly different for subjects with ADHD+D, but not for subjects with dyslexia-only (Supplementary Table 2) (see online Supplementary Material at http://www.liebertonline.com).
When data were analyzed without an adjustment for baseline scores, no significant improvements during treatment with atomoxetine on the ADHDRS-Parent:Inv Total and subscale scores were observed for subjects with dyslexia-only, wheras improvements from baseline were significant for subjects with ADHD+D and ADHD-only (Table 1).
Table 1.
Acute Phase: ADHDRS-IV-Parent:Inv, ADHDRS-IV-Teacher Version, and K-SCT Interview
| |
|
ADHD+D |
Dyslexia-only |
ADHD-only |
|
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure | Tx | n | Baseline | LSMean (mean) changea | pb(effect Size) | n | Baseline | LSMean (mean) changea | pb(effect size) | n | Baseline | LSMean (mean) change | pc | pd |
| ADHDRS-IV-Parent Version:Inv (no adjustment for baseline score) | ||||||||||||||
| Total | A | 62 | 37.22 | −17.52*** (−18.87) | 0.002 (−0.53) | 26 | 13.62 | −3.57 (−4.50) | 0.425 (−0.23) | 27 | 35.26 | −15.58*** (−16.59) | <0.001 | 0.546 |
| P | 58 | 37.57 | −11.27*** (−12.98) | 28 | 14.25 | −1.57 (−2.68) | ||||||||
| Inattentive | A | 62 | 22.32 | −9.82*** (−10.64) | 0.020 (−0.40) | 26 | 9.50 | −2.13 (−2.81) | 0.530 (−0.15) | 27 | 21.89 | −9.50*** (−10.33) | <0.001 | 0.982 |
| P | 58 | 22.52 | −6.83*** (−7.79) | 28 | 9.82 | −0.90 (−1.86) | ||||||||
| Hyp/Imp | A | 62 | 14.90 | −7.71*** (−8.23) | 0.002 (−0.52) | 26 | 4.12 | −1.44 (−1.69) | 0.602 (−0.22) | 27 | 13.37 | −6.08*** (−6.26) | <0.001 | 0.240 |
| P | 58 | 15.05 | −4.44*** (−5.18) | 28 | 4.43 | −0.66 (−0.82) | ||||||||
| ADHDRS-IV-Teacher Version (no adjustment for baseline score) | ||||||||||||||
| Total | A | 21 | 24.38 | −8.26** (−7.19) | 0.149 (−0.47) | 12 | 14.00 | −2.97 (−3.36) | 0.700 (0.21) | 11 | 22.09 | −3.46 (−3.09) | 0.085 | 0.407 |
| P | 22 | 24.09 | −3.60 (−2.98) | 11 | 17.90 | −1.99 (−4.49) | ||||||||
| Inattentive | A | 21 | 16.00 | −5.24*** (−4.48) | 0.024 (−0.68) | 12 | 9.25 | −1.25 (−1.86) | 0.779 (0.46) | 11 | 13.64 | −1.90 (−2.27) | 0.206 | 0.443 |
| P | 22 | 13.32 | −1.08 (−0.99) | 11 | 13.18 | −1.72 (−3.68) | ||||||||
| Hyp/Imp | A | 21 | 8.38 | −3.03 (−2.71) | 0.809 (−0.14) | 12 | 4.75 | −1.71 (−1.50) | 0.432 (−0.27) | 11 | 8.45 | −1.56 (−0.82) | 0.171 | 0.526 |
| P | 22 | 10.77 | −2.52 (−1.99) | 11 | 4.72 | −0.27 (−0.81) | ||||||||
| K-SCT Interview (adjusted for baseline scores) | ||||||||||||||
| Parent | A | 56 | 21.58 | −8.23*** (−7.82) | 0.012 (−0.31) | 25 | 9.81 | −2.51 (−1.93) | 0.251 (−0.13) | 23 | 22.11 | −7.07*** (−8.24) | 0.012 | 0.642 |
| P | 57 | 22.63 | −3.93** (−4.64) | 27 | 13.59 | −0.08 (−1.11) | ||||||||
| Teacher | A | 22 | 23.23 | −6.88** (−8.82) | 0.017 (−0.77) | 11 | 12.22 | −6.69* (−2.77) | 0.147 (0.01) | 11 | 18.18 | −4.78 (−3.47) | 0.017 | 0.979 |
| P | 22 | 14.41 | −0.66 (−1.64) | 12 | 16.00 | −1.87 (−2.83) | ||||||||
LSMean values were derived from a model comparing atomoxetine-treated subjects with placebo-treated subjects within the diagnostic group.
Atomoxetine versus placebo p value.
ADHD+D (atomoxetine) versus dyslexia-only (atomoxetine) p value.
ADHD+D (atomoxetine) versus ADHD-only (atomoxetine) p value.
Intra-group p values: *p<0.05; **p<0.01; ***p<0.001; p values from t tests on LSMean change.
A, atomoxetine; ADHD, attention deficit/hyperactivity disorder; ADHDRS-IV-Parent:Inv, ADHD Rating Scale-IV-Parent-Version:Investigator-Administered and Scored; ADHDRS-IV-Teacher Version, ADHD Rating Scale-IV-Teacher-Version; K-SCT, Kiddie-Sluggish Cognitive Tempo; Hyp/Imp, Hyperactive/Impulsive; LSMean, least squares mean; P, placebo; Tx, treatment.
Improvements on the ADHDRS-IV-Teacher-Version Total score, and Inattentive and Hyperactive/Impulsive subscales, after acute treatment with atomoxetine, were significant for subjects with ADHD+D, but not for subjects with ADHD-only when analyzed with an adjustment for baseline scores; subjects with dyslexia-only showed significant improvements only on the Inattentive subscale (Supplementary Table 2). When data were not adjusted for baseline scores, only subjects with ADHD+D showed significant improvements during treatment with atomoxetine on ADHDRS-IV-Teacher-Version Total scores and Inattentive subscale scores (Table 1).
On the LPS, changes from baseline, during treatment with atomoxetine, were significant for subjects with ADHD+D for the Self-Control subscale and the Total score, when data were analyzed either adjusted or unadjusted for baseline scores (Supplementary Tables 2 and 3) (see online Supplementary Material at http://www.liebertonline.com). For subjects with ADHD-only, changes from baseline were significant during treatment with atomoxetine on the Self-Control subscale and the LPS Total score, when data were analyzed adjusted for baseline scores (Supplementary Table 2). Analysis of data unadjusted for baseline scores also showed significant changes on the Happy/Social subscale (Supplementary Table 3). It was assumed that analyses of score changes on the K-SCT, MSCS and WMTB-C were not biased as these scales did not specifically measure ADHD symptoms. The MSCS and WMTB-C have been used in assessments of patients with multiple disease states (Bracken 1992; Pickering and Gathercole 2001). The K-SCT is a construct that is currently being researched, and there are some data to support SCT as a separate disorder from ADHD (Penny et al. 2009; Garner et al. 2010; Barkley and Fischer 2011). Therefore, analyses of changes on K-SCT, MSCS, and WMTB-C were only performed with the a priori defined model, including an adjustment for baseline scores. Subjects with ADHD+D experienced significantly greater improvements during treatment with atomoxetine compared with placebo on K-SCT Parent and Teacher subscales (Table 1). On MSCS subscales, no significant treatment group differences were observed for subjects with ADHD+D, and on WMTB-C, only the Central Executive component score was significantly more improved during treatment with atomoxetine than with placebo in subjects with ADHD+D (Supplementary Table 3).
Most effect sizes ranked from moderate to large for statistically significant differences between atomoxetine and placebo treatment (Table 1 and Supplementary Table 3). Comparison of score changes during atomoxetine treatment among subjects with ADHD+D, dyslexia-only, and ADHD-only yielded no significant differences in either the baseline score-adjusted or -unadjusted analyses (Table 1 and Supplementary Table 3).
After 16 weeks, change in the K-SCT Parent subscale score was significantly correlated with changes in ADHDRS-IV-Parent:Inv scores (correlation coefficient of 0.40–0.54, p<0.001); and change in the K-SCT Teacher subscale score was significantly correlated with changes in ADHDRS-IV-Teacher-Version scores (correlation coefficient of 0.33–0.61, p≤0.004) (Supplementary Table 4) (see online supplementary material at http://www.liebertonline.com). All correlations were positive, showing that as ADHDRS scores improved so did K-SCT scores. The change in the K-SCT Youth subscale score showed a significant, but weak, correlation with changes in ADHDRS-Parent:Inv scores (correlation coefficient of 0.16–0.19, p≤0.032), but not in ADHDRS-IV-Teacher-Version scores. None of the examined baseline demographic parameters showed significant correlations with any of the presented outcome measures.
Efficacy results—extension phase
When analyzed with an adjustment for baseline scores, significant (p<0.05) improvements on the ADHDRS-Parent:Inv Total score, and Inattentive and Hyperactive/Impulsive subscale scores, were seen in response to treatment with atomoxetine in subjects with ADHD+D, Dyslexia-only, and ADHD-only, after 32 weeks (Supplementary Table 2). When data were analyzed unadjusted for baseline scores, improvements remained significant for subjects with ADHD+D and ADHD-only for ADHDRS-Parent:Inv Total and subscale scores; in subjects with dyslexia-only, only changes from baseline on the Inattentive subscale remained significant (Table 2). Total score changes and changes on both subscales of the ADHDRS-Parent:Inv were significantly different between subjects with ADHD+D and those with dyslexia-only, when data were not adjusted for baseline scores.
Table 2.
Efficacy Results—Week 32
| |
ADHD+D |
Dyslexia-only |
ADHD-only |
|
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure | n | Baseline | LSMean (mean) change | n | Baseline | LSMean (mean) change | n | Baseline | LSMean (mean) change | pa | pb |
| ADHDRS-IV-Parent Version: Inv (no adjustment for baseline score) | |||||||||||
| Total | 45 | 37.18 | −21.59*** (−23.64) | 18 | 15.00 | −5.68 (−8.61) | 21 | 34.95 | −19.57*** (−22.62) | <0.001 | 0.453 |
| Inattentive | 45 | 22.42 | −13.84*** (−12.92) | 18 | 10.28 | −4.14* (−5.67) | 21 | 22.38 | −12.12*** (−14.33) | <0.001 | 0.659 |
| Hyp/Imp | 45 | 14.76 | −8.67*** (−9.80) | 18 | 4.72 | −1.54 (−2.94) | 21 | 12.57 | −7.45*** (−8.29) | <0.001 | 0.443 |
| ADHDRS-IV-Teacher Version (no adjustment for baseline score) | |||||||||||
| Total | 23 | 23.97 | −9.05** (−6.15) | 11 | 15.55 | −6.88 (−6.76) | 9 | 21.00 | −8.39 (−7.08) | 0.903 | 0.875 |
| Inattentive | 23 | 16.02 | −5.19* (−4.28) | 11 | 10.27 | −3.65 (−4.03) | 9 | 13.00 | −3.96 (−4.19) | 0.713 | 0.707 |
| Hyp/Imp | 23 | 7.96 | −3.86** (−1.87) | 11 | 5.27 | −3.23 (−2.73) | 9 | 8.00 | −4.43* (−2.89) | 0.695 | 0.752 |
| K-SCT Interview (adjusted for baseline score) | |||||||||||
| Parent | 45 | 22.09 | −9.97*** (−10.40) | 18 | 8.12 | −14.67***(−3.68) | 21 | 21.03 | −10.50*** (−10.83) | 0.038 | 0.812 |
| Teacher | 23 | 22.87 | −7.56** (−7.00) | 11 | 14.49 | −11.53* (−5.40) | 9 | 17.00 | −10.62* (−7.89) | 0.455 | 0.547 |
| Youth | 45 | 18.71 | −4.96** (−4.36) | 18 | 14.61 | −4.17 (−2.00) | 21 | 19.29 | −4.78** (−5.43) | 0.921 | 0.929 |
ADHD+D (atomoxetine) versus Dyslexia-only (atomoxetine) p value.
ADHD+D (atomoxetine) versus ADHD-only (atomoxetine) p value.
Intra-group p values: *p<0.05; **p<0.01; ***p<0.001; p values from t tests on LSMean change.
ADHD, attention deficit/hyperactivity disorder; ADHDRS-IV-Parent:Inv, ADHD Rating Scale-IV-Parent-Version:Investigator-Administered and Scored; ADHDRS-IV-Teacher Version, ADHD Rating Scale-IV-Teacher-Version; K-SCT, Kiddie-Sluggish Cognitive Tempo; Hyp/Imp, Hyperactive/Impulsive; LSMean, least squares mean.
Improvements on the ADHDRS-IV-Teacher-Version Total score, and Inattentive and Hyperactive/Impulsive subscales, during extension phase treatment with atomoxetine, were significant for subjects with ADHD+D, when analyzed with an adjustment for baseline scores; subjects with dyslexia-only showed significant improvements on the Total score and Inattentive subscale score, while subjects with ADHD-only showed significant improvements on the Hyperactive/Impulsive subscale score (Supplementary Table 2). When data were not adjusted for baseline scores, only subjects with ADHD+D showed significant improvements during treatment with atomoxetine on the ADHDRS-IV-Teacher-Version Total score and Inattentive subscale score, and subjects with ADHD-only showed significant improvements during treatment with atomoxetine on the Hyperactive/Impulsive subscale score (Table 2).
Changes from baseline on LPS during extension phase treatment with atomoxetine were significant for subjects with ADHD+D for the Happy/Social subscale, the Self-Control subscale, and the Total scores, when data were analyzed adjusted for baseline scores (Supplementary Table 5) (see online Supplementary Material at http://www.liebertonline.com); without adjustment for baseline scores, changes on LPS Total and both subscales were significant (Supplementary Table 6) (see online Supplementary Material at http://www.liebertonline.com). For subjects with ADHD-only, changes were significant on the Self-Control subscale and the LPS Total score when data were analyzed adjusted or unadjusted for baseline scores (Supplementary Tables 2 and 5). In subjects with dyslexia-only, changes were significant on the Self-Control subscale score and the LPS Total score when data were analyzed adjusted for baseline scores (Supplementary Table 6); no significant changes on the LPS Total score or either of the subscale scores were observed in subjects with dyslexia-only when data were not adjusted for baseline scores (Supplementary Table 5).
Similar to the acute treatment phase, in the extension phase it was assumed that analyses of score changes on the K-SCT Interview, MSCS, and WMTB-C were not biased, as these tests do not specifically measure ADHD symptoms; therefore, analyses were performed only with the a priori defined model that included an adjustment for baseline scores. Subjects with ADHD+D and ADHD-only experienced significant improvements on all K-SCT Interview subscales, whereas changes reached significance only for the Parent and Teacher subscales for subjects with dyslexia-only; changes were significantly different between subjects with ADHD+D and subjects with dyslexia-only for the K-SCT Parent subscale (Table 2). On the MSCS, changes in the Total score and all subscales, except the Family subscale, reached significance for subjects with ADHD+D; for subjects with dyslexia-only, no significant changes were observed; for subjects with ADHD-only, the Academic and the Competence subscales showed significant changes. On the WMTB-C, only the Phonological Loop component score was significantly improved in subjects with ADHD+D; in subjects with dyslexia-only, changes on the Phonological Loop component and on the Central Executive component reached significance; in subjects with ADHD-only, no significant changes were observed (Supplementary Table 5).
After 32 weeks, change in the K-SCT Interview Parent subscale score was significantly correlated with changes in ADHDRS-Parent:Inv scores (correlation coefficient of 0.48–0.63, p<0.001), and change in the K-SCT Interview Teacher subscale score was significantly correlated with changes in ADHDRS-IV-Teacher-Version scores (correlation coefficient of 0.46–0.71, p≤0.003) (Supplementary Table 7) (see online Supplementary Material at http://www.liebertonline.com). All correlations were positive, and showed that as K-SCT scores improved so did ADHDRS scores. The change in the K-SCT Youth subscale score showed a significant, but weak, correlation with changes in ADHDRS-Parent:Inv Inattentive and Total scores (correlation coefficient of 0.20–0.24, p≤0.016), but not the ADHDRS-IV-Teacher-Version scores. The baseline demographic parameter “ADHD subtype” was negatively correlated with ADHDRS-Parent:Inv scores (correlation coefficient of −0.70 to −0.48, p≤0.031) in ADHD-only patients, as well as with the MSCS Academic subscale score in dyslexia-only patients (correlation coefficient of −0.62, p=0.041). No other baseline demographic parameters showed strong and significant correlations to any of the presented outcome measures.
Safety
Overall, atomoxetine was well tolerated and the treatment-emergent adverse event (TEAE) profiles in both acute and extension phases were consistent with previous reports (Sumner et al. 2009). The most frequently observed TEAEs with atomoxetine treatment were nausea, fatigue, and upper abdominal pain (Table 3).
Table 3.
Treatment-Emergent Adverse Events in ≥5% of Subjects in Either Treatment Group and Statistically Significantly Differences Between Treatment Groups
| |
Acute phase |
Extension phase |
|||
|---|---|---|---|---|---|
| ATX (n=120) | PLB (n=89) | p value | ATX/ATX (n=84) | PLB/ATX (n=71) | |
| Subjects with ≥1 event | 108 (90.0) | 71 (79.8) | 0.046 | 40 (47.6) | 46 (64.8) |
| Nausea | 34 (28.3) | 5 (5.6) | <0.001 | 2 (2.4) | 8 (11.3) |
| Fatigue | 31 (25.8) | 9 (10.1) | 0.004 | 3 (3.6) | 9 (12.7) |
| Upper abdominal pain | 23 (19.2) | 6 (6.7) | 0.014 | 1 (1.2) | 6 (8.5) |
| Decreased appetite | 22 (18.3) | 4 (4.5) | 0.003 | 2 (2.4) | 9 (12.7) |
| Somnolence | 10 (8.3) | 0 | 0.006 | NA | NA |
| Aggression | 6 (5.0) | 1 (1.1) | 0.039 | NA | NA |
ATX, atomoxetine; NA, not available; PLB, placebo.
Discussion
In this randomized, placebo-controlled trial, we tested the a priori hypothesis that atomoxetine QD for ∼16 weeks would provide superior efficacy compared with placebo for the treatment of ADHD in children and adolescents with ADHD+D. Atomoxetine treatment resulted in significant improvements of several well-established measures of ADHD symptoms in children and adolescents with ADHD+D or ADHD-only, but, as expected, not in subjects with dyslexia-only. These ADHD symptom improvements were maintained during an open-label extension phase. Neither during the acute nor during the open-label treatment phases were significant differences in ADHD symptom improvements noted between atomoxetine-treated subjects with ADHD+D and those with ADHD-only. Our results support the findings of previous, smaller studies that show efficacy of atomoxetine treatment in children with ADHD+D (de Jong et al. 2009; Sumner et al. 2009). Demonstrating efficacy of atomoxetine in children with a comorbidity of ADHD+D comparable to its efficacy in children with ADHD-only is an important finding for clinicians faced with treatment decisions.
Adjustment for baseline disease characteristics
In the a priori analysis plan of this study, an adjustment for baseline disease characteristics was included to control for potential baseline differences between treatment groups; however, the authors realized, retrospectively, that this adjustment might have overcorrected these between-treatment-group differences, especially for the subjects with dyslexia-only. This subject group was not symptomatic for ADHD, and all ADHD-specific measures produced signals within the background noise level. Although this result was expected, the adjustment for baseline disease characteristic resulted in an unexpected effect—it amplified ADHD symptom signals within this group of subjects, and it artificially created significant changes. Therefore, the authors decided to repeat the analyses without an adjustment for baseline disease characteristics, which eliminated this artificial signal.
SCT
SCT has been shown to be responsive to psychosocial treatment (Pfiffner et al. 2007); however, to our knowledge, this is the first study to report a significant effect of any medication on SCT. Although this finding might be the result of chance because of the high number of comparisons that were performed in the current analyses, our results are interesting, in light of recent studies that identified a subset of patients with ADHD who have SCT, marked by sluggish-lethargic behavior, hypoactivity, and mental confusion (Barkley 2012). Currently, no information is available to indicate which percentage of patients with ADHD+D and ADHD-only could be classified as SCT. It is not yet clear whether SCT is a subtype or a completely different entity of ADHD (Penny et al. 2009). Some research supports the hypothesis that SCT and ADHD are distinct disorders with a high rate of comorbidity in affected individuals (Barkley 2012; Lee et al. 2013). Based on this research, we decided not to adjust SCT scores for baseline levels within our analyses. In consideration of shared genetic variables between ADHD and dyslexia, which seem to mainly connect reading difficulties and ADHD inattention symptoms (Paloyelis et al. 2010), one might expect a significant percentage of patients with ADHD+D to be affected by SCT. Future studies that examine those disease characteristics, and the potential differences in treatment response that might be associated with these classifications, are warranted.
Study limitations
Several factors limit the interpretation of our results. Overall, a higher percentage of subjects with Inattentive ADHD subtype participated in this study compared with previous studies, which, therefore, limits its comparisons with previous results. Excluding 6–10-year-old subjects contributes to a higher percentage of subjects with Inattentive ADHD; however, this observation might also reflect a higher likelihood of comorbidity with dyslexia in subjects with inattentive ADHD, and this likelihood would be supported by the connection of reading difficulties and ADHD inattention symptoms and by shared genetic variables between ADHD and dyslexia (Paloyelis et al. 2010). The results of our study also heavily relied on parent ratings, with very few measures in academic settings and low teacher participation, which could account for teacher ratings not reaching significance, whereas parent ratings reached significance on a number of measures. During individual clinic visits, a relatively large number of measures were administered to the subjects typically late in the afternoon after school, and this might have promoted exhaustion and biased the data. Finally, the validity of our results is limited to subjects 10–16 years of age.
Conclusions
This study demonstrates the efficacy of atomoxetine in the treatment of ADHD core symptoms as observed by parents, in children and adolescents with ADHD+D and ADHD-only.
Clinical Significance
The inattention dimension of ADHD symptoms has been associated with the experimental construct of SCT. This is the first study to report a significant effect of any medication on SCT.
Supplementary Material
Acknowledgments
The authors thank Dr. Alexandra Heinloth, Ms. Maria Rovere, and Ms. Angela Lorio, all full-time employees of PharmaNet/i3, an inVentiv Health Company, for their assistance in the preparation of this manuscript.
Disclosures
Ms. Wietecha is a full-time employee and minor stockholder of Eli Lilly and Company. Mr. Williams is a full-time employee of PharmaNet/i3, inVentiv Health Clinical, LLC, and was a full-time employee of Eli Lilly and Company until October 2010. Drs. Shaywitz and Shaywitz received research support from Eli Lilly and Company. Dr. Hooper is a consultant for and received research support from Eli Lilly and Company. Dr. Wigal received research support from Addrenex Pharmaceuticals, Inc., Eli Lilly and Company, McNeil Consumer Healthcare, National Institute of Child Health and Human Development, NextWave, PsychoGenics, Quintiles, Rhodes Pharmaceuticals, L.P., Otsuka America Pharmaceutical, Inc., Shionogi & Co. Ltd., and Shire. Dr. Wigal is also a consultant for Eli Lilly and Company, McNeil Consumer Healthcare, National Institutes of Health, NextWave, Noven Pharmaceuticals, Inc., NuTec, Shire, and Taisho Pharmaceutical Co., Ltd., and is on the speaker's bureau of McNeil Consumer Healthcare, Noven Pharmaceuticals, Inc., Shionogi & Co. Ltd., and Shire. Dr. Dunn received research support from Eli Lilly and Company, GlaxoSmithKline, and Supernus Pharmaceuticals. Dr. McBurnett received research support from Abbott Laboratories, Cephalon Inc., Eli Lilly and Company, Johnson & Johnson, McNeil Consumer Healthcare, National Institute of Mental Health, New River Pharmaceuticals Inc., Otsuka America Pharmaceutical, Inc., Shire, and Sigma-Tau Pharmaceuticals Inc. Dr. McBurnett is also a consultant for Eli Lilly and Company, Lexicon Pharmaceuticals, Inc., McNeil Consumer Healthcare, and Shire, and he received an honorarium from Lexicon Pharmaceuticals, Inc. The data were analyzed by Mr. David Williams who served as the statistical expert. The manuscript was written by Dr. Alexandra Heinloth in collaboration with all listed authors. Mr. Williams and Dr. Heinloth are full-time employees of Inventiv Health Clinical, LLC, which was contracted by Eli Lilly and Company to support the statistical analyses for and writing of this manuscript.
References
- American Psychiatric Association. 4th. Washington, DC: American Psychiatric Association; 2000. Diagnostic and Statistical Manual of Mental Disorders. Text Revision. [Google Scholar]
- Barkley RA. Distinguishing sluggish cognitive tempo from attention-deficit/hyperactivity disorder in adults. J Abnorm Psychol. 2012;121:978–990. doi: 10.1037/a0023961. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Fischer M. Predicting impairment in major life activities and occupational functioning in hyperactive children as adults: Self-reported executive function (EF) deficits versus EF tests. Dev Neuropsychol. 2011;36:137–161. doi: 10.1080/87565641.2010.549877. [DOI] [PubMed] [Google Scholar]
- Bracken BA. Austin, TX: PRO-ED; 1992. Multidimensional Self Concept Scale (MSCS) Examiner's Manual. [Google Scholar]
- Brod M. Schmitt E. Goodwin M. Hodgkins P. Niebler G. ADHD burden of illness in older adults: a life course perspective. Qual Life Res. 2012;21:795–799. doi: 10.1007/s11136-011-9981-9. [DOI] [PubMed] [Google Scholar]
- Carlson CL. Mann M. Sluggish cognitive tempo predicts a different pattern of impairment in the attention deficit hyperactivity disorder, predominantly inattentive type. J Clin Child Adolesc Psychol. 2002;31:123–129. doi: 10.1207/S15374424JCCP3101_14. [DOI] [PubMed] [Google Scholar]
- de Jong CG. Van De Voorde S. Roeyers H. Raymaekers R. Allen AJ. Knijff S. Verhelst H. Temmink AH. Smit LM. Rodriques–Pereira R. Vandenberghe D. van Welsen I. ter Schuren L. Al–Hakim M. Amin A. Vlasveld L. Oosterlaan J. Sergeant JA. Differential effects of atomoxetine on executive functioning and lexical decision in attention-deficit/hyperactivity disorder and reading disorder. J Child Adolesc Psychopharmacol. 2009;19:699–707. doi: 10.1089/cap.2009.0029. [DOI] [PubMed] [Google Scholar]
- DuPaul G. Power T. Anastopolous A. Reid R. New York: Guilford Publications; 1998. ADHD Rating Scale-IV: Checklists, Norms, Clinical Interpretation. [Google Scholar]
- Garner AA. Marceaux JC. Mrug S. Patterson C. Hodgens B. Dimensions and correlates of attention deficit/hyperactivity disorder and sluggish cognitive tempo. J Abnorm Child Psychol. 2010;38:1097–1107. doi: 10.1007/s10802-010-9436-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germano E. Gagliano A. Curatolo P. Comorbidity of ADHD and dyslexia. Dev Neuropsychol. 2010;35:475–493. doi: 10.1080/87565641.2010.494748. [DOI] [PubMed] [Google Scholar]
- Hartman CA. Willcutt EG. Rhee SH. Pennington BF. The relation between sluggish cognitive tempo and DSM-IV ADHD. J Abnorm Child Psychol. 2004;32:491–503. doi: 10.1023/b:jacp.0000037779.85211.29. [DOI] [PubMed] [Google Scholar]
- Hinshaw SP. Carte ET. Sami N. Treuting JJ. Zupan BA. Preadolescent girls with attention-deficit/hyperactivity disorder: II. Neuropsychological performance in relation to subtypes and individual classification. J Consult Clin Psychol. 2002;70:1099–1111. doi: 10.1037//0022-006x.70.5.1099. [DOI] [PubMed] [Google Scholar]
- Houck G. Kendall J. Miller A. Morrell P. Wiebe G. Self-concept in children and adolescents with attention deficit hyperactivity disorder. J Pediatr Nurs. 2011;26:239–247. doi: 10.1016/j.pedn.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang–Pollock CL. Nigg JT. Carr TH. Deficient attention is hard to find: Applying the perceptual load model of selective attention to attention deficit hyperactivity disorder subtypes. J Child Psychol Psychiatry. 2005;46:1211–1218. doi: 10.1111/j.1469-7610.2005.00410.x. [DOI] [PubMed] [Google Scholar]
- Kaufman J. Birmaher B. Brent D. Rao U. Flynn C. Moreci P. Williamson D. Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Lee S. Burns GL. Snell J. McBurnett K. Validity of the sluggish cognitive tempo symptom dimension in children: Sluggish cognitive tempo, ADHD-inattention as distinct symptom dimensions. J Abnorm Child Psychol. 2013 doi: 10.1007/s10802-013-9714-3. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- McBurnett K. Pfiffner LJ. Frick PJ. Symptom properties as a function of ADHD type: An argument for continued study of sluggish cognitive tempo. J Abnorm Child Psychol. 2001;29:207–213. doi: 10.1023/a:1010377530749. [DOI] [PubMed] [Google Scholar]
- Paloyelis Y. Rijsdijk F. Wood AC. Asherson P. Kuntsi J. The genetic association between ADHD symptoms and reading difficulties: The role of inattentiveness and IQ. J Abnorm Child Psychol. 2010;38:1083–1095. doi: 10.1007/s10802-010-9429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny AM. Waschbusch DA. Klein RM. Corkum P. Eskes G. Developing a measure of sluggish cognitive tempo for children: content validity, factor structure, and reliability. Psychol Assess. 2009;21:380–389. doi: 10.1037/a0016600. [DOI] [PubMed] [Google Scholar]
- Pfiffner LJ. Yee Mikami A. Huang–Pollock C. Easterlin B. Zalecki C. McBurnett K. A randomized, controlled trial of integrated home-school behavioral treatment for ADHD, predominantly inattentive type. J Am Acad Child Adolesc Psychiatry. 2007;46:1041–1050. doi: 10.1097/chi.0b013e318064675f. [DOI] [PubMed] [Google Scholar]
- Pickering S. Gathercole S. Working Memory Test Battery for Children (WMTB-C) Manual. London: Pearson Assessment; 2001. [Google Scholar]
- Reeves CB. Palmer S. Gross AM. Simonian SJ. Taylor L. Willingham E. Mulhern RK. Brief report: Sluggish cognitive tempo among pediatric survivors of acute lymphoblastic leukemia. J Pediatr Psychol. 2007;32:1050–1054. doi: 10.1093/jpepsy/jsm063. [DOI] [PubMed] [Google Scholar]
- Ridley C. The experiences of nursing students with dyslexia. Nurs Stand. 2011;25:35–42. doi: 10.7748/ns2011.02.25.24.35.c8342. [DOI] [PubMed] [Google Scholar]
- Sexton CC. Gelhorn H. Bell J. Classi P. The co-occurrence of reading disorder and ADHD: Epidemiology, treatment, psychosocial impact, and economic burden. J Learn Disabil. 2012;45:538–564. doi: 10.1177/0022219411407772. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE. Shaywitz BA. Paying attention to reading: The neurobiology of reading and dyslexia. Dev Psychopathol. 2008;20:1329–1349. doi: 10.1017/S0954579408000631. [DOI] [PubMed] [Google Scholar]
- Skirbekk B. Hansen BH. Oerbeck B. Kristensen H. The relationship between sluggish cognitive tempo, subtypes of attention-deficit/hyperactivity disorder, and anxiety disorders. J Abnorm Child Psychol. 2011;39:513–525. doi: 10.1007/s10802-011-9488-4. [DOI] [PubMed] [Google Scholar]
- Smith–Spark JH. Fawcett AJ. Nicolson RI. Fisk JE. Dyslexic students have more everyday cognitive lapses. Memory. 2004;12:174–182. doi: 10.1080/09658210244000450. [DOI] [PubMed] [Google Scholar]
- Sumner CR. Gathercole S. Greenbaum M. Rubin R. Williams D. Hollandbeck M. Wietecha L. Atomoxetine for the treatment of attention-deficit/hyperactivity disorder (ADHD) in children with ADHD and dyslexia. Child Adolesc Psychiatry Ment Health. 2009;3:40. doi: 10.1186/1753-2000-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstedt C. Bohlin G. DSM-IV-defined inattention and sluggish cognitive tempo: independent and interactive relations to neuropsychological factors and comorbidity. Child Neuropsychol. 2010;16:350–365. doi: 10.1080/09297041003671176. [DOI] [PubMed] [Google Scholar]
- Willcutt EG. Betjemann RS. McGrath LM. Chhabildas NA. Olson RK. DeFries JC. Pennington BF. Etiology and neuropsychology of comorbidity between RD and ADHD: The case for multiple-deficit models. Cortex. 2010;46:1345–1361. doi: 10.1016/j.cortex.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt EG. Pennington BF. Olson RK. DeFries JC. Understanding comorbidity: A twin study of reading disability, attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:709–714. doi: 10.1002/ajmg.b.30310. [DOI] [PubMed] [Google Scholar]
- Yee Mikami A. Huang–Pollock CL. Pfiffner LJ. McBurnett K. Hangai D. Social skills differences among attention-deficit/hyperactivity disorder types in a chat room assessment task. J Abnorm Child Psychol. 2007;35:509–521. doi: 10.1007/s10802-007-9108-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.